Effect of Cu(NO3)2 and Cu(CH3COO)2 Activating Additives on Combustion Characteristics of Anthracite and Its Semi-Coke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Samples
2.2. Introduction of the Activating Additives
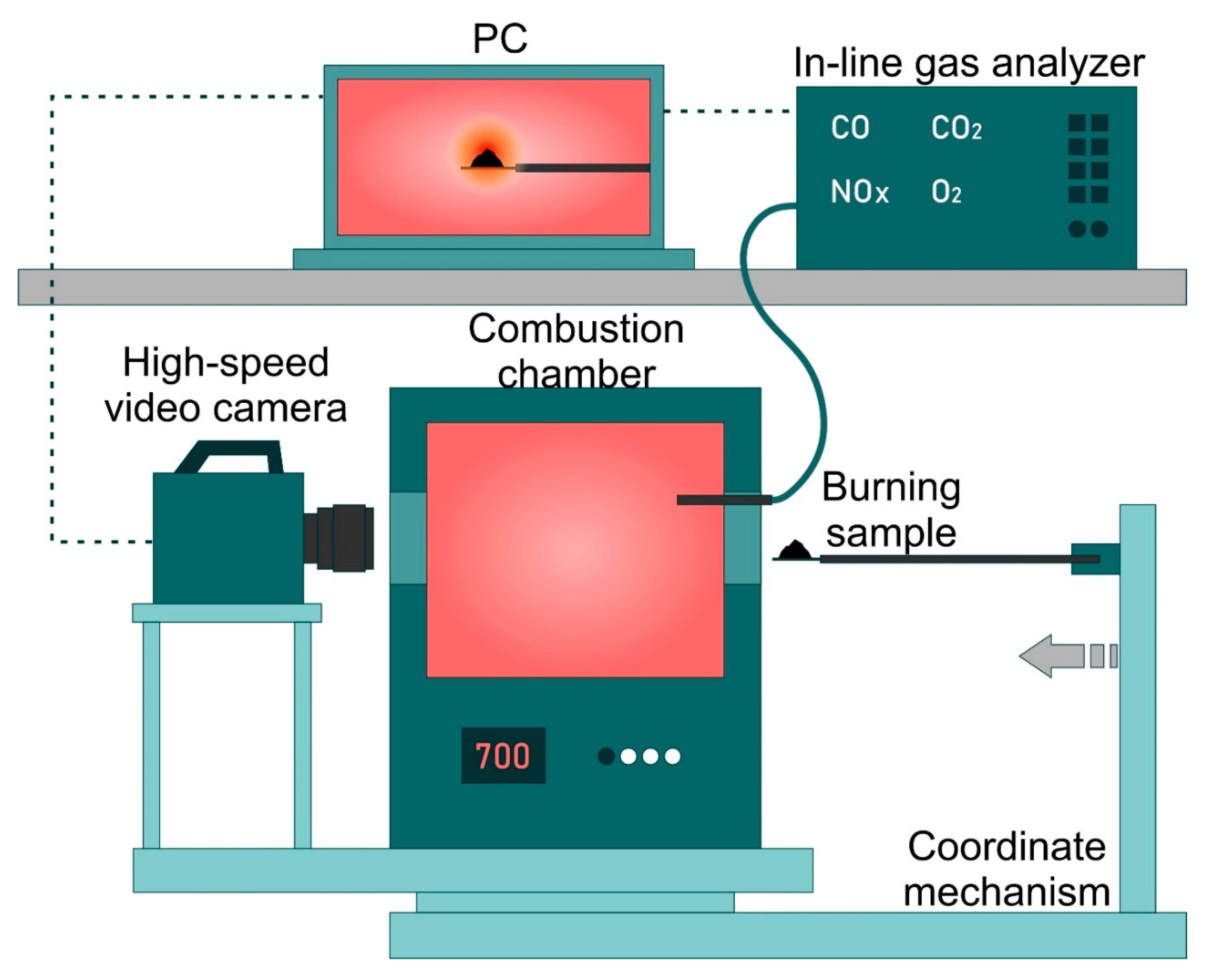
2.3. Combustion Experiments
2.4. Determination of the Minimum Ignition Temperature
3. Results
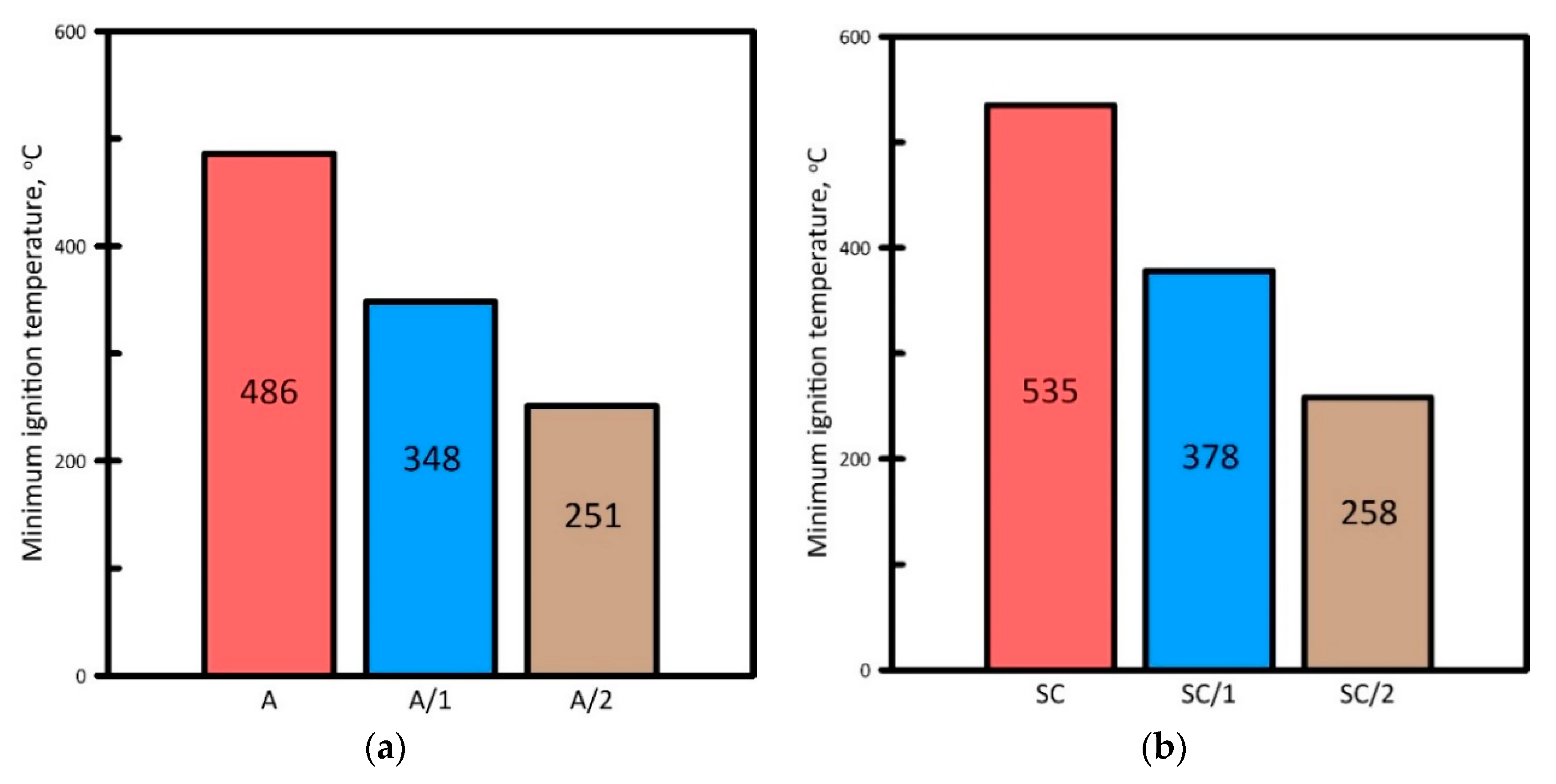
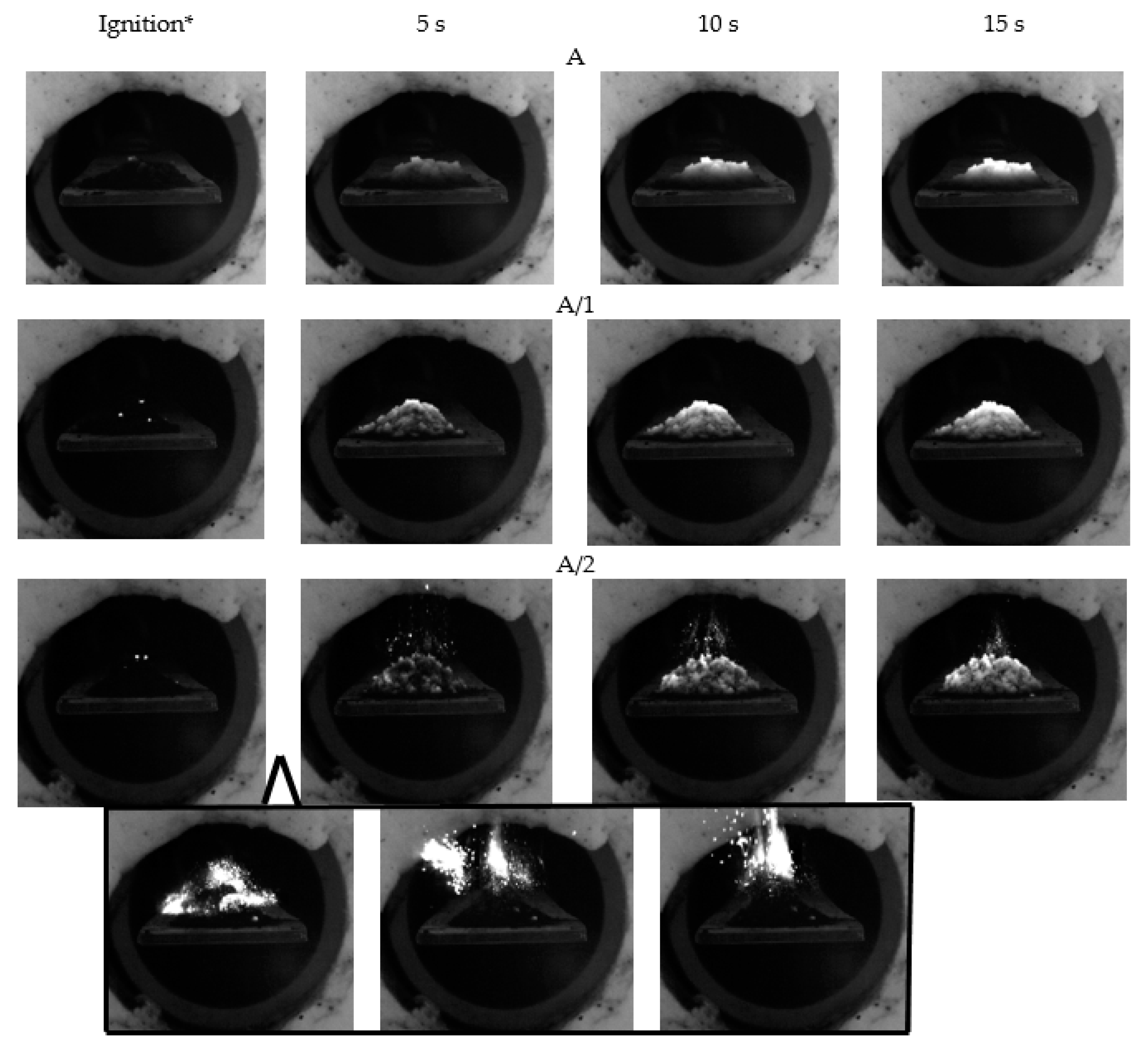
3.1. Ignition Characteristics of the Fuel Samples
3.2. Combustion Characteristics of the Fuel Samples
3.3. Gas-Phase Combustion Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Köpsel, R.F.W.; Halang, S. Catalytic influence of ash elements on NOx formation in char combustion under fluidized bed conditions. Fuel 1997, 76, 345–351. [Google Scholar] [CrossRef]
- Statistical Review of World Energy. 2020. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 17 September 2020).
- Ma, Z.; Deng, J.; Li, Z.; Li, Q.; Zhao, P.; Wang, L.; Sun, Y.; Zheng, H.; Pan, L.; Zhao, S.; et al. Characteristics of NOx emission from Chinese coal-fired power plants equipped with new technologies. Atmos. Environ. 2016, 131, 164–170. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, J.; Li, X.; Shi, R. Effects of catalysts on combustion reactivity of anthracite and coal char with low combustibility at low/high heating rate. J. Therm. Anal. Calorim. 2016, 126, 1469–1480. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Z.; Wang, Z. Reactivity of pulverized coals during combustion catalyzed by CeO2 and Fe2O3. Combust. Flame 2010, 157, 351–356. [Google Scholar] [CrossRef]
