Potential of an Automated- and Image-Based Cell Counter to Accelerate Microalgal Research and Applications
Abstract
:1. Introduction
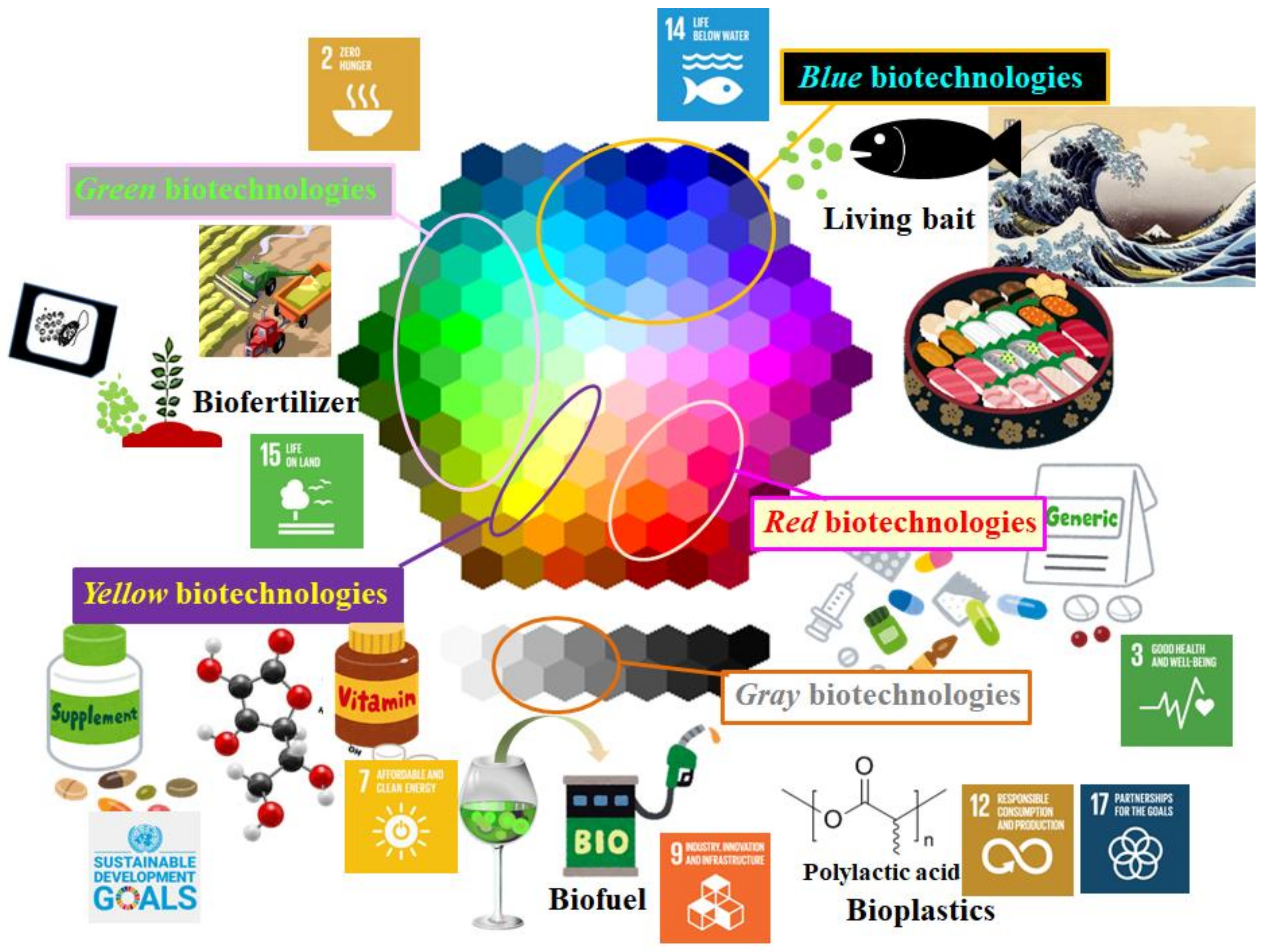
2. Present Applications of Microalgae and a Smoldering Challenge from a Practical Application Perspective
3. General Evaluation Methods of Microalgae
4. Automated Cell Counter Method for Cell Counting in Cell Biology
5. Applicability of an Automated Cell Counter for the Evaluation of Microalgae
5.1. Selectivity to Detect Microalgae
5.2. Multifaceted Evaluation of Microalgae
6. Conclusions
Funding
Conflicts of Interest
References
- Kaferski, P. Rainbow code of biotechnology. Chemik 2012, 66, 811–816. [Google Scholar]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe. In JRC Scientific and Policy Reports; Vigani, M., Parisi, C., Cerezo, E.R., Eds.; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Febris, M.; Abbriano, R.M.; Pernicce, M.; Sutheriand, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryo, A. Characteristics of the microalgae Euglena and its applications in foods and ecological fields. Jap. Soc. Photosynth. Res. 2012, 22, 33–38. [Google Scholar]
- Lee, H.-R.; Kim, K.; Mun, S.C.; Chang, Y.K.; Choi, S.Q. A new method to produce cellulose nanofibrils from microalgae and the measurement of their mechanical strength. Carbohydr. Polym. 2018, 180, 276–285. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Nikhil, K. Effect of algal bio-fertilizer on the Vigna radiate: A Critical Review. Int. J. Eng. Res. Appl. 2016, 6, 85–94. [Google Scholar]
- Faheed, F.A.; Fattah, Z.A.-E. Effects of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- Ghayal, M.S.; Pandya, M.T. Microalgae biomass: A renewable source of energy. Energy Procedia 2013, 32, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.-S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef]
- Seyidoglu, N.; Inan, S.; Aydin, C. A Prominent superfood: Spirulina platensis. In Superfood and Functional Food-Development of Superfood and its Role in Medicine, 1st ed.; Shiomi, N., Waisundara, V.Y., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 1–27. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.A.; Oh, T.-H.; Choi, J.-S.; Chang, D.-J.; Joo, C.-K. Impact of β-1,3-glucan Isolated From Euglena gracilis on Corneal Epithelial Cell Migration and on Wound Healing in a Rat Alkali Burn Model. Curr. Eye Res. 2013, 38, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Applicability of automated cell counter with a chlorophyll detector in routine management of microalgae. Sci. Rep. 2018, 8, 4967. [Google Scholar] [CrossRef] [PubMed]
