A Comparison of the Influence of Kraft Lignin and the Kraft Lignin/Silica System as Cell Carriers on the Stability and Efficiency of the Anaerobic Digestion Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Carriers
2.2. Preparation of Carriers
2.3. Bacillus Amyloliquefaciens Cell Biomass
2.4. Experimental Set-Up of Continuous Systems
2.4.1. Structure and Operation of Digester
2.4.2. Anaerobic Digestion
2.5. Analytical Methods
2.5.1. Physicochemical Analysis of Substrate, Digestate and Biogas Samples
2.5.2. Physicochemical Characteristics of Carrier Materials
2.5.3. Microbiological and Biochemical Analysis of Digestate
2.5.4. Statistical Analyses
3. Results and Discussion
3.1. Characterisation of Substrate and Inoculum
3.2. Characterisation of Carrier Materials
3.2.1. Dispersive and Morphological Properties
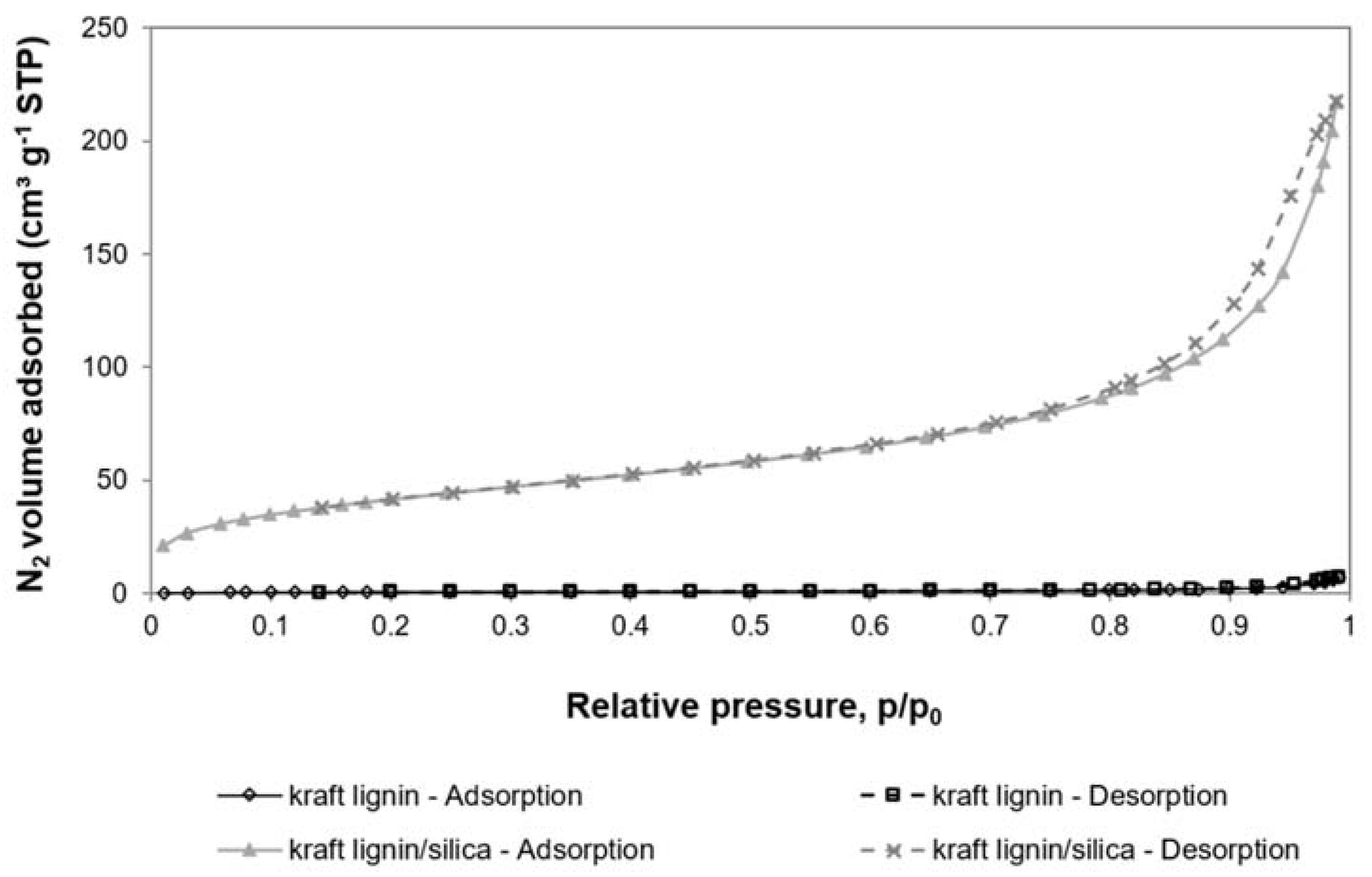
3.2.2. Porous Structure Properties
3.2.3. FTIR Spectroscopy and Elemental Analysis
3.2.4. Thermal Analysis
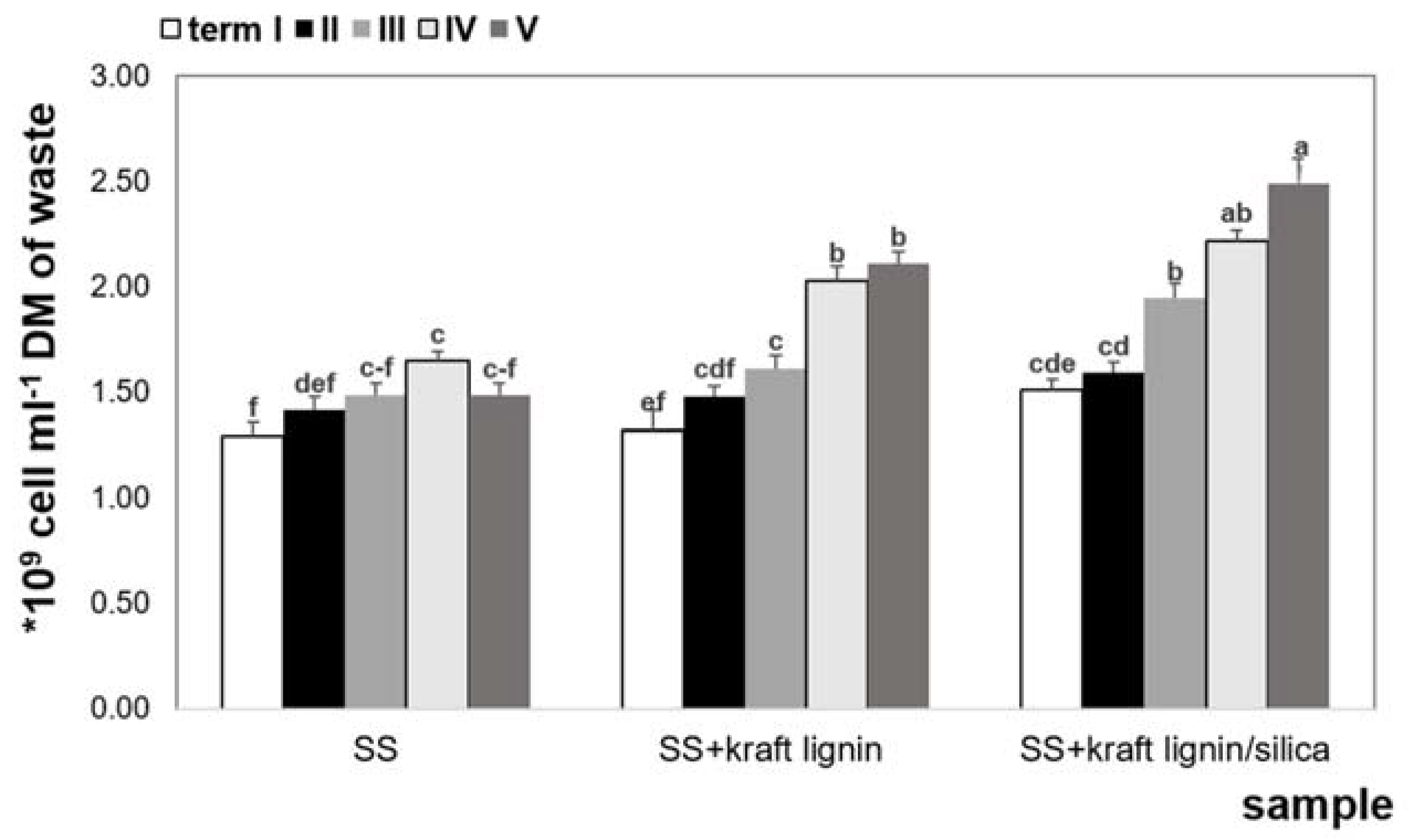
3.3. Cell Growth and Cell Biomass
3.4. Analysis of Digestate Samples
3.4.1. Total Bacterial Count
3.4.2. Dehydrogenase Activity
3.4.3. Zinc and Lead Concentration
3.5. Continuous Mesophilic Anaerobic Digestion
3.5.1. Monitoring Process Stability
3.5.2. Process Efficiency
4. Conclusions
- The kraft lignin/silica material (1:4 by weight) was characterised by better properties than pure kraft lignin as a cell carrier: very well-developed BET specific surface, high pore volume, high thermal stability.
