Effective Combustion of Glycerol in a Compression Ignition Engine Equipped with Double Direct Fuel Injection
Abstract
:1. Introduction
1.1. Glycerol Properties
1.2. Combustion Applications
1.3. Combustion in the Internal Combustion Engine
2. Research Methodology
- To obtain knowledge on glycerol combustion under compression ignition conditions with diesel fuel pilot for combustion initiation. As glycerol features relatively high viscosity, ethanol was proposed to dilute it that results in a decrease of its kinematic viscosity.
- To obtain knowledge on the toxicity of the engine exhaust gases. Hence, the tests were focused on emissions of the following toxic exhaust gases: NOx, CO, UHC and smoke.
- To test the dual fuel injection system with two independently working high pressure injectors for diesel fuel pilot and glycerol-ethanol, respectively, mounted in the engine cylinder.
2.1. Parameters Varied
2.2. Parameters Maintained Constant
- The basic fuel was a mixture of 50% ethanol and 50% glycerol by volume. The 50/50 ratio was determined on a basis of maximum injector flowrate capacity under acceptable kinematic viscosity. Hence, the kinematic viscosity varied in narrow range between 3.3× 10-6 and 3.7× 10-6 m2/s.
- Engine run at a constant speed of 970 rpm. The speed was limited by a belt transmission from the engine to the power generator and synchronous speed of that generator.
- Injection pressures for both injectors were constant and adjusted to 2000 bar. Hence, each fuel dose was precisely regulated by injection timing.
- The entire fuel energy per cycle was constant and it was in the range between 2820 and 2890 J/cycle, as previously depicted in Figure 2. This condition required simultaneously changing both the glycerol-ethanol and diesel pilot dose. Hence, with an increase of the diesel pilot dose, the glycerol-ethanol dose systematically decreased. It was important to maintain constant the total energy of entire fuel injected into the cylinder in regards to obtaining knowledge on the following: engine overheating, exhaust gases temperature and thermal efficiency.
- Injection timing for the diesel pilot fuel was fixed. Start of injection (SOI) was at 26 CA deg bTDC (before Top Dead Center—bTDC). This optimal SOI for diesel fuel was determined during preliminary tests regarding the highest efficiency of the engine working on diesel fuel only.
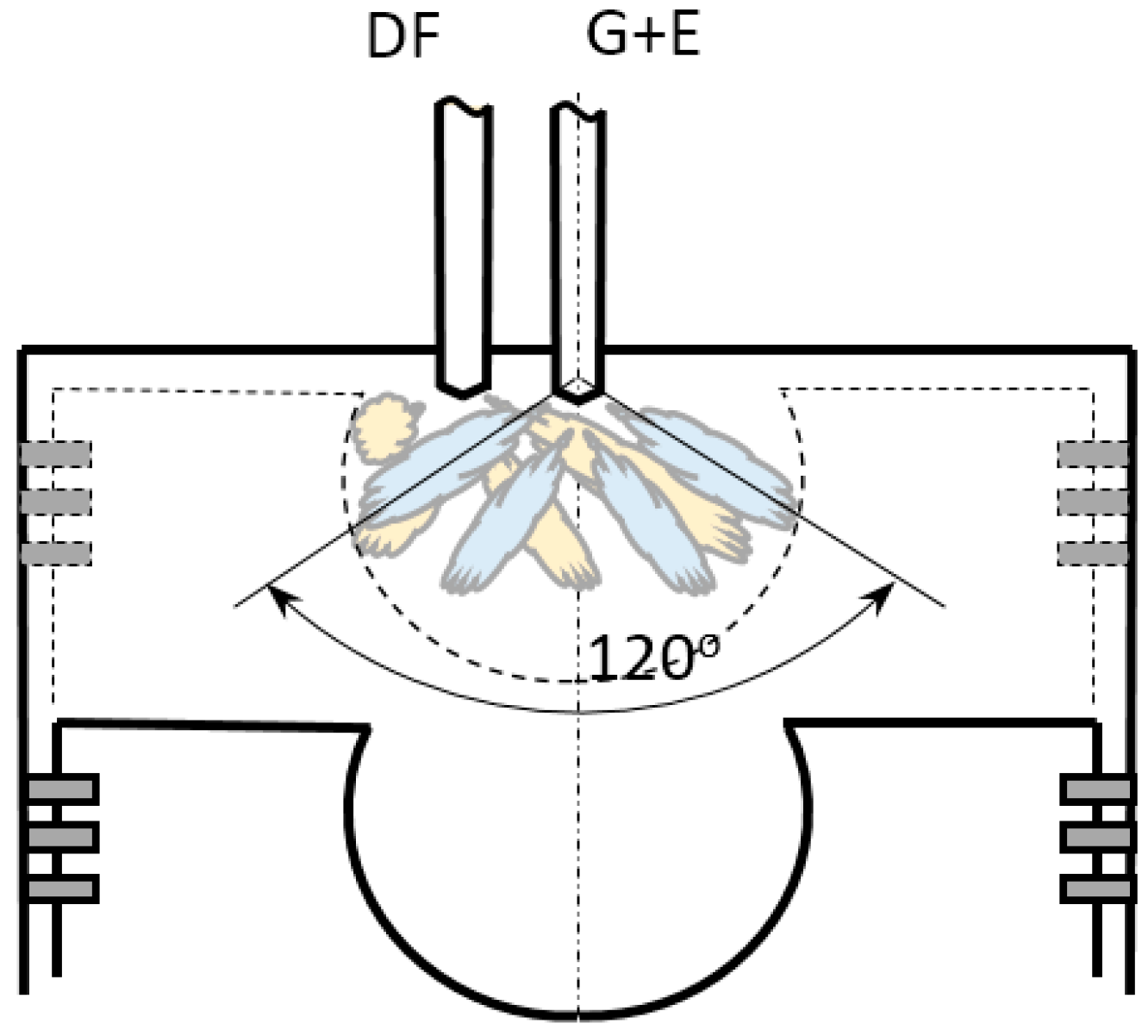
- With respect to glycerol-ethanol injection timings, the fixed SOI strategy was applied due to maintaining the same pressure-volume initial conditions at ignition point, that affects total combustion process. As known, combustion products among others depend on combustion initial parameters. Hence, SOI for glycerol-ethanol was fixed at 10 CA deg bTDC. A summary of the operating parameters is presented in Table 4.
