Passive Regulation of the Water Content at the Anode Chamber under Dead-Ended Conditions: Innovative Design of an Air-Breathing Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ex-Situ Permeability Tests
2.2. Fuel Cell Tests
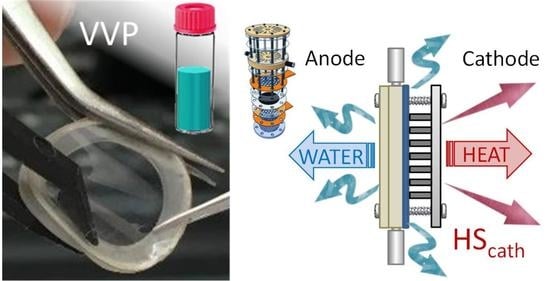
2.2.1. Fuel Cell Description
2.2.2. In Situ Permeation Tests
3. Results and Discussion
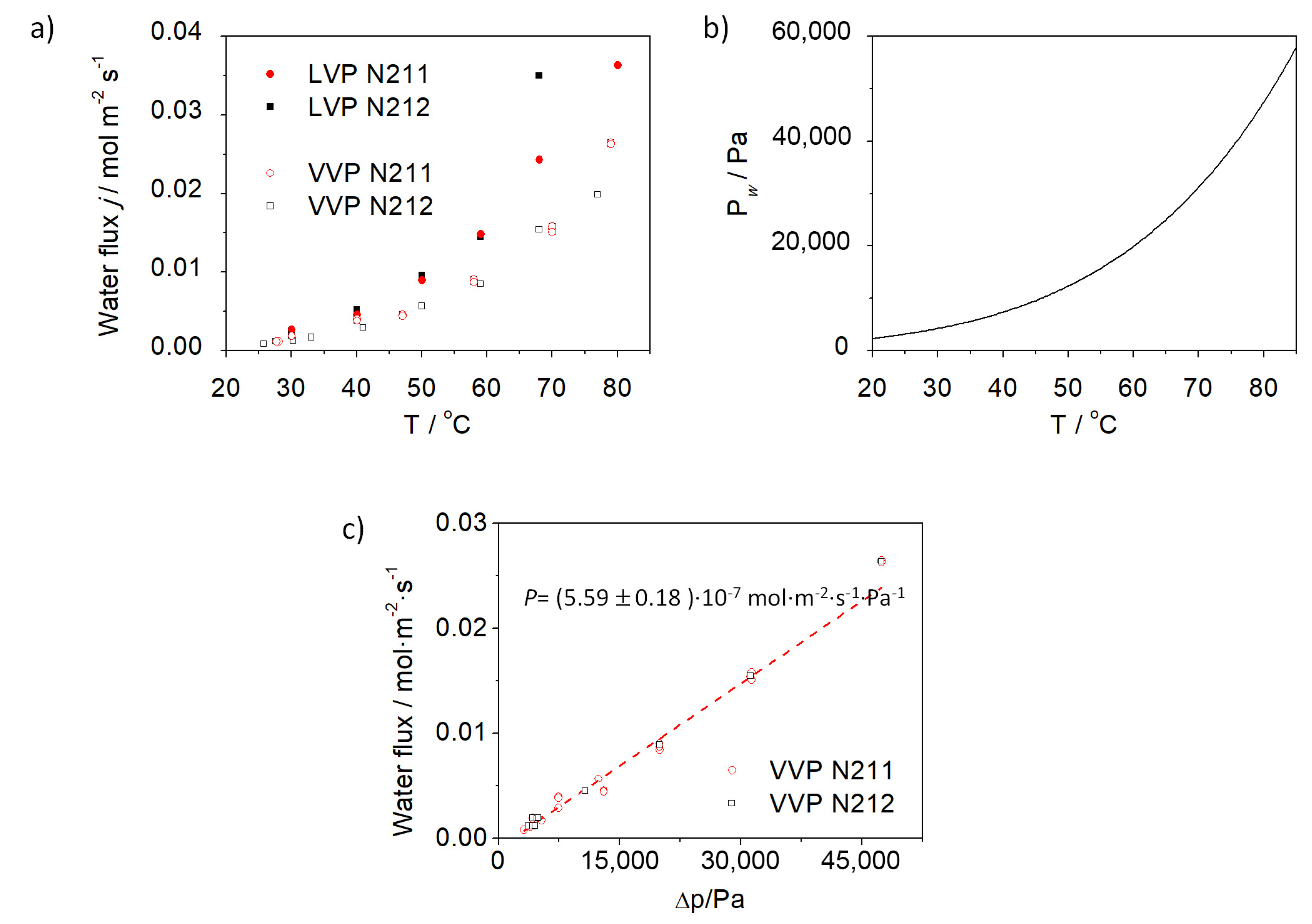
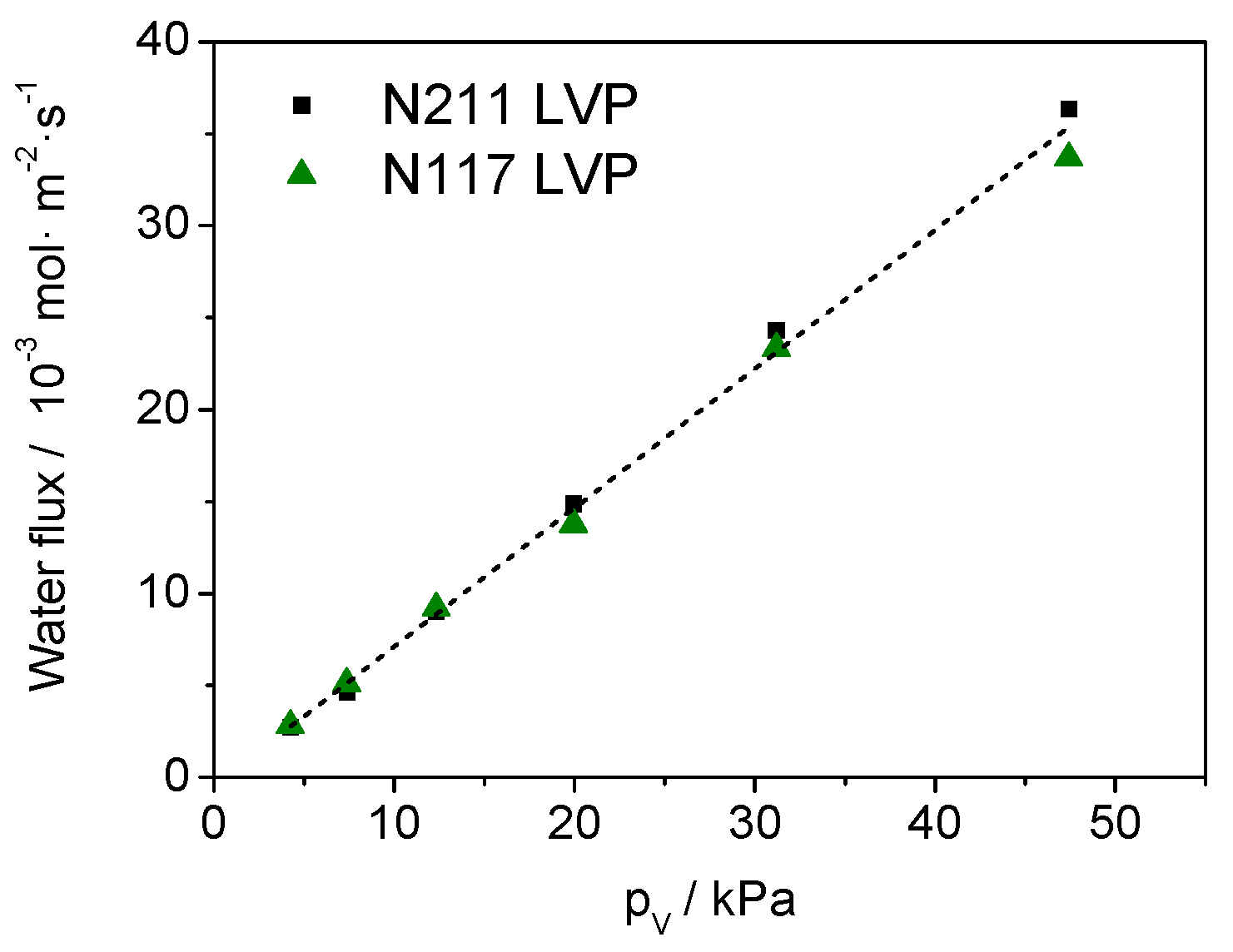
3.1. Permeability Tests on Nafion Membranes
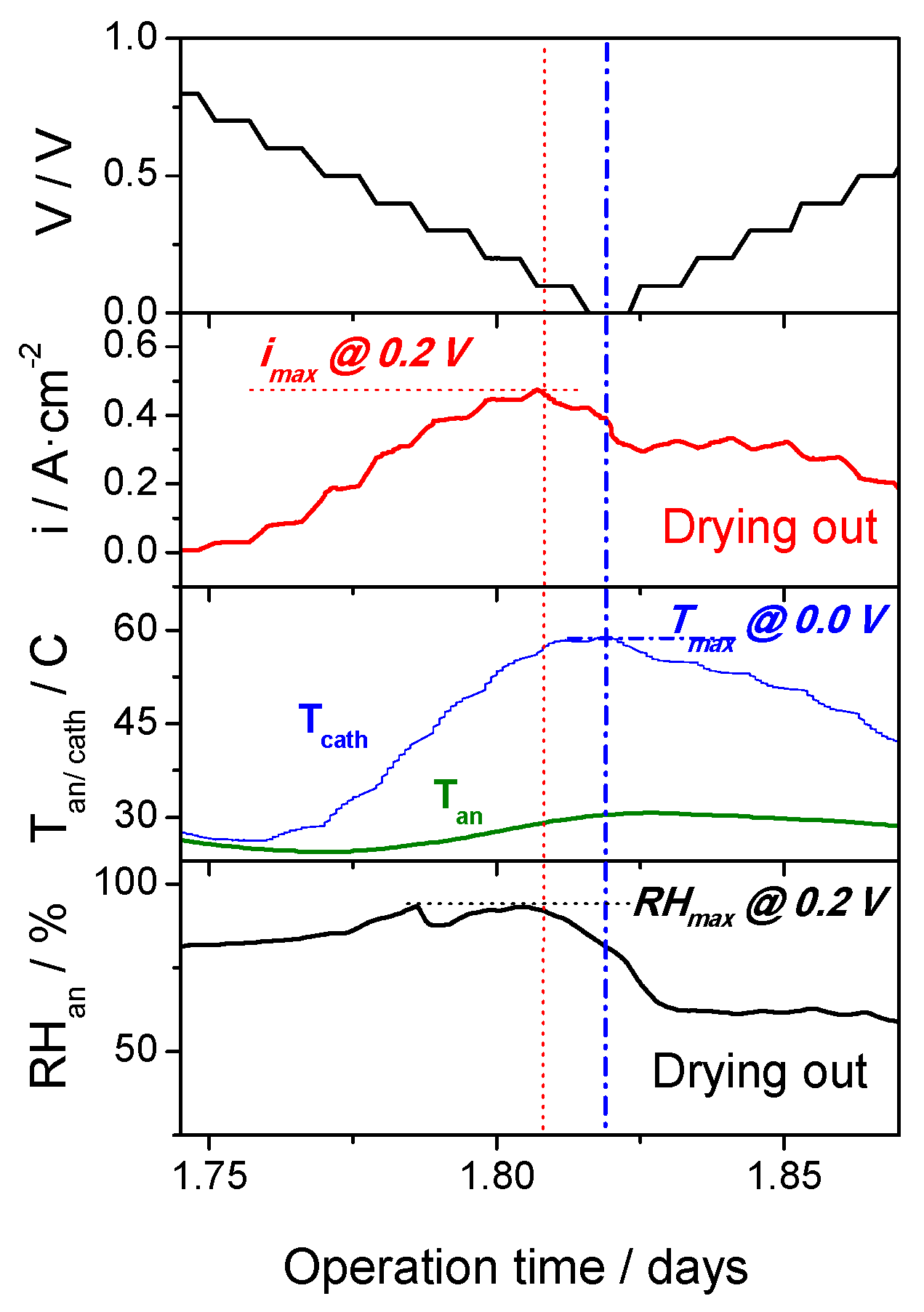
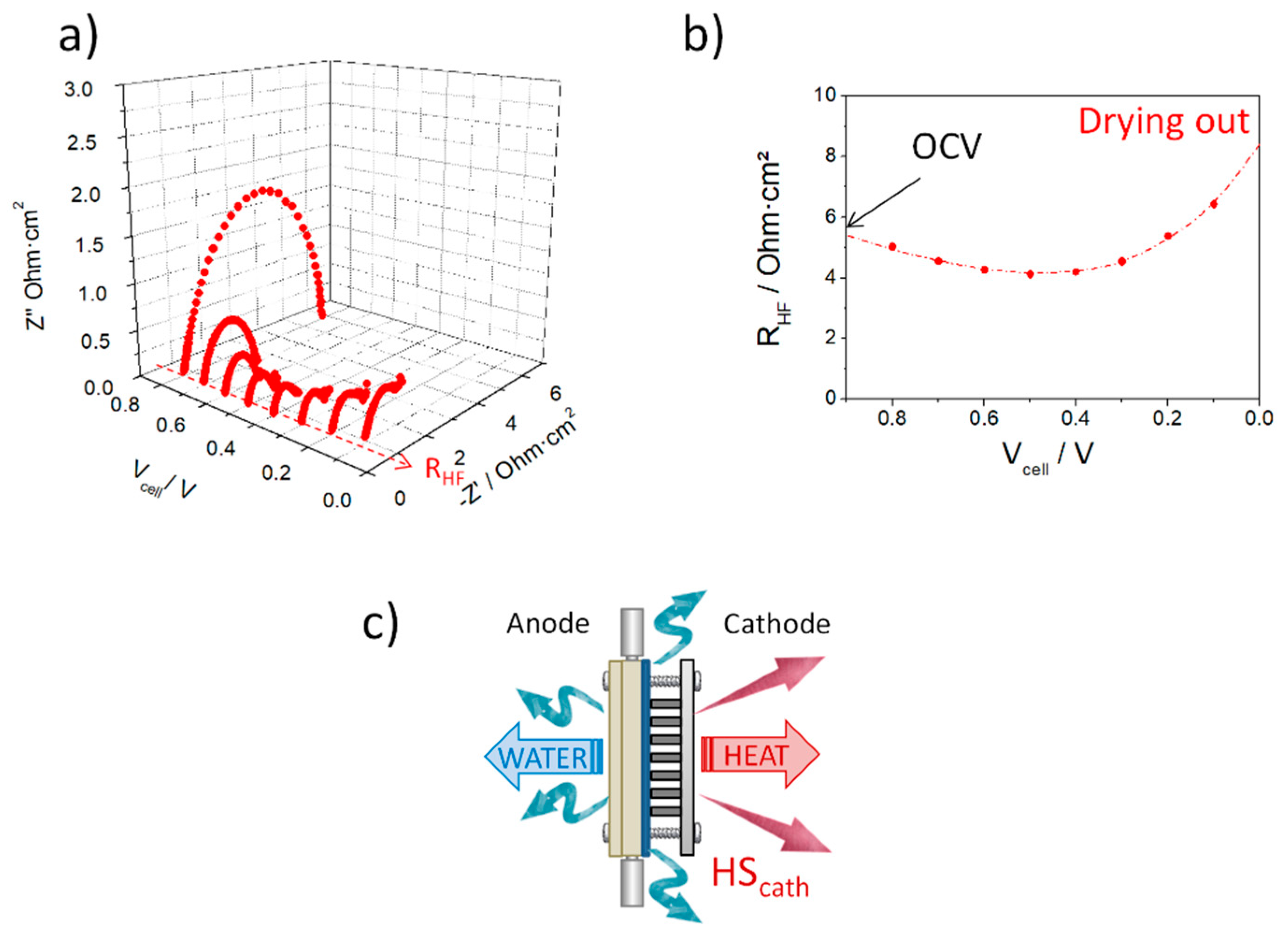