- The proliferation of Bacillus amyloliquefaciens bacterial cells was more intensive in the kraft lignin/silica system, where the cellular biomass was twice as large as in the culture with pure kraft lignin.
- The quantitative analysis conducted by means of in situ fluorescence showed increased proliferation of microorganisms during the anaerobic digestion in the SS + kraft lignin/silica variant. The addition of pure kraft lignin also increased cell proliferation, but to a lesser extent.
- The highest dehydrogenase activity was observed during the decomposition of the sample with silica. In the course of the process, the value of this parameter tended to decrease in each experimental variant.
- As indicated by the values of monitoring parameters, the course of the AD process was stable in the SS and SS + kraft lignin/silica samples. There was a temporary increase in the VFA/TA ratio accompanied by simultaneous inhibition of biogas/methane production in the SS + kraft lignin sample. It is most likely that this effect was caused by the release of lignin decomposition products, i.e., furfural and/or derivatives of phenolic alcohols.
- During the continuous anaerobic decomposition of three samples based on sewage sludge, the largest amount of biogas was produced from the SS + kraft lignin/silica system (689 m3 Mg−1 VS, including 413 m3 Mg−1 VS methane). There were comparable amounts of biogas produced from the SS sample (526 m3 Mg−1 VS biogas, including 51% methane) and the SS + kraft lignin sample (586 m3 Mg−1 VS biogas, including 54% methane).
Author Contributions
Funding
Conflicts of Interest
References
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar]
- Weiß, S.; Zankel, A.; Lebuhn, M.; Petrak, S.; Somitsch, W.; Guebitz, G.M. Investigation of microorganisms colonising activated zeolites during anaerobic biogas production from grass silage. Bioresour. Technol. 2011, 102, 4353–4359. [Google Scholar] [PubMed]
- Purnomo, C.W.; Mellyanawaty, M.; Budhijanto, W. Simulation and experimental study on iron impregnated microbial immobilization in zeolite for production of biogas. Waste Biomass Valor. 2017, 8, 2413–2421. [Google Scholar]
- Ivanova, G.; Rákhely, G.; Kovács, K.L. Hydrogen production from biopolymers by Caldicellulosiruptor saccharolyticus and stabilization of the system by immobilization. Int. J. Hydrogen Energy 2008, 33, 6953–6961. [Google Scholar]
- Gong, W.; Ran, Z.; Ye, F.; Zhao, G. Lignin from bamboo shoot shells as an activator and novel immobilizing support for α-amylase. Food Chem. 2017, 228, 455–462. [Google Scholar] [PubMed]
- Ge, Y.; Qin, L.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar]
- Milczarek, G.; Inganas, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y.; Sergienko, V.I. Hydrolysis lignin-based organic electrode material for primary lithium batteries. J. Solid State Electrochem. 2014, 17, 2611–2621. [Google Scholar]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar]
- Jasiukaitytė Grojzdek, E.; Huš, M.; Grilc, M.; Likozar, B. Acid catalysed α-O-4 aryl-ether bond cleavage in methanol/(aqueous) ethanol: Understanding depolymerisation of a lignin model compound during organosolv pretreatment. Sci. Rep. 2020, 10, 11037. [Google Scholar] [PubMed]
- Gao, C.; Zhou, L.; Yao, S.; Qin, C.; Fatehi, P. Phosphorylated kraft lignin with improved thermal stability. Int. J. Biol. Macromol. 2020, 162, 1642–1652. [Google Scholar]
- Settera, C.; Costa, K.L.S.; de Oliveira, T.J.P.; Mendes, R.F. The effects of kraft lignin on the physicomechanical quality of briquettes produced with sugarcane bagasse and on the characteristics of the bio-oil obtained via slow pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar]