- Gong, X.-Z.; Guo, Z.-C.; Wang, Z. Anthracite combustion catalyzed by Ca-Fe-Ce series catalyst. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2009, 37, 421–426. [Google Scholar]
- Gong, X.; Guo, Z.; Wang, Z. Variation on anthracite combustion efficiency with CeO2 and Fe2O3 addition by Differential Thermal Analysis (DTA). Energy 2010, 35, 506–511. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, S. Catalytic Effects of CeO2/Fe2O3 and Inherent Mineral Matter on Anthracite Combustion Reactions and Its Kinetic Analysis. Energy Fuels 2017, 31, 12867–12874. [Google Scholar] [CrossRef]
- Yazykov, N.A.; Simonov, A.D.; Dubinin, Y.V.; Zaikina, O.O. Catalytic Co-Combustion of Peat and Anthracite in a Fluidized Bed. Catal. Ind. 2019, 11, 342–348. [Google Scholar] [CrossRef]
- Yang, L.; Luo, H.; Zhang, K.; Gong, Z.; Wu, W. Effect of limonite additive on combustion and NOx emission characteristics of anthracite. Meitan Xuebao J. China Coal Soc. 2019, 44, 305–312. [Google Scholar]
- Gong, X.Z.; Guo, Z.C.; Wang, Z. Effect of K2CO3 and Fe2O3 on combustion reactivity of pulverized coal by thermogravimetry analysis. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2009, 37, 42–48. [Google Scholar]