- Okauchi, M. Selection of algal strains with high-growth rate as food organisms and development of their effective production techniques. Nippon Suisan Gakkaishi 2014, 80, 323–326. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T. Relationship between Algal Blooms and Marine-Ecosystem Services. In Book Marine Ecology: Current and Future Developments, 1st ed.; Takahashi, T., Ed.; Bentham Science Publishers: Sharjah, UAE, 2020; Volume 2, pp. 30–40. [Google Scholar]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Barzkar, N.; Jahromi, S.T.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drag 2019, 17, 464. [Google Scholar] [CrossRef] [Green Version]
- Shiho, M.; Kawachi, M.; Horioka, K.; Nishita, Y.; Ohashi, K.; Kaya, K.; Watanabe, M.M. Business evaluation of a green microalgae Botryococcus braunii oil production system. Procedia Environ. Sci. 2012, 15, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical Conversion of Microalgal Biomass Into Biofuels: A Review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Kaplan, D. Absorption and adsorption of heavy metals by microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 602–611. [Google Scholar]
- Ongena, K.; Das, C.; Smith, J.L.; Gil, S.; Johnston, G. Determining cell number during cell culture using the Scepter cell counter. J. Vis. Exp. 2010, 45, e2204. [Google Scholar] [CrossRef]
- Koch, A.L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal. Biochem. 1970, 38, 252–259. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Validation Technol. 2011, 17, 46–49. [Google Scholar]
- Chiocciodi, M.; Hankamer, B.; Ross, I.L. Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weith in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar]
- Takahashi, T. Routine Management of Microalgae Using Autofluorescence from Chlorophyll. Molecules 2019, 24, 4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T. Quality assessment of microalgae exposed to trace metals using flow cytometry. In Superfood and Functional Food-Development of Superfood and its Role in Medicine, 1st ed.; Shiomi, N., Waisundara, V.Y., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 29–45. [Google Scholar]
- Takahashi, T.; Yokoyama, S. Bioassay of components eluted from electric arc furnace steel slag using microalgae Chlorella. ISIJ Int. 2016, 56, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T. Direct evaluation of endosymbiotic status in Paramecium bursaria using capillary flow cytometry. Cytom. Part A 2014, 85, 911–914. [Google Scholar] [CrossRef]
- Takahashi, T. Application of phytoplankton. In Corrosion Control and Surface Finishing–Environmentally Friendly Approaches, 1st ed.; Kanematsu, H., Barry, D.M., Eds.; Springer Japan: Tokyo, Japan, 2016; pp. 213–224. [Google Scholar]
- Doan, M.; Vorobjev, I.; Rees, P.; Filby, A.; Wolkenhauer, O.; Goldfeld, A.E.; Lieberman, J.; Barteneva, N.; Carpenter, A.E.; Hennig, H. Diagnostic Potential of Imaging Flow Cytometry. Cell Press Rev. 2018, 36, 649–652. [Google Scholar] [CrossRef] [Green Version]