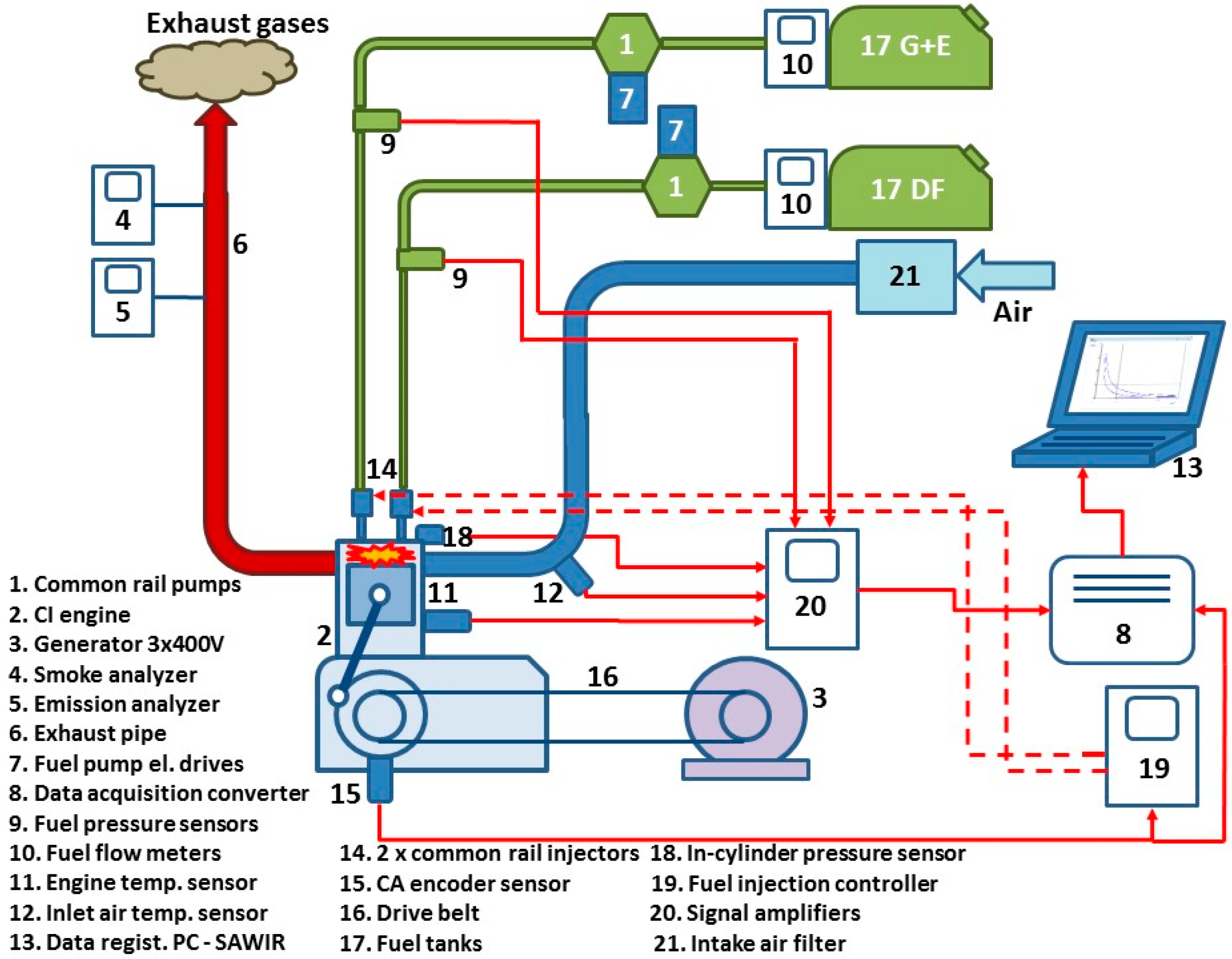
2.3. Test Bench Description
- A 415SE smoke meter from AVL (Graz, Austria) with a measuring range to 10 filter smoke number (FSN) [29];
- A Measurement Computing USB-1608HS—16 bits resolution data acquisition module (Measurement Computing Corporation, Norton, MA, USA), with a sampling frequency of 200 kHz;
- A 6061 SN 298131piezo-ceramic pressure transducer (Kistler, Winterthur, Switzerland) with ±0.5% sensitivity;
- Kistler 5011 charge amplifier with FS < ±0.5% linearity;
- Crank angle encoder with resolution 1024 pulses/rev,
- MSO2014 signal analysis unit (Tektronix, Beaverton, OR, USA);
- Air/fuel LM-2 with lambda sensor, range 7.35–22.38 (Ratio Meter, Sycamore, IL, USA)
- BEA 350THC, CO, CO2 gas analyzer (Bosch, Gerlingen, German) (CO, CO2 and THC are measured based on the NDIR method, while the measuring principle of the oxygen concentration is based on an electrochemical O2 cell).
2.4. Errors and Accuracies in Tests
3. Results and Discussion
- cooling by higher amounts of ,
- and oxygenation by oxygen bounded in the mixture.
4. Conclusions
- Blending crude glycerol with ethanol at a volumetric ratio of 1:1 (50/50%) is a reasonable way to burn large amounts of glycerol in reciprocating internal combustion engines with diesel fuel pilot for self-ignition. Glycerol-ethanol blends also provide mixture stability during their storage.
- The proposed injection system consisting of two high pressure injectors with in-cylinder direct injection can be successfully applied to the engine for effective combustion of glycerol-ethanol blends.