- Betancur, Y.; López, D.; Du, Z.-Y.; Li, W.-Y. The role of K2CO3 on structural changes of coal, biomass and coal/biomass blends during pre-treatment using N2 and CO2. Differences in the reactivity under oxy-combustion conditions. In Proceedings of the 2018 International Pittsburgh Coal Conference The Thirty-Fifth Annual Pittsburgh Coal Conference, Xuzhou, China, 15–18 October 2018. [Google Scholar]
- Cheng, J.; Zhou, F.; Xuan, X.; Liu, J.; Zhou, J.; Cen, K. Cascade chain catalysis of coal combustion by Na–Fe–Ca composite promoters from industrial wastes. Fuel 2016, 181, 820–826. [Google Scholar] [CrossRef]
- Zou, C.; Wen, L.; Zhang, S.; Bai, C.; Qiu, G.; Lü, X.; Tan, X. Effects of catalysts on the conbustion behavior of pulverized coal injection (PCI) anthracite and its mechanism. Metal Int. 2011, 16, 53–60. [Google Scholar]
- Belyaev, A.A. Effect of alkali metal carbonate additives on the rate of oxidation of the organic matter of anthracite. Solid Fuel Chem. 2013, 47, 226–230. [Google Scholar] [CrossRef]
- Sen, K.; Dash, P.S. Quantum chemical perspective of coal molecular modeling: A review. Fuel 2020, 279, 118539. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Z.; Wang, Z. Experimental study on mechanism of lowering ignition temperature of anthracite combustion catalyzed by Fe2O3. Huagong Xuebao/CIESC J. 2009, 60, 1707–1713. [Google Scholar]