3.2. Evaluation of the Self-Regulation Humidity Anode under Fuel Cell Operation
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, L.; Huang, B.; Cheng, Z.; Jian, Q. Improved water management by alternating air flow directions in a proton exchange membrane fuel cell stack. J. Power Sources 2020, 466, 228311. [Google Scholar] [CrossRef]
- Ji, M.; Wei, Z. A review of water management in polymer electrolyte membrane fuel cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Degradation, Efficiency, and Equilibrium of a Dead-Ended Anode Fuel Cell. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2013. [Google Scholar]
- Chen, B.; Ke, W.; Luo, M.; Wang, J.; Tu, Z.; Pan, M.; Zhang, H.; Liu, X.; Liu, W. Operation characteristics and carbon corrosion of PEMFC (Proton exchange membrane fuel cell) with dead-ended anode for high hydrogen utilization. Energy 2015, 91, 799–806. [Google Scholar] [CrossRef]
- Ge, S.; Wang, C.-Y. Liquid Water Formation and Transport in the PEFC Anode. J. Electrochem. Soc. 2007, 154, B998. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Conde, J.J.; Chaparro, A.M. 2-Fundamentals and components of portable hydrogen fuel-cell systems. In Portable Hydrogen Energy Systems; Ferreira-Aparicio, P., Chaparro, A.M.B.T.