- Yan, Q.; Boardman, C.R.; Cai, Z. Thermal stability of metal-lignin composites prepared by coprecipitation method. Thermochim. Acta 2020, 690, 178659. [Google Scholar]
- Bjelić, A.; Likozar, B.; Grilc, M. Scaling of lignin monomer hydrogenation, hydrodeoxygenation and hydrocracking reaction micro-kinetics over solid metal/acid catalysts to aromatic oligomers. Chem. Eng. J. 2020, 399, 125712. [Google Scholar]
- Bartocci, P.; Tschentscher, R.; Stensrød, R.E.; Barbanera, M.; Fantozzi, F. Kinetic analysis of digestate slow pyrolysis with the application of the master-plots method and independent parallel reactions scheme. Molecules 2019, 24, 1657–1672. [Google Scholar]
- Jiao, G.J.; Xu, Q.; Cao, S.L.; Peng, P.; She, D. Controlled-release fertilizer with lignin used to trap urea/hydroxymethylurea/urea-formaldehyde polymers. Bioresources 2018, 13, 1711–1728. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A. Cell immobilization on lignin–polyvinylpyrrolidone material used for anaerobic digestion of waste wafers and sewage sludge. Environ. Eng. Sci. 2019, 36, 478–490. [Google Scholar]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K. Kraft lignin grafted with polyvinylpyrrolidone as a novel microbial carrier in biogas production. Energies 2018, 11, 3246–3268. [Google Scholar]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073–2093. [Google Scholar]
- Jesionowski, T. Characterisation of pigments obtained by adsorption of C.I. Basic Blue 9 and C.I. Acid Orange 52 dyes onto silica particles precipitated via the emulsion route. Dyes Pigment. 2005, 67, 81–92. [Google Scholar]
- Jesionowski, T.; Nowacka, M.; Ciesielczyk, F. Electrokinetic properties of hybrid pigments obtained via adsorption of organic dyes on the silica support. Pigment Resin Technol. 2012, 41, 9–19. [Google Scholar]
- Hayashi, J.; Shoji, T.; Watada, Y.; Muroyama, K. Preparation of silica–lignin xerogel. Langmuir 1997, 13, 4185–4186. [Google Scholar]
- Štandeker, S.; Novak, Z.; Knez, Ž. Adsorption of toxic organic compounds from water with hydrophobic silica aerogels. J. Colloidal Interface Sci. 2007, 310, 362–368. [Google Scholar]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [PubMed]
- Saikia, J.; Yazdimamaghani, M.; Moghaddam, S.P.H.; Ghandehari, H. Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS Appl. Mater. Interfaces 2016, 8, 34820–34832. [Google Scholar] [PubMed] [Green Version]
- Clemments, A.M.; Botella, P.; Landry, C.C. Protein adsorption from biofluids on silica nanoparticles: Corona analysis as a function of particle diameter and porosity. ACS Appl. Mater. Interfaces 2015, 7, 21682–21689. [Google Scholar]
- Dai, X.; Xu, Y.; Dong, B. Effect of the micron-sized silica particles (MSSP) on biogas conversion of sewage sludge. Water Res. 2017, 115, 220–228. [Google Scholar]
- Chen, S.; Dong, B.; Yang, D.; Li, N.; Dai, X. Micron-sized silica particles in wastewater influenced the distribution of organic matters in sludge and their anaerobic degradation. J. Hazard. Mater. 2020, 393, 122340. [Google Scholar]
- Chauhan, A.; Ogram, A. Evaluation of support matrices for immobilization of anaerobic consortia for efficient carbon cycling in waste regeneration. Biochem. Biophys. Res. Commun. 2005, 327, 884–893. [Google Scholar]
- German Institute for Standardization. Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Norm DIN 38 414-S8; German Institute for Standardization: Berlin, Germany, 1985. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37–50. [Google Scholar]
- Amann, R.I.; Krumholz, L.; Stahl, D.A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 1990, 172, 762–770. [Google Scholar]