- Gong, X.Z.; Guo, Z.C.; Wang, Z. Effect of coal characteristics on its combustion catalyzed by CeO2. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2010, 38, 29–34. [Google Scholar]
- Li, X.G.; Ma, B.G.; Xu, L.; Luo, Z.T.; Wang, K. Catalytic effect of metallic oxides on combustion behavior of high ash coal. Energy Fuels 2007, 21, 2669–2672. [Google Scholar] [CrossRef]
- Larionov, K.B.; Zenkov, A.V.; Slyusarsky, K.V.; Mishakov, I.V. Intensification of thermal coal and coke oxidation by metal oxides and their precursors. J. Phys. Conf. Ser. 2020, 1565, 012037. [Google Scholar] [CrossRef]
- Larionov, K.B.; Mishakov, I.V.; Slyusarskiy, K.V.; Vedyagin, A.A. Intensification of bituminous coal and lignite oxidation by copper-based activating additives. Int. J. Coal Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Chai, Y.F.; Ning, X.J.; Zhang, J.L.; Qi, C.L.; Li, F.G.; Wang, S. Study on pulverized coal combustion catalyzed by CeO2 under different heating rates. Chin. Rare Earths 2014, 35, 1–6. [Google Scholar]
- Zhang, S.; Chen, Z.D.; Chen, X.J.; Gong, X.Z. Effects of ash/K2CO3/Fe2O3 on ignition temperature and combustion rate of demineralized anthracite. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2014, 42, 166–174. [Google Scholar] [CrossRef]
- Zhu, Q. Effect of calcium-based composite additives on combustion and sulfur retention of an anthracite coal. Chin. J. Environ. Eng. 2014, 8, 5413–5418. [Google Scholar]