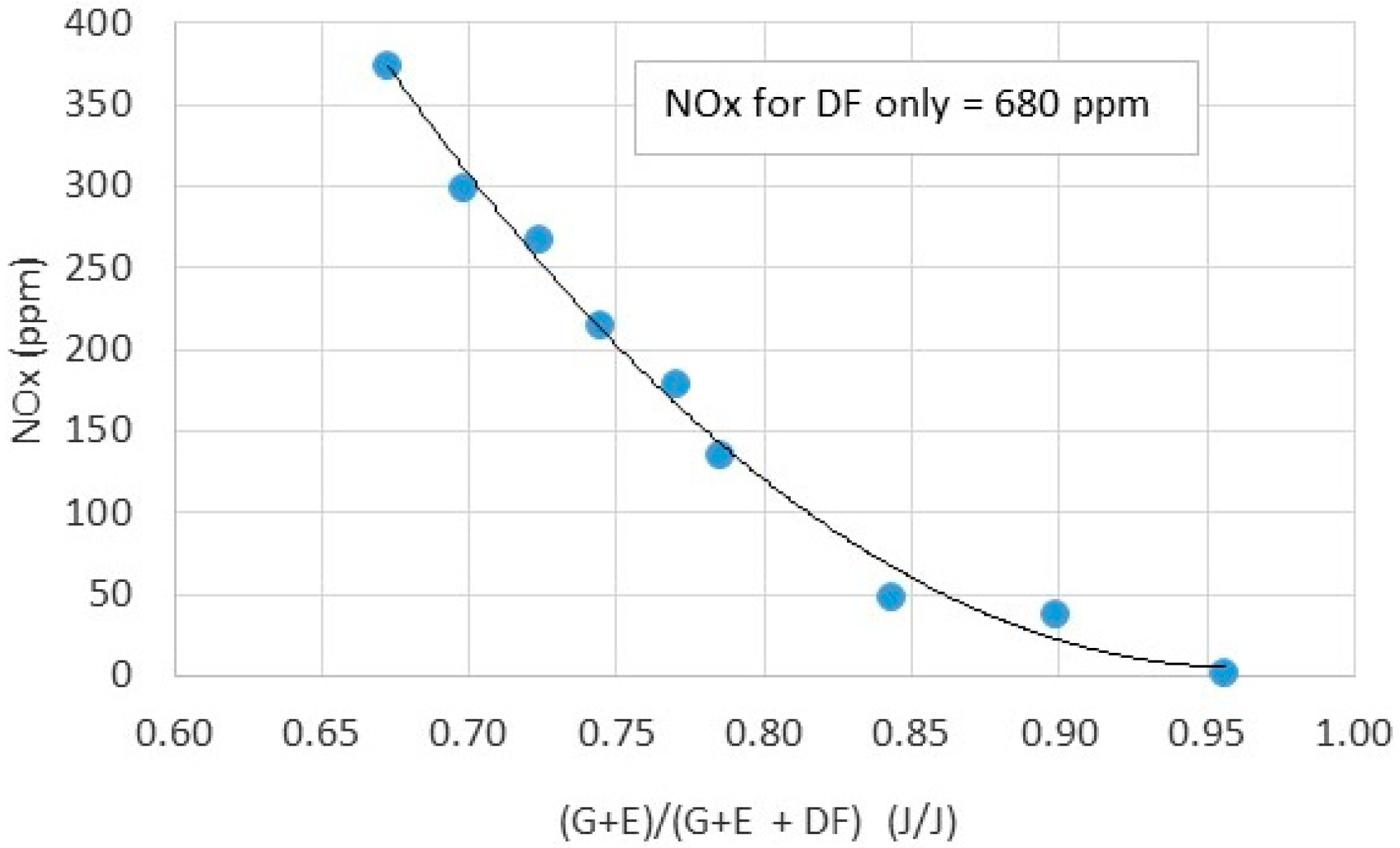
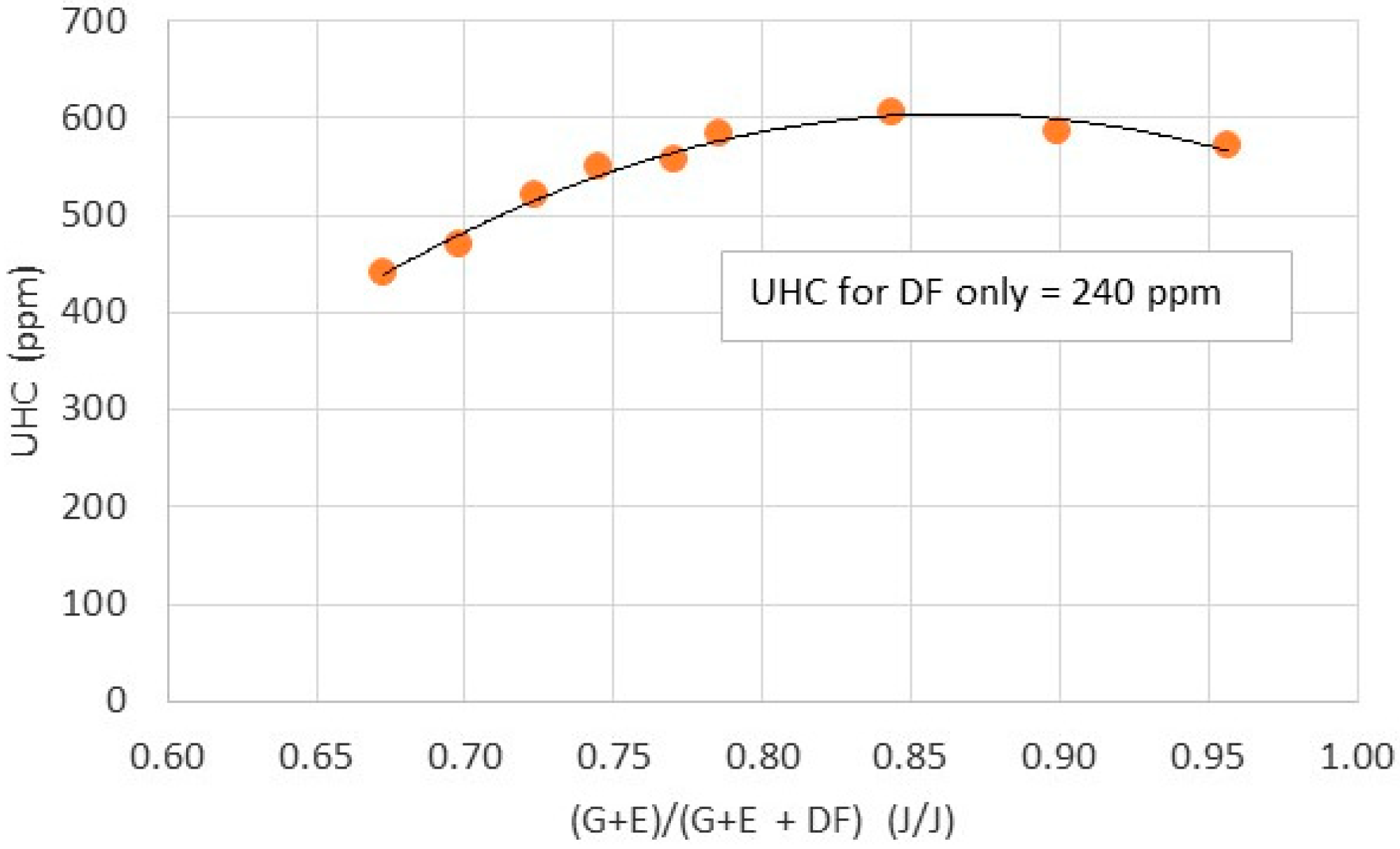
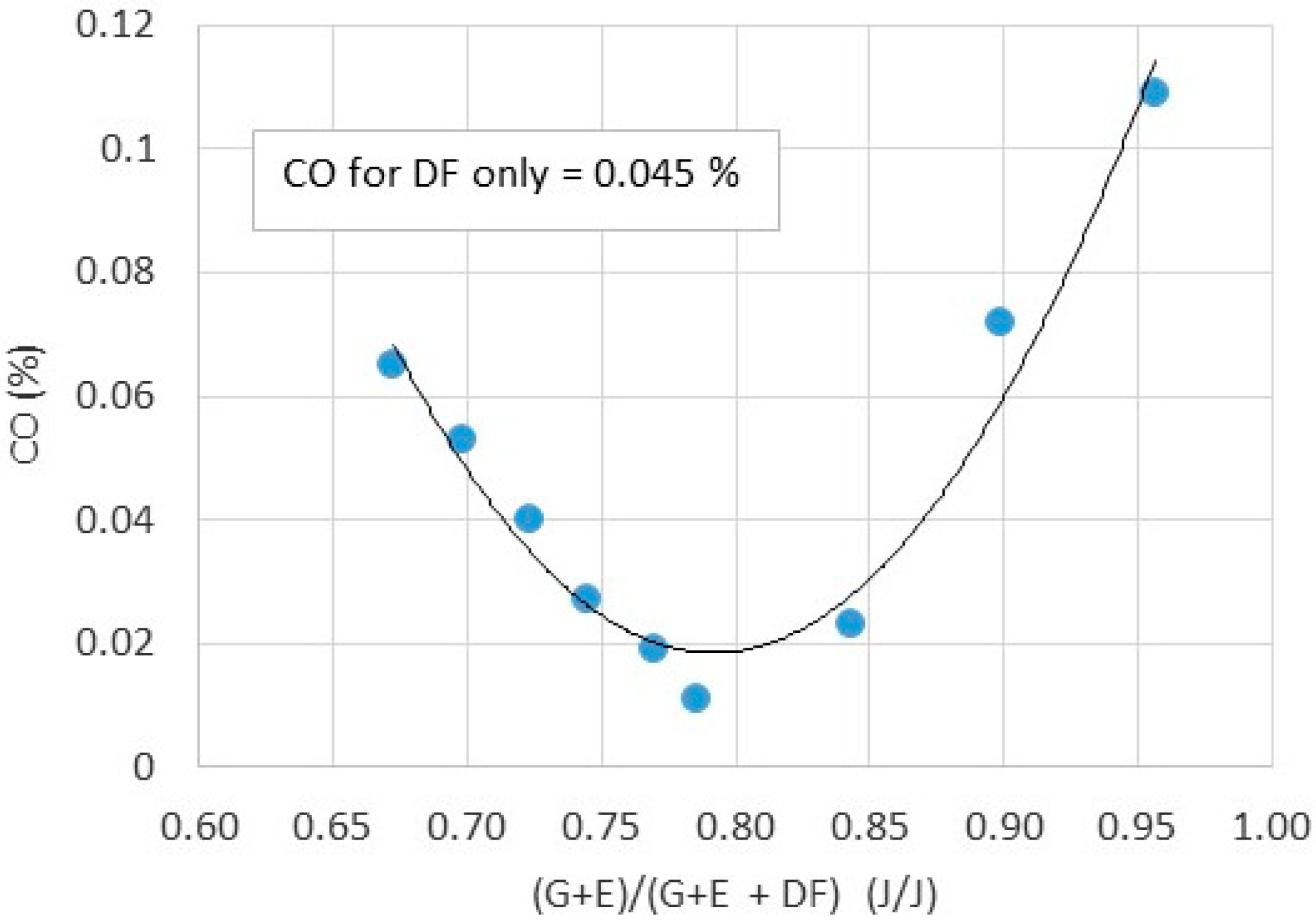
- Preliminary investigation on engine exhaust toxic emissions shows that both NOx, UHC and CO are at satisfactory levels in comparison to emissions from diesel fueled engines.