-P.H.E.S., Eds.; Academic Press: London, UK, 2018; pp. 15–39. ISBN 978-0-12-813128-2. [Google Scholar]
- Ziegler, C.; Schmitz, A.; Tranitz, M.; Fontes, E.; Schumacher, J.O. Modeling planar and self-breathing fuel cells for use in electronic devices. J. Electrochem. Soc. 2004, 151, A2028–A2041. [Google Scholar] [CrossRef]
- Liu, W.; Wan, L.; Liu, J.; Zhao, M.; Zou, Z. Performance improvement of the open-cathode proton exchange membrane fuel cell by optimizing membrane electrode assemblies. Int. J. Hydrogen Energy 2015, 40, 7159–7167. [Google Scholar] [CrossRef]
- Chen, J.; Siegel, J.B.; Stefanopoulou, A.G.; Waldecker, J.R. Optimization of purge cycle for dead-ended anode fuel cell operation. Int. J. Hydrogen Energy 2013, 38, 5092–5105. [Google Scholar] [CrossRef]
- Matsuura, T.; Chen, J.; Siegel, J.; Stefanopoulou, A. Degradation phenomena in PEM fuel cell with dead-ended anode. Int. J. Hydrogen Energy 2013, 38, 11346–11356. [Google Scholar] [CrossRef]
- Chaparro, A.M.; Ferreira-Aparicio, P. WO2015025070—Fuel Cell. Available online: https://patentimages.storage.googleapis.com/19/d1/3c/93f911a503b2b3/WO2015025070A1.pdf (accessed on 7 November 2020).