- Zhou, H.; Liu, R.P.; Liu, Z.H.; Cheng, M.; Cen, K.F. Influence of alkali metal on the evolution of NOx during coke combustion. Meitan Xuebao J. China Coal Soc. 2015, 40, 1160–1164. [Google Scholar]
- Dong, L.; Han, S.; Yu, W.; Lei, Z.; Kang, S.; Zhang, K.; Yan, J.; Li, Z.; Shui, H.; Wang, Z.; et al. Effect of volatile reactions on the yield and quality of tar from pyrolysis of Shenhua bituminous coal. J. Anal. Appl. Pyrolysis 2019, 140, 321–330. [Google Scholar] [CrossRef]
- Larionov, K.B.; Gromov, A.A. Non-isothermal oxidation of coal with Ce(NO3)3 and Cu(NO3)2 additives. Int. J. Coal Sci. Technol. 2019, 6, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Tokareva, I.V.; Mishakov, I.V.; Vedyagin, A.A.; Korneev, D.V.; Petuhkova, E.S.; Savvinova, M.E. Modification of carbon fibers forrein forcingpipe polyethylene. Compos. Nanostruct. 2014, 6, 158–167. [Google Scholar]
- Zhang, L.; Hu, S.; Chen, Q.; Xiao, L.; Shatir, A.; Syed-Hassan, S.S.A.; Jiang, L.; Wang, Y.; Su, S.; Xiang, J. Molecular structure characterization of the tetrahydrofuran-microwave-extracted portions from three Chinese low-rank coals. Fuel 2017, 189, 178–185. [Google Scholar] [CrossRef]
- Liu, J.; Luo, L.; Ma, J.; Zhang, H.; Jiang, X. Chemical properties of superfine pulverized coal particles. 3. Nuclear magnetic resonance analysis of carbon structural features. Energy Fuels 2016, 30, 6321–6329. [Google Scholar] [CrossRef]