- Amann, R.I.; Binder, B.J.; Olson, R.J.; Chisholm, S.W.; Devereux, R.; Stahl, D.A. Combination of 16SrRNAtargeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 1990, 56, 1919–1925. [Google Scholar]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of dehydrogenase activity in acid soilsrich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasiński, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: A pilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2019–2030. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. S 2016, 23, 99–115. [Google Scholar]
- Ralph, J.; Lundguist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Wood Chemistry and Wood Biotechnology; Walter de Gruyter: Berlin, Germany, 2009. [Google Scholar]
- Kupiec, K.; Konieczka, P. Charakterystyka, procesy chemicznej modyfikacji oraz zastosowanie krzemionki. Ecol. Chem. Eng. 2007, 14, 473–487. [Google Scholar]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar]
- Klapiszewski, L.; Rzemieniecki, T.; Krawczyk, M.; Malina, D.; Norman, M.; Zdarta, J.; Majchrzak, I.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. Kraft lignin/silica–AgNPs as a functional material with antibacterial activity. Colloids Surf. B Biointerfaces 2015, 134, 220–228. [Google Scholar]
- Ciesielczyk, F.; Klapiszewski, Ł.; Szwarc-Rzepka, K.; Jesionowski, T. A novel method of combination of Kraft lignin with synthetic mineral suport. Adv. Powder Technol. 2014, 25, 695–703. [Google Scholar]
- Yamamoto, T.; Yabushita, S.; Irisawa, T.; Tanabe, Y. Enhancement of bending strength, thermal stability and recyclability of carbon-fiber-reinforced thermoplastics by using silica colloids. Compos. Sci. Technol. 2019, 18, 107665. [Google Scholar]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar]
- Bhatt, B.; Prajapati, V.; Patel, K.; Trivedi, U. Kitchen waste for economical amylase production using Bacillus amyloliquefaciens KCP2. Biocatal. Agric. Biotechnol. 2020, 26, 101654. [Google Scholar]
- Mei, J.; Shen, X.; Gang, L.; Xu, H.; Wu, F.; Sheng, L. A novel lignin degradation bacteria-Bacillus amyloliquefaciens SL-7 used to degrade straw lignin efficiently. Bioresour. Technol. 2020, 310, 123445. [Google Scholar] [PubMed]
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119. [Google Scholar]
- Shaheen, R.; Asgher, M.; Hussain, F.; Bhatti, H.N. Immobilized lignin peroxidase from Ganoderma lucidum ibl-05 with improved dye decolorization and cytotoxicity reduction properties. Int. J. Biol. Macromol. 2017, 103, 57–64. [Google Scholar]
- Choromański, P.; Karwowska, E.; Łebkowska, M. The influence of petroleum products on the methane fermentation process. J. Hazard. Mater. 2016, 301, 327–331. [Google Scholar]
- Liang, Y.G.; Xu, L.; Bao, J.; Firmin, K.A.; Zong, W. Attapulgite enhances methane production from anaerobic digestion of pig slurry by changing enzyme activities and microbial community. Renew. Energy 2020, 145, 222–232. [Google Scholar]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.; Zhang, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar]
- Astals, S.; Nolla-Ardčvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar]
- Quintas, M.; Guimarães, C.; Baylina, J.; Brandão, T.R.S.; Silva, C.L.M. Multiresponse modelling of the caramelisation reaction. Innov. Food Sci. Emerg. Technol. 2007, 8, 306–315. [Google Scholar]
- Witaszek, K.; Pilarska, A.A.; Pilarski, K. Selected methods of vegetable raw material pretreatment used in biogas production. Econom. Environ. 2015, 2, 138–152. [Google Scholar]
- Pilarska, A.A. Anaerobic co-digestion of waste wafers from the confectionery production with sewage sludge. Pol. J. Environ. Stud. 2018, 27, 237–245. [Google Scholar]
- Han, D.; Lee, C.Y.; Soon, W.; Chang, S.W.; Kim, D.J. Enhanced methane production and wastewater sludge stabilization of a continuous full scale thermal pretreatment and thermophilic anaerobic digestion. Bioresour. Technol. 2017, 245, 1162–1167. [Google Scholar]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.T. Anaerobic co-digestion of wastewater sludge: A review of potential co-substrates and operating factors for improved methane yield. Processes 2020, 8, 39–60. [Google Scholar]