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M.; et al. A Critical Review of Modeling Transport Phenomena in Polymer-Electrolyte Fuel Cells. J. Electrochem. Soc. 2014, 161, F1254–F1299. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Wang, H.; Benziger, J. Transport of liquid water through Nafion membranes. J. Memb. Sci. 2012, 392–393, 88–94. [Google Scholar] [CrossRef]
- Adachi, M.; Navessin, T.; Xie, Z.; Frisken, B.; Holdcroft, S. Correlation of In Situ and Ex Situ Measurements of Water Permeation Through Nafion NRE211 Proton Exchange Membranes. J. Electrochem. Soc. 2009, 156, B782. [Google Scholar] [CrossRef] [Green Version]
- Motupally, S.; Becker, A.J.; Weidner, J.W. Diffusion of Water in Nafion 115 Membranes. J. Electrochem. Soc. 2000, 147, 3171. [Google Scholar] [CrossRef]
- Fuel Cell Store Nafion NR211 and NR212 Ion Exchange Materials Solution Cast Membranes. Available online: https://www.fuelcellstore.com/spec-sheets/chemours-nafion-211-212-spec-sheet.pdf (accessed on 7 November 2020).
- Fuel Cell Store—Nafion 115, 117, 1110 Ion Exchange Materials. Available online: https://www.fuelcellstore.com/spec-sheets/chemours-nafion-115-117-1110-spec-sheet.pdf (accessed on 7 November 2020).
- Folgado, M.A.; Conde, J.J.; Ferreira-Aparicio, P.; Chaparro, A.M. Single Cell Study of Water Transport in PEMFCs with Electrosprayed Catalyst Layers. Fuel Cells 2018, 18, 602–612. [Google Scholar] [CrossRef]
- Adachi, M. Proton Exchange Membrane Fuel Cells: Water Permeation Through Nafion Membranes. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2010. [Google Scholar]
- Majsztrik, P.W. Mechanical and Transport Properties of Nafion® for PEM Fuel Cells: Temperature and Hydration Effects; Princeton University: Princeton, NJ, USA, 2008. [Google Scholar]
- Zhao, Q.; Majsztrik, P.; Benziger, J. Diffusion and interfacial transport of water in Nafion. J. Phys. Chem. B 2011, 115, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Majsztrik, P.; Bocarsly, A.; Benziger, J. Water permeation through nafion membranes: The role of water activity. J. Phys. Chem. B 2008, 112, 16280–16289. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Klaus, S.; Benziger, J. Wetting and Absorption of Water Drops on Nafion Films. Langmuir 2008, 24, 8627–8633. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Ingham, D.B.; Hughes, K.J.; Ma, L.; Pourkashanian, M. Thermal modelling of the cathode in air-breathing PEM fuel cells. Appl. Energy 2013, 111, 529–537. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Guizado, P.A.; Fernández-Sotillo, A.M.; Ferreira-Aparicio, P. Passive Regulation of the Water Content at the Anode Chamber under Dead-Ended Conditions: Innovative Design of an Air-Breathing Proton Exchange Membrane Fuel Cell. Energies 2020, 13, 5880. https://doi.org/10.3390/en13225880
Pérez-Guizado PA, Fernández-Sotillo AM, Ferreira-Aparicio P. Passive Regulation of the Water Content at the Anode Chamber under Dead-Ended Conditions: Innovative Design of an Air-Breathing Proton Exchange Membrane Fuel Cell. Energies. 2020; 13(22):5880. https://doi.org/10.3390/en13225880
Chicago/Turabian StylePérez-Guizado, Pedro A., Alba María Fernández-Sotillo, and Paloma Ferreira-Aparicio. 2020. "Passive Regulation of the Water Content at the Anode Chamber under Dead-Ended Conditions: Innovative Design of an Air-Breathing Proton Exchange Membrane Fuel Cell" Energies 13, no. 22: 5880. https://doi.org/10.3390/en13225880