- Wayne, H.R. Copper Compounds. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Kramer, H. Mathematical Methods of Statistics; Almquist and Wiskell: Stockholm, Sweden, 1975. [Google Scholar]
- Wang, Y.; Wang, J.; Chen, H.; Yao, M.; Li, Y. Preparation and NOx-assisted soot oxidation activity of a CuO–CeO2 mixed oxide catalyst. Chem. Eng. Sci. 2015, 135, 294–300. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Li, G. Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2008, 464, 532–536. [Google Scholar] [CrossRef]
- Arii, T.; Masuda, Y. Thermal decomposition of calcium copper acetate hexahydrate by simultaneous measurement of controlled-rate thermogravimetry and mass spectrometry (CRTG-MS). Thermochim. Acta 1999, 342, 139–146. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, J.; Cao, G.; Zhan, D.; Tao, Y.; Cong, C. The kinetics of thermal dehydration of copper(II) acetate monohydrate in air. Thermochim. Acta 2005, 437, 145–149. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Paushkina, K.K.; Shabardin, D.P. Co-combustion of coal processing waste, oil refining waste and municipal solid waste: Mechanism, characteristics, emissions. Chemosphere 2020, 240, 124892. [Google Scholar] [CrossRef]
- Larionov, K.B.; Mishakov, I.V.; Gromov, A.A. Research of thermal destruction dynamics of coal particles in oxidizing medium with copper nitrate. J. Phys. Conf. Ser. 2018, 1128, 012070. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhang, X.; Zhang, D.; Peng, Q.; Zhang, Q.; Dobashi, R. Flame propagation behaviours in nano-metal dust explosions. Powder Technol. 2017, 321, 154–162. [Google Scholar] [CrossRef]
- Larionov, K.B.; Mishakov, I.V.; Slyusarsky, K.V. Influence of inorganic salt on the characteristics of oxidation, ignition and combustion of bituminous coal. J. Phys. Conf. Ser. 2019, 1359, 012058. [Google Scholar] [CrossRef]
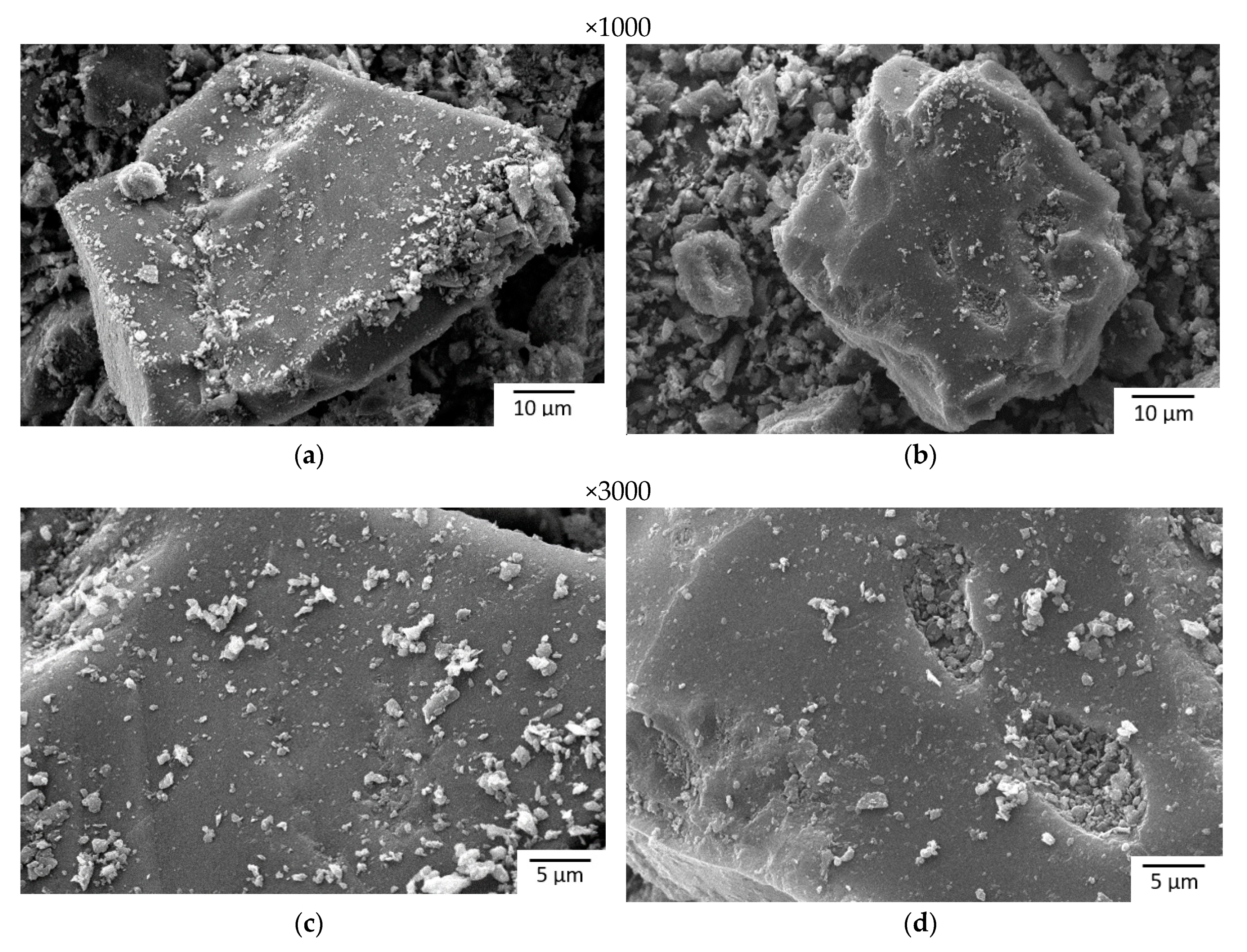


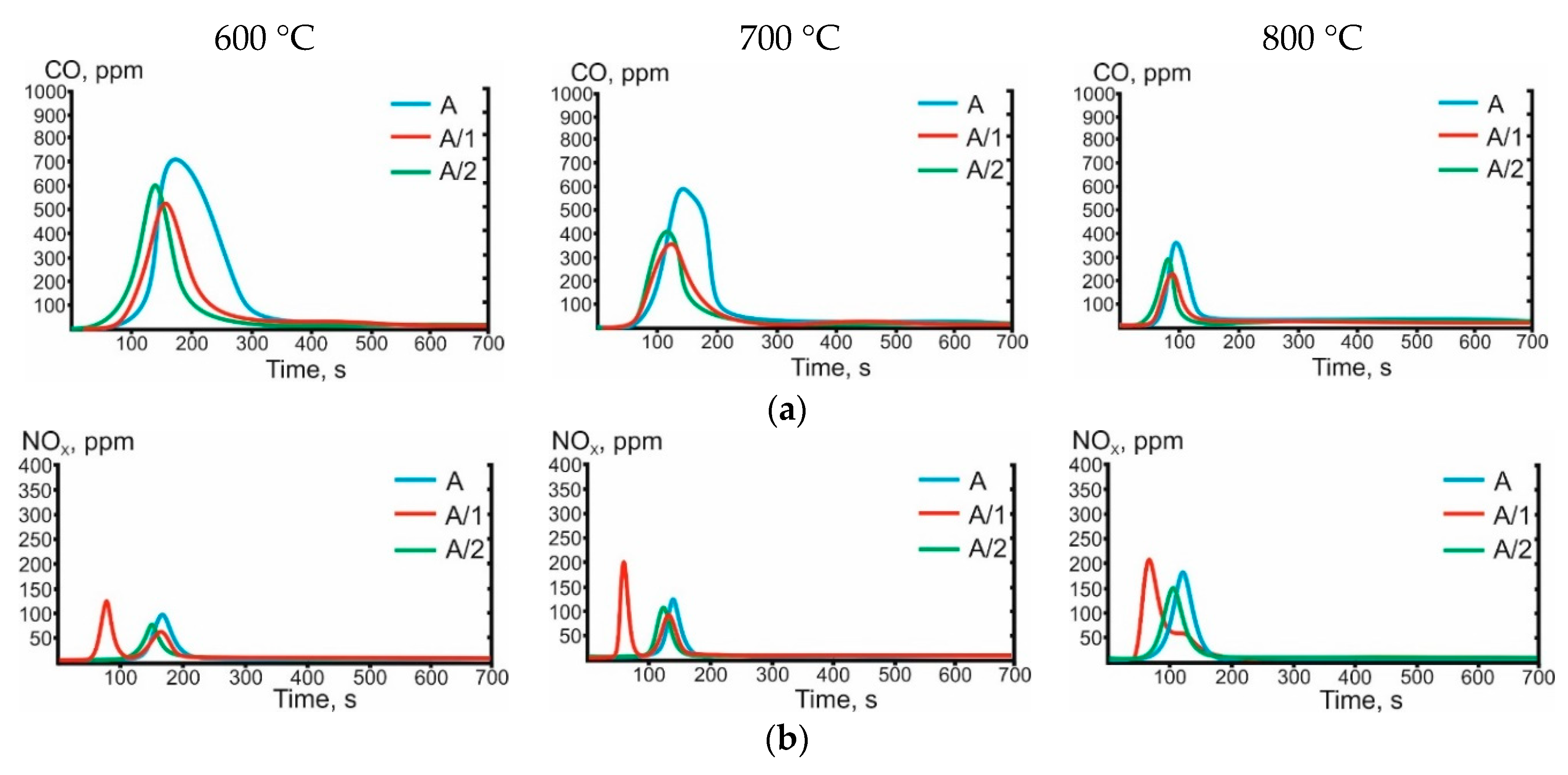
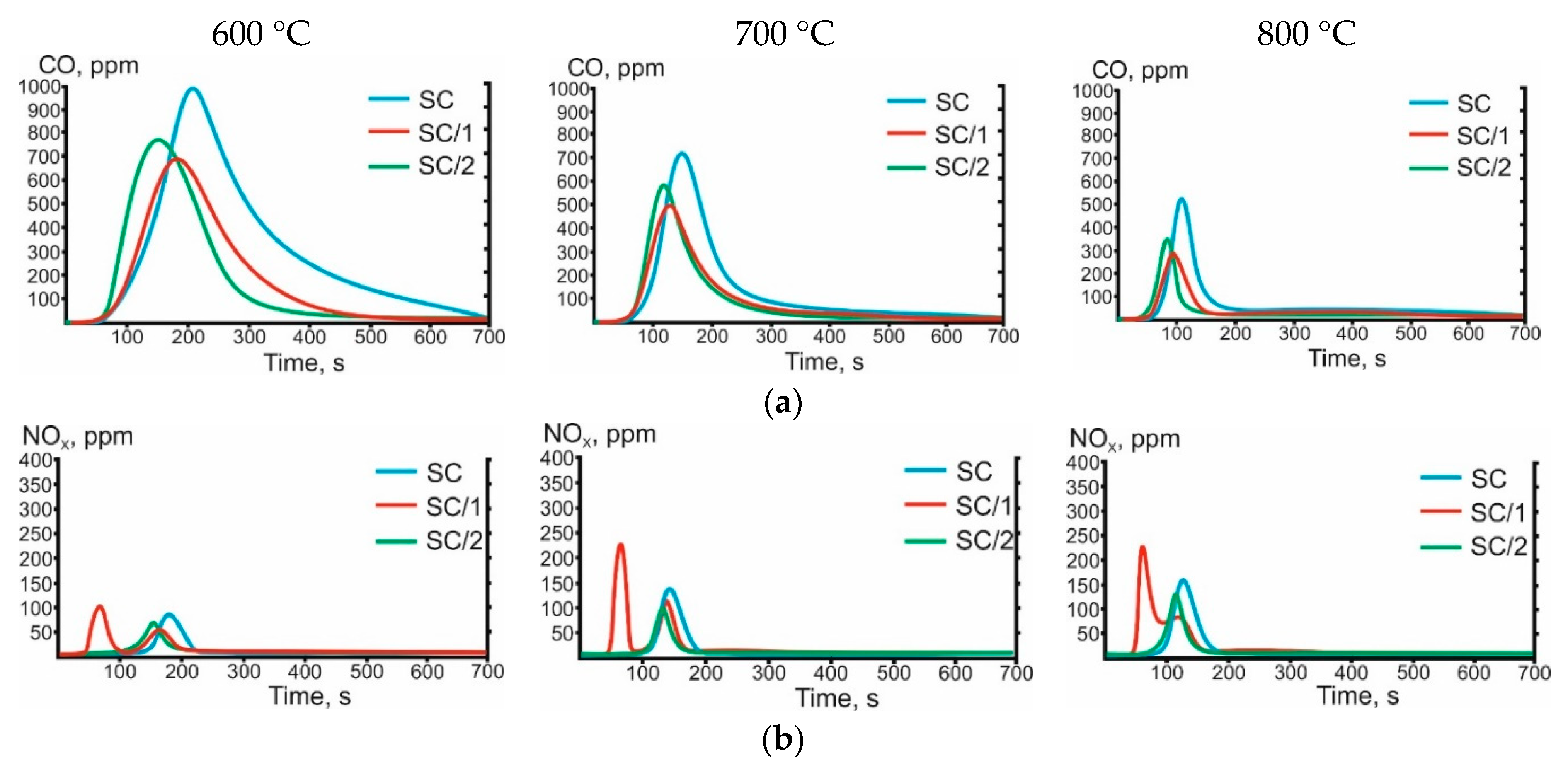
| Characteristic | A | SC |
|---|---|---|
| Moisture content W a, wt.% | 2.1 | 0.7 |
| Ash content A d, wt.% | 17.7 | 21.4 |
| Volatile matter content V daf, wt.% | 7.2 | 1.7 |
| Net heating value, MJ/kg | 24.8 | 26.2 |
| Type and Content of Additive | Tag | Elemental Composition 1, wt.% | Ash Content A 1, wt.% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | H/C | O/C | |||
| Anthracite | |||||||||
| No additive | А | 69.9 | 1.4 | 1.2 | 0.2 | 9.6 | 0.24 | 0.09 | 17.7 |
| 5 wt.% Cu(NO3)2 | А/1 | 62.8 | 1.4 | 2.1 | 0.2 | 13.4 | 0.27 | 0.16 | 20.1 |
| 5 wt.% Cu(CH3COO)2 | А/2 | 64.4 | 1.7 | 1.0 | 0.1 | 12.9 | 0.32 | 0.15 | 19.9 |
| Anthracite Semi-Coke | |||||||||
| No additive | SC | 73.8 | 1.0 | 1.1 | 0.1 | 2.6 | 0.16 | 0.03 | 21.4 |
| 5 wt.% Cu(NO3)2 | SC/1 | 68.6 | 1.1 | 1.7 | 0.1 | 5.0 | 0.19 | 0.05 | 23.4 |
