Numerical and Experimental Investigations on Combustion Characteristics of Premixed Lean Methane–Air in a Staggered Arrangement Burner with Discrete Cylinders
Abstract
:1. Introduction
2. Experiment
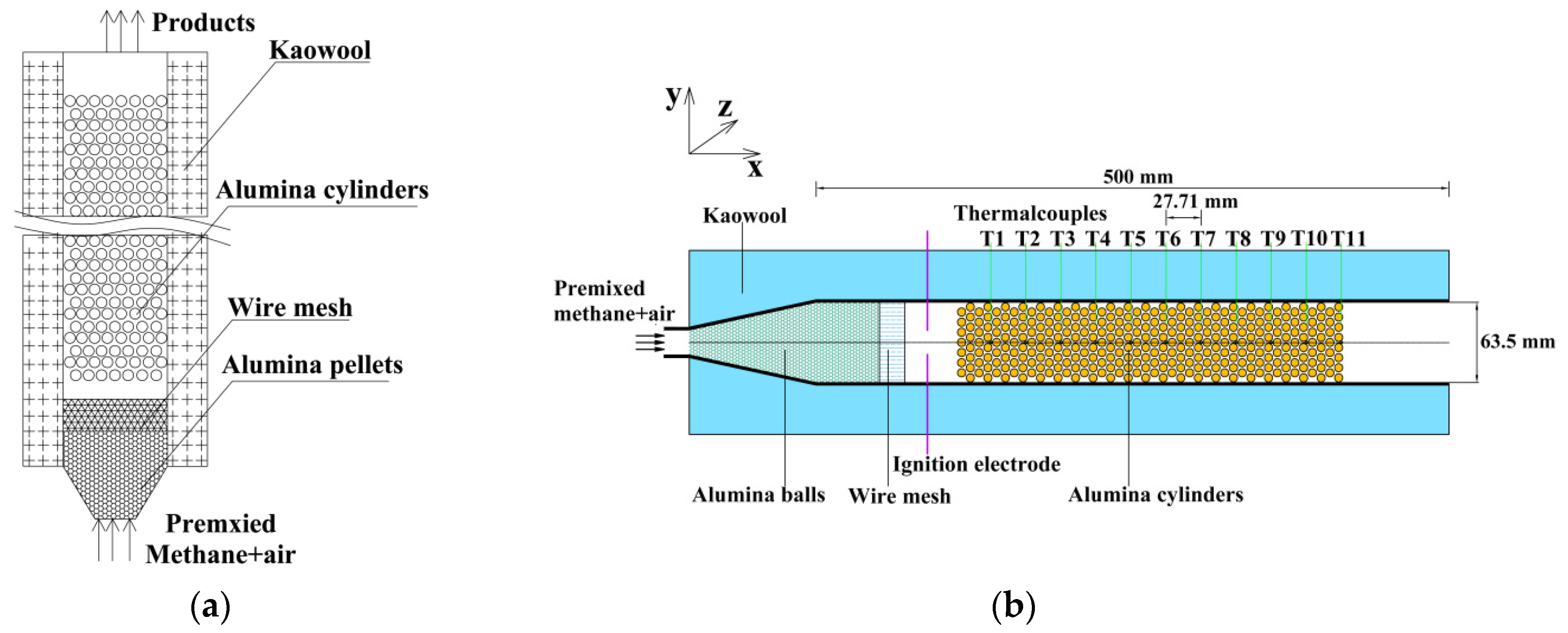
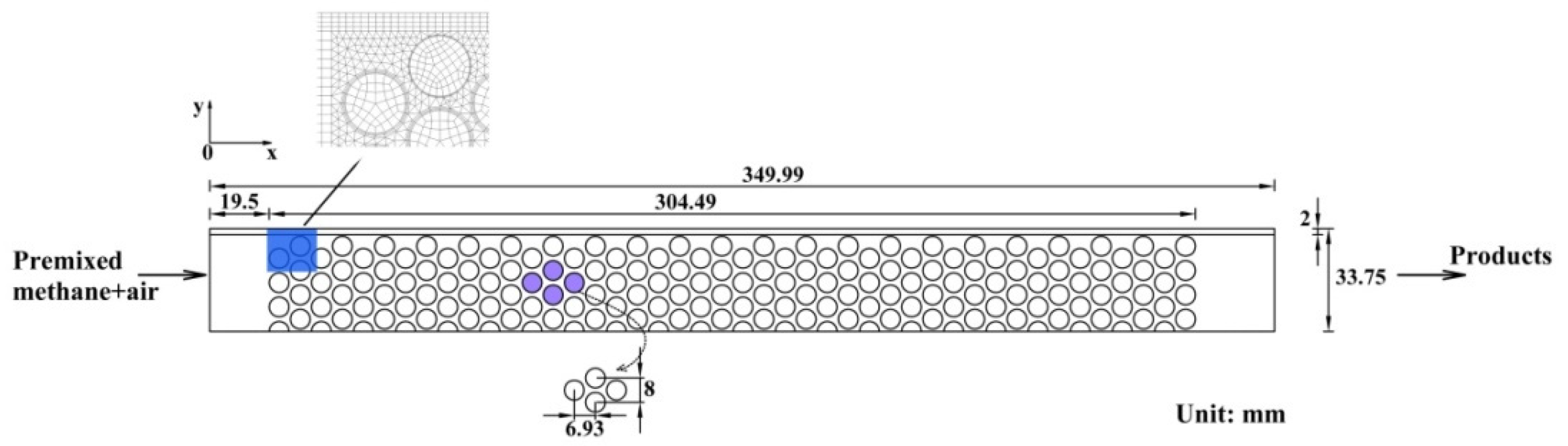
2.1. Experimental Setup
2.2. Experimental Steps
3. Mathematical Model
3.1. Governing Equations
- (1)
- The matrix was considered as opaque and inert homogenous porous media.