- Poulton, N.J. Imaging Flow Cytometry for Phytoplankton Analysis: Instrumentation and Applications. J. Biomol. Tech. 2019, 30, S52. [Google Scholar]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. Countess II FL Automated Cell Counter. Available online: https://www.thermofisher.com/jp/en/home/life-science/cell-analysis/cell-analysis-instruments/automated-cell-counters/countess-ii-fl-automated-cell-counter.html (accessed on 20 September 2020).
- Nikon Instruments Inc. MICROSCOPY U: The Source for Microscopy Education. Available online: https://www.microscopyu.com/microscopy-basics/field-of-view (accessed on 20 September 2020).
- Satinover, S.J.; Dove, J.D.; Eorden, M.A. Single-Particle Optical Sizing of Microbubbles. Ultrasound Med. Biol. 2014, 40, 138–147. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll fluorescence emission spectrum inside a leaf. Photochem. Photobiol. Sci. 2008, 7, 498–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chroma Technology Japan. Spectra Viewer. Available online: https://jp.chroma.com/spectra-viewer#tabs-spectra_viewer_plot-left-2 (accessed on 2 November 2020).
- Thermo Fisher Scientific Inc. Tali Image-Based Cytometer. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp10796.pdf (accessed on 2 November 2020).
- Logos Biosystems Inc. LUNA-FL Automated Fluorescence Cell Counter. Available online: https://www.biocat.com/logos/manuals/LUNA-FL_User_Manual_VL1712-01.pdf#search='LUNAFL%E2%84%A2+Automated+fluorescence+cell+counter (accessed on 2 November 2020).
- DeNovix Inc. CellDrop FL Automated Cell Counters. Available online: https://www.denovix.com/products/celldrop (accessed on 2 November 2020).
- Nexcelom Inc. Cellometer Spectrum Image Cytometry Systems. Available online: https://www.nexcelom.com/nexcelom-products/cellometer-and-celigo-image-cytometers/cellometer-spectrum-image-cytometry-system/#specs (accessed on 2 November 2020).
- Transparence Market Research. ALGAE MARKET TO REACH VALUATION OF ~US$ 10.7 BN BY 2027, Press Releases. Available online: https://www.transparencymarketresearch.com/pressrelease/algae-market.htm (accessed on 30 April 2020).







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T. Potential of an Automated- and Image-Based Cell Counter to Accelerate Microalgal Research and Applications. Energies 2020, 13, 6019. https://doi.org/10.3390/en13226019
Takahashi T. Potential of an Automated- and Image-Based Cell Counter to Accelerate Microalgal Research and Applications. Energies. 2020; 13(22):6019. https://doi.org/10.3390/en13226019
Chicago/Turabian StyleTakahashi, Toshiyuki. 2020. "Potential of an Automated- and Image-Based Cell Counter to Accelerate Microalgal Research and Applications" Energies 13, no. 22: 6019. https://doi.org/10.3390/en13226019
APA StyleTakahashi, T. (2020). Potential of an Automated- and Image-Based Cell Counter to Accelerate Microalgal Research and Applications. Energies, 13(22), 6019. https://doi.org/10.3390/en13226019