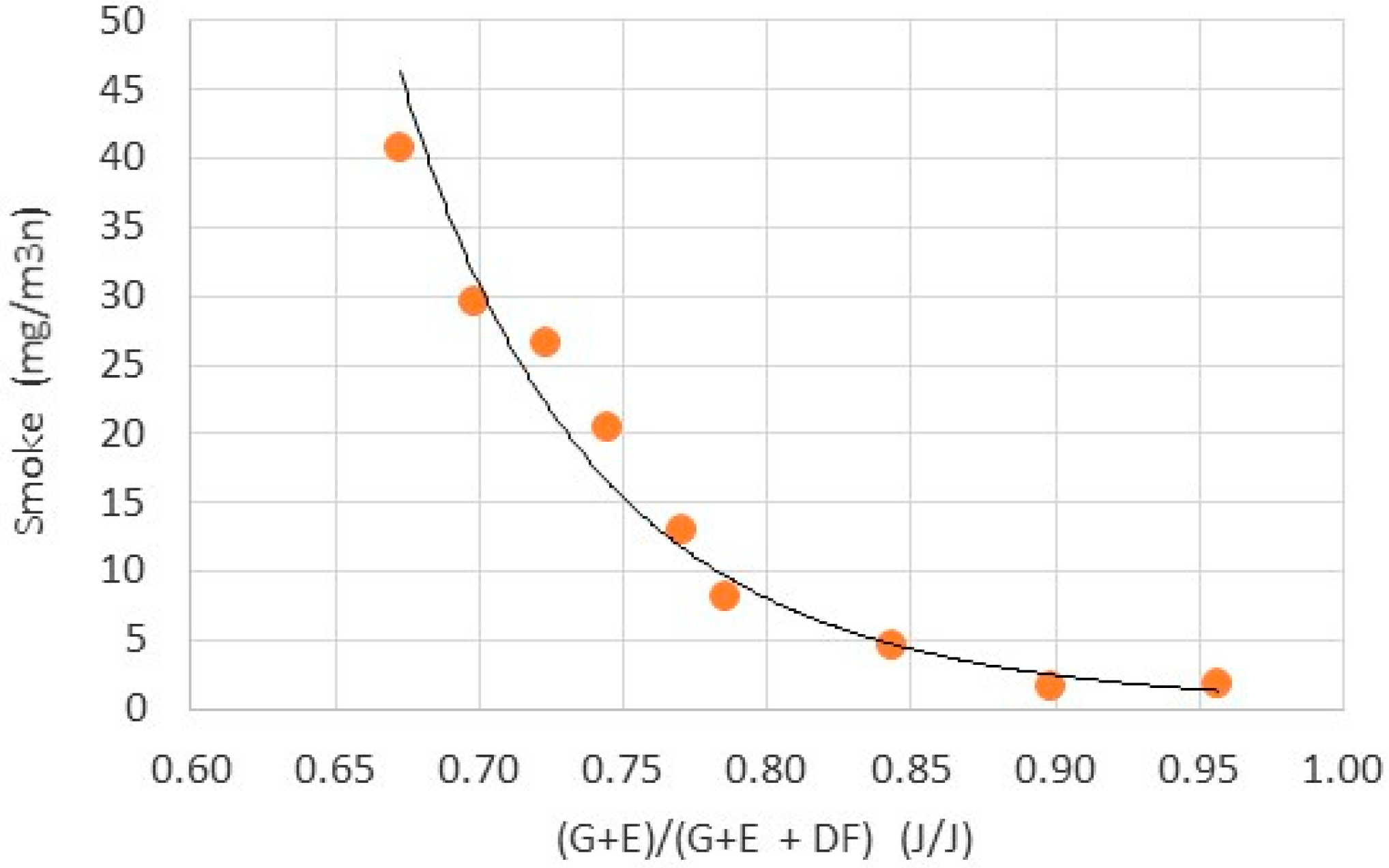
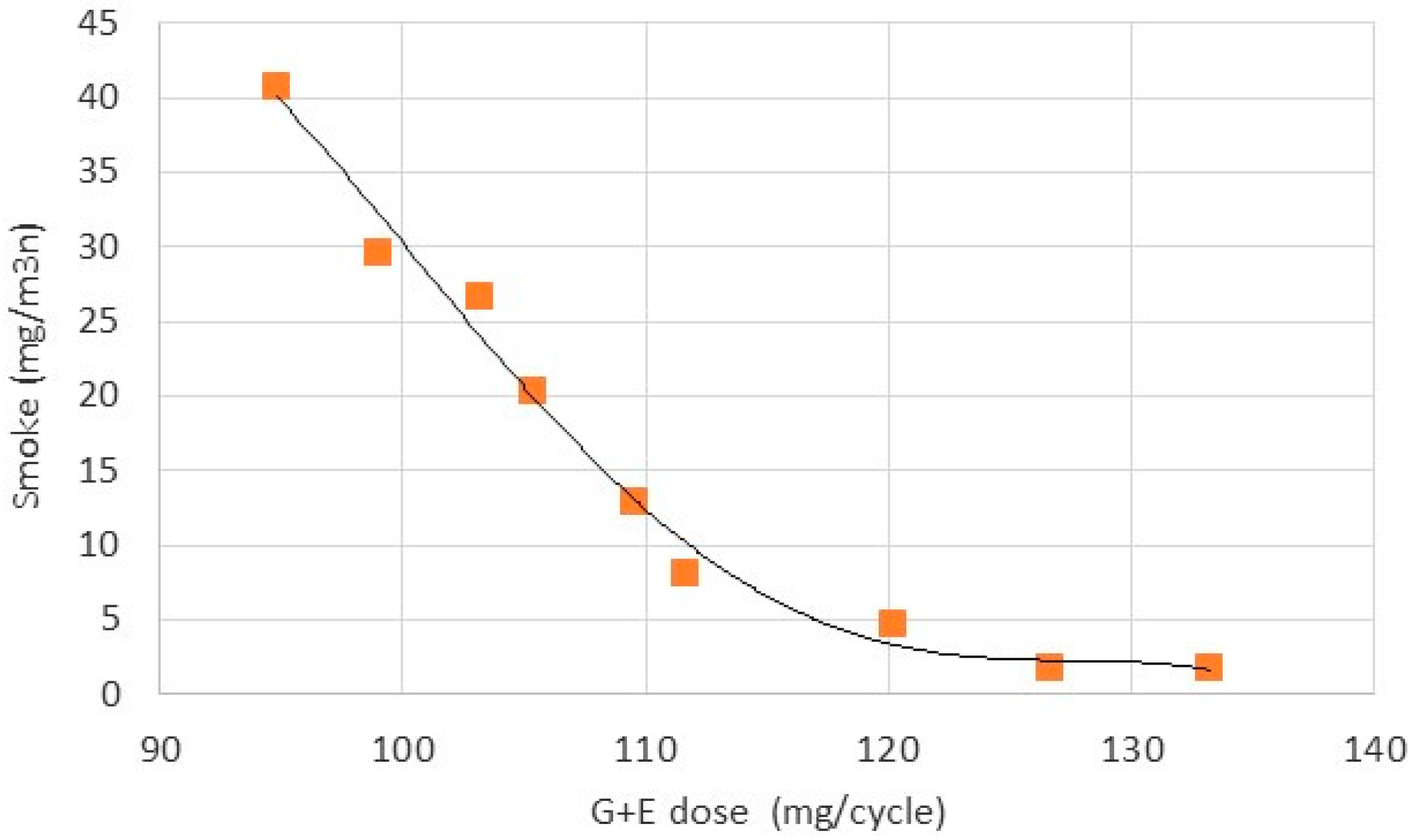
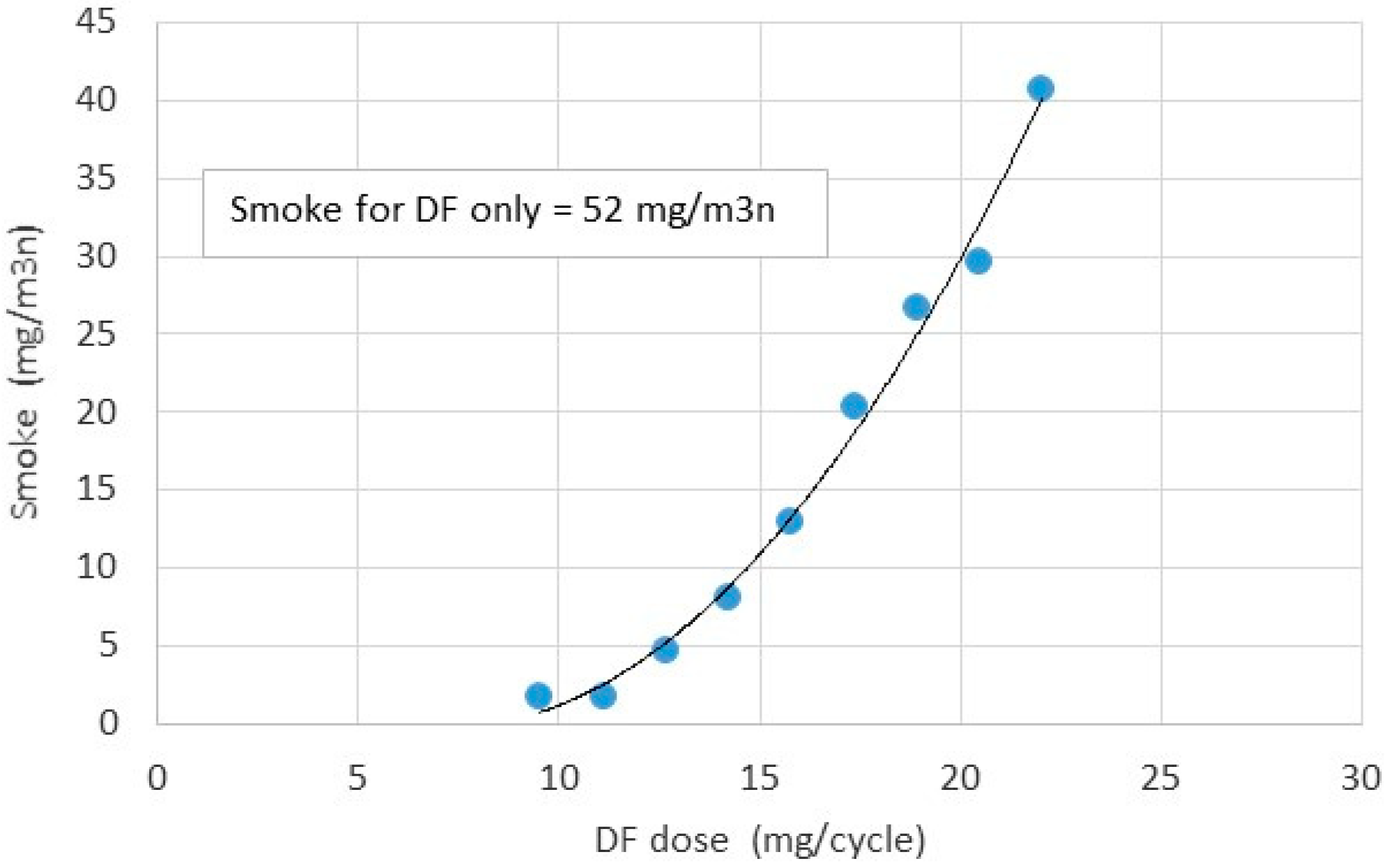
- The most interesting issue in the toxic emissions was the significant decrease in smoke with increase in glycerol-ethanol blend in the total fuel charge injected into the engine cylinder. As found smoke was produced from combustion of the diesel fuel pilot. Blend of glycerol and ethanol reduced smoke several times from 40 to 3 mg/cycle. This ratio was higher than diesel fuel decrease ratio from 22 to 9.5 mg/cycle. As concluded, it was caused by the oxygen content in the blend.