- (2)
- Given the lower gas flow velocities, it was assumed to be laminar for all the cases to be discussed.
- (3)
- The heat loss by the burner outer walls to the surroundings was neglected.
- (4)
- Pressure drop in the burner was ignored.
- (1)
- Continuity equations:
- (2)
- Momentum equations:
- (3)
- Species conservation equation:
- (4)
- Gas phase energy equation
- (5)
- Solid phase energy equation
- (6)
- Ideal gas state equation
3.2. Boundary Conditions
3.3. Computation Scheme
4. Results and Analysis
4.1. Flame Temperature Distributions
4.2. Species Fractions and Reaction Rate
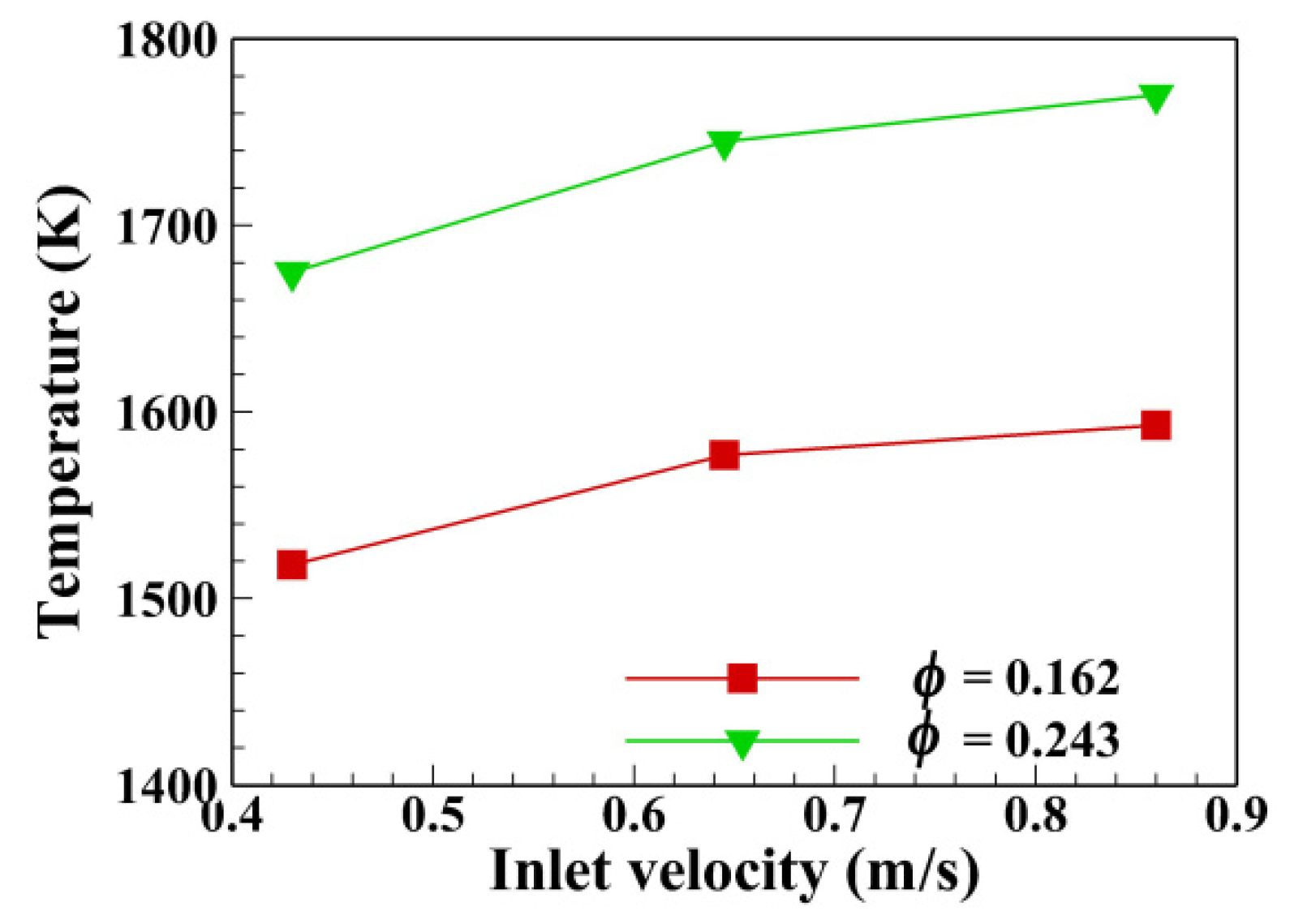
4.3. Effect of u0 on the Peak Flame Temperature