- Klapiszewski, Ł.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar]
- De Oliveira, L.L.; Duarte, I.C.S.; Sakamoto, I.K.; Varesche, M.B.A. Influence of support material on the immobilization of biomass for the degradation of linear alkylbenzene sulfonate in anaerobic reactors. J. Environ. Manag. 2009, 90, 1261–1268. [Google Scholar]
- Abbas, Y.; Yun, S.; Wang, Z.; Zhang, Y.; Zhang, X.; Wang, K. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2021, 135, 110378. [Google Scholar]
- Watanabe, R.; Tada, C.; Baba, Y.; Fukuda, Y.; Nakai, Y. Enhancing methane production during the anaerobic digestion of crude glycerol using Japanese cedar charcoal. Bioresour. Technol. 2013, 150, 387–392. [Google Scholar]
- Carrasco, J.; Kovács, K.; Czech, V.; Fodor, F.; Lucena, J.J.; Vértes, A.; Hernandez-Apaolaza, L. Influence of pH, iron source, and Fe/ligand ratio on iron speciation in lignosulfonate complexes studied using mössbauer spectroscopy. Implications on their fertilizer properties. J. Agric. Food Chem. 2012, 60, 3331–3340. [Google Scholar]














| Material | pH | TS | VS | TKN |
|---|---|---|---|---|
| (-) | (wt %) | (wt %TS) | (wt %TS) | |
| SS | 6.83 | 4.6 | 81.4 | 0.33 |
| Inoculum | 7.15 | 2.4 | 65.7 | 0.19 |
| Carriers | ABET | Vp | Sp |
|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (nm) | |
| Kraft lignin | 1.9 | 0.01 | 18.4 |
| Kraft lignin/silica | 151.5 | 0.35 | 10.8 |
| Carriers | C | H | S | O |
|---|---|---|---|---|
| (%) | (%) | (%TS) | (%TS) | |
| Kraft lignin | 60.29 | 5.61 | 0.57 | ˗ |
| Kraft lignin/silica | 4.03 | 0.4 | ˗ | 3.41 |
| Parameter | Term | Combination | Interaction |
|---|---|---|---|
| Bacteria | 168.77 *** | 296.91 *** | 32.59 *** |
| Dehydrogenase | 133.57 *** | 38.21 *** | 1.01 ns |
| Fractions Separation (%) | Concentrations Balance (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Zn | Pb | ||||||
| Supernat. | Sludge | Supernat. | Sludge | Total | Supernat. | Sludge | Total |
| SS Sample | |||||||
| 63 | 37 | 0.31 | 41.10 | 41.42 | 0.013 | 2.765 | 2.778 |
| 63 | 37 | 0.28 | 41.04 | 41.32 | 0.017 | 2.754 | 2.771 |
| 65 | 35 | 0.36 | 42.22 | 42.58 | 0.022 | 2.731 | 2.753 |
| SS + Kraft Lignin Sample | |||||||
| 45 | 55 | 1.50 | 46.48 | 47.97 | 0.020 | 2.725 | 2.745 |
| 45 | 55 | 2.13 | 48.18 | 50.31 | 0.024 | 2.647 | 2.887 |
| 46 | 54 | 2.24 | 47.92 | 50.16 | 0.028 | 2.708 | 2.736 |
| SS + Kraft Lignin/Silica Sample | |||||||
| 52 | 48 | 0.45 | 44.29 | 44.74 | 0.010 | 1.781 | 1.792 |
| 52 | 48 | 1.26 | 46.17 | 47.43 | 0.031 | 1.761 | 1.793 |
| 58 | 42 | 1.23 | 45.76 | 46.99 | 0.029 | 1.414 | 1.444 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarski, K.; Olesienkiewicz, A. A Comparison of the Influence of Kraft Lignin and the Kraft Lignin/Silica System as Cell Carriers on the Stability and Efficiency of the Anaerobic Digestion Process. Energies 2020, 13, 5803. https://doi.org/10.3390/en13215803
Pilarska AA, Wolna-Maruwka A, Niewiadomska A, Pilarski K, Olesienkiewicz A. A Comparison of the Influence of Kraft Lignin and the Kraft Lignin/Silica System as Cell Carriers on the Stability and Efficiency of the Anaerobic Digestion Process. Energies. 2020; 13(21):5803. https://doi.org/10.3390/en13215803
Chicago/Turabian StylePilarska, Agnieszka A., Agnieszka Wolna-Maruwka, Alicja Niewiadomska, Krzysztof Pilarski, and Artur Olesienkiewicz. 2020. "A Comparison of the Influence of Kraft Lignin and the Kraft Lignin/Silica System as Cell Carriers on the Stability and Efficiency of the Anaerobic Digestion Process" Energies 13, no. 21: 5803. https://doi.org/10.3390/en13215803