- The only problem reported was the combustion got longer with an increase in content in the total fuel injected into the cylinder. Under fixed injection timings, it caused the diffusion combustion phase to shift far away from TDC location. As result, the thermal efficiency decreased.
Author Contributions
Funding
Conflicts of Interest
References
- Presciutti, A.; Asdrubali, F.; Baldinelli, G.; Rotilia, A.; Malavasi, M.; Di Salvia, G. Energy and exergy analysis of glycerol combustion in an innovative flameless power plant. J. Clean. Prod. 2018, 172, 3817–3824. [Google Scholar] [CrossRef]
- Brock, D.; Koder, A.; Rabl, H.-P.; Touraud, D.; Kunz, W. Optimising the biodiesel production process: Implementation of glycerol derivatives into biofuel formulations and their potential to form hydrofuels. Fuel 2020, 264, 116695. [Google Scholar] [CrossRef]
- Asuquo, G.; Toronjo, D.; Sastri, B.; Kouch, C. Crude Glycerol as Cost-Effective Fuel for Combined Heat and Power to Replace Fossil Fuels; Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Quispe, C.A.; Coronado, C.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, N. Scope and opportunities of using glycerol as an energy source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- Available online: http://www.met.reading.ac.uk/~sws04cdw/viscosity_calc.html (accessed on 31 August 2020).
- Available online: https://www.engineeringtoolbox.com/ethanol-dynamic-kinematic-viscosity-temperature-pressure-d_2071.html?vA=40°ree=C# (accessed on 31 August 2020).
- Sliwinski, K.; Marek, W. The use of glycerin as motor fuel. Combust. Engines 2019, 178, 166–172. [Google Scholar]
- Ferreira, A.G.M.; Egas, A.P.V.; Fonseca, I.M.A.; Costa, A.C.; Abreu, D.C.; Lobo, L.Q. The viscosity of glycerol. J. Chem. Thermodyn. 2017, 113, 162–182. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.; Zhang, Y.; Setyawan, H.; Liu, P.; Zhang, D. An experimental study of the ignition and combustion characteristics of single droplets of biochar-glycerol-water slurry fuels. Proc. Combust. Inst. 2017, 36, 2475–2482. [Google Scholar] [CrossRef]
- Queirós, P.; Costa, M.; Carvalho, R.H. Co-combustion of crude glycerin with natural gas and hydrogen. Proc. Combust. Inst. 2013, 34, 2759–2767. [Google Scholar] [CrossRef]
- Bohon, M.D.; Metzger, B.A.; Linak, L.P.; King, C.J.; Roberts, L.W. Glycerol combustion and emissions. Proc. Combust. Inst. 2011, 33, 2717–2724. [Google Scholar] [CrossRef]
- Roberts, L.W. Crude Glycerol as Cost-Effective Fuel for Combined Heat and Power to Replace Fossil Fuels, DOE Final Technical Report. 2011. Available online: https://www.osti.gov/servlets/purl/1053951 (accessed on 31 October 2012). [CrossRef] [Green Version]
- Coronado, C.R.; Carvalho, J.A., Jr.; Quispe, C.A.; Sotomonte, C.R. Ecological efficiency in glycerol combustion. Appl. Therm. Eng. 2014, 63, 97–104. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Borisov, R.S.; Stolonogova, T.I.; Zarezin, D.P.; Maximov, A.L.; Bermeshev, M.V.; Chernysheva, E.A.; Kapustin, V.M. Glycerol to renewable fuel oxygenates. Part II: Gasoline-blending characteristics of glycerol and glycol derivatives with C3-C4 alkyl(idene) substituents. Fuel 2020, 280, 118585. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Meurah, T.I.M.; Muraza, O. Glycerol to Solketal for Fuel Additive: Recent Progress in Heterogeneous Catalysts. Energies 2019, 12, 2872. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.; Bordado, J.C.; Galhano dos Santos, R. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals-A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Stenhede, T. Combined Technologies Wärtsilä Power Plants; International Seminar on Gasification: Malmo, Sweden, 2008; Available online: http://www.sgc.se/ckfinder/userfiles/files/SGC193.pdf (accessed on 10 October 2008).