| 5 wt.% Cu(CH3COO)2 | SC/2 | 67.5 | 1.5 | 0.9 | 0.1 | 6.0 | 0.27 | 0.07 | 24.0 |
| Sample | Temperature of Heating Medium, °C | |||||
|---|---|---|---|---|---|---|
| 600 | 700 | 800 | 600 | 700 | 800 | |
| SCOMS/SCOref | SNOxMS/SNOxref | |||||
| A/1 | 0.58 | 0.52 | 0.48 | 1.63 | 1.49 | 1.06 |
| A/2 | 0.81 | 0.59 | 0.56 | 0.60 | 0.66 | 0.74 |
| SC/1 | 0.72 | 0.58 | 0.39 | 1.45 | 1.27 | 1.03 |
| SC/2 | 0.82 | 0.63 | 0.43 | 0.53 | 0.60 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larionov, K.; Slyusarskiy, K.; Tsibulskiy, S.; Tolokolnikov, A.; Mishakov, I.; Bauman, Y.; Vedyagin, A.; Gromov, A. Effect of Cu(NO3)2 and Cu(CH3COO)2 Activating Additives on Combustion Characteristics of Anthracite and Its Semi-Coke. Energies 2020, 13, 5926. https://doi.org/10.3390/en13225926
Larionov K, Slyusarskiy K, Tsibulskiy S, Tolokolnikov A, Mishakov I, Bauman Y, Vedyagin A, Gromov A. Effect of Cu(NO3)2 and Cu(CH3COO)2 Activating Additives on Combustion Characteristics of Anthracite and Its Semi-Coke. Energies. 2020; 13(22):5926. https://doi.org/10.3390/en13225926
Chicago/Turabian StyleLarionov, Kirill, Konstantin Slyusarskiy, Svyatoslav Tsibulskiy, Anton Tolokolnikov, Ilya Mishakov, Yury Bauman, Aleksey Vedyagin, and Alexander Gromov. 2020. "Effect of Cu(NO3)2 and Cu(CH3COO)2 Activating Additives on Combustion Characteristics of Anthracite and Its Semi-Coke" Energies 13, no. 22: 5926. https://doi.org/10.3390/en13225926