- Grab-Rogaliński, K.; Szwaja, S. The Possibility of Use a waste Product of Biofuels Production-Glycerol as a fuel to the Compression Ignition Engine. J. Kones 2016, 23, 157–164. [Google Scholar]
- Lábaj, J.; Barta, D.; Lenhard, R. CFD simulation of glycerol combustion in diesel engine. In Colloquium Fluid Dynamics 2008; Institute of Thermomechanics AS CR v.v.i.: Prague, Czech Republic, 2008; pp. 35–50. ISBN 978-80-87012-14-7. [Google Scholar]
- Oprescu, E.E.; Dragomir, R.E.; Radu, E.; Radu, A.; Velea, S.; Bolocan, I.; Stepan, E.; Rosca, P. Performance and emission characteristics of diesel engine powered with diesel-glycerol derivatives blends. Fuel Process. Technol. 2014, 126, 460–468. [Google Scholar] [CrossRef]
- Eaton, S.J.; Harakas, G.N.; Kimball, R.W.; Smith, J.A.; Pilot, K.A.; Kuflik, M.T.; Bullard, J.M. Formulation and Combustion of Glycerol–Diesel Fuel Emulsions. Energy Fuels 2014, 28, 3940–3947. [Google Scholar] [CrossRef]
- Beatrice, C.; Di Blasio, G.; Guido, C.; Cannilla, C.; Bonura, G.; Frusteri, F. Mixture of glycerol ethers as diesel bio-derivable oxy-fuel: Impact on combustion and emissions of an automotive engine combustion system. Appl. Energy 2014, 132, 236–247. [Google Scholar] [CrossRef]
- Munsin, R.; Laoonual, Y.; Jugjai, S.; Matsuki, M.; Kosaka, H. Effect of glycerol ethoxylate as an ignition improver on injection and combustion characteristics of hydrous ethanol under CI engine condition. Energy Convers. Manag. 2015, 98, 282–289. [Google Scholar] [CrossRef]
- McNeil, J.; Day, P.; Sirovski, F. Glycerine from biodiesel: The perfect diesel fuel. Process Saf. Environ. Prot. 2012, 90, 180–188. [Google Scholar] [CrossRef]
- Grab-Rogaliński, K.; Szwaja, S. The combustion properties analysis of various liquid fuels based on crude oil and renewables. Iop Conf. Ser. Mater. Sci. Eng. 2016, 148, 012066. [Google Scholar] [CrossRef] [Green Version]
- Chwist, M.; Gruca, M.; Pyrc, M.; Szwaja, M. By-products from thermal processing of rubber waste as fuel for the internal combustion piston engine. Combust. Engines 2020, 181, 11–18. [Google Scholar]
- Lapuerta, M.; Rodríguez-Fernández, J.; García-Contreras, R. Effect of a glycerol-derived advanced biofuel -FAGE (fatty acid formal glycerol ester)- on the emissions of a diesel engine tested under the New European Driving Cycle. Energy 2015, 93, 568–579. [Google Scholar] [CrossRef]
- Available online: https://www.avl.com/zh/-/avl-smoke-meter (accessed on 31 August 2020).
- Zeldovich, Y.B. The oxidation of nitrogen in combustion explosions. Acta Phys. Ussr 1946, 21, 577–628. [Google Scholar]






| Property | Unit | Number | |
|---|---|---|---|
| 1. | Higher heating value (HHV) | MJ/kg kJ/mol | 18 1662 |
| 2. | Lower heating value (LHV) | MJ/kg | 16 |
| 3. | Dynamic viscosity at 25 °C | Pa∙s | 1.3–1.7 |
| 4. | Kinematic viscosity at 25 °C and 40 °C | m2/s | (450–750) × 10−66 (350–580) × 10−6 |
| 5. | Density at 0 °C | kg/dm3 | 1.26 |
| 6. | Cetane number (CN) | - | 0–10 |
| 7. | Auto-ignition temperature | K | 643 |
| Component Content | % (kg/kg) | |
|---|---|---|
| 1. | Glycerol | 70–75 |
| 2. | Water | 14–17 |
| 3. | Mineral content (ash) | 6–8 |
| 4. | MONG (other organic compounds except glycerol) | 5 |
| 5. | Methanol | <1 |
| Parameter | Unit | Glycerol | Ethanol | G + E (50/50) | Diesel Fuel |
|---|---|---|---|---|---|
| LHV | MJ/kg | 20.3 | 33.4 | 25.8 | 42.5 |
| Density at NTP | kg/dm3 | 1.248 | 0.785 | 1.016 | 0.825 |
| Kinematic viscosity at 40 °C | m2/s | 226 × 10−6 | 1.06 × 10−6 | 3.52 × 10−6 | 2.50 × 10−6 |
| Parameter | Unit | Value |
|---|---|---|
| Parameters varied | ||
| G + E fraction in total fuel dose | - | 0.67–0.96 |
| G + E dose | mg/cycle | 95–133 |
| DF dose | mg/cycle | 22–9.5 |
| Parameters maintained constant | ||
| Glycerol to ethanol ratio | % | 50/50 |
| Entire fuel dose by energy | J | 2820–2890 |
| Engine speed | rpm | 970 |
| Injection pressure for G + E and DF | bar | 2000 |
| Start of injection for DF | CA deg bTDC | 26 |
| Start of injection for G+E | CA deg bTDC | 10 |
| Parameter | Value |
|---|---|
| Type of the engine | Four stroke compression ignition |
| Number of cylinders | 1 |
| Compression ratio | 17 |
| Engine rotational speed | 970 rpm |
| Displacement | 1810 cm3 |
| Max. injection pressure | 250 MPa |
| Max. rated power | 13.3 kW |
| Max. engine torque | 104 Nm |
| Bore × Stroke | 120 × 160 mm |
| Measured Parameters | Absolute Error | Uncertainty (%) | |
|---|---|---|---|
| Rate of flow of diesel fuel | 0.25 g/min | 2.5 | |
| Rate of flow of glycerol/ethanol blend | 0.25 g/min | 0.5 | |
| Dose of diesel fuel | 0.5 mg/cycle | 2.5 | |
| Dose of glycerol/ethanol fuel | 0.5 mg/cycle | 0.5 | |
| Gas analyser | |||
| UHC | range 0–9999 ppm vol | 12 ppm vol | |
| CO | range 0–10% vol | 0.4% vol | |
| NO | range 0–9999 ppm vol | 10 ppm vol | |
| Smoke | range: 0–10 FSN | 0.002 FSN or 0.02 mg/m3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruca, M.; Pyrc, M.; Szwaja, M.; Szwaja, S. Effective Combustion of Glycerol in a Compression Ignition Engine Equipped with Double Direct Fuel Injection. Energies 2020, 13, 6349. https://doi.org/10.3390/en13236349
Gruca M, Pyrc M, Szwaja M, Szwaja S. Effective Combustion of Glycerol in a Compression Ignition Engine Equipped with Double Direct Fuel Injection. Energies. 2020; 13(23):6349. https://doi.org/10.3390/en13236349
Chicago/Turabian StyleGruca, Michal, Michal Pyrc, Magdalena Szwaja, and Stanislaw Szwaja. 2020. "Effective Combustion of Glycerol in a Compression Ignition Engine Equipped with Double Direct Fuel Injection" Energies 13, no. 23: 6349. https://doi.org/10.3390/en13236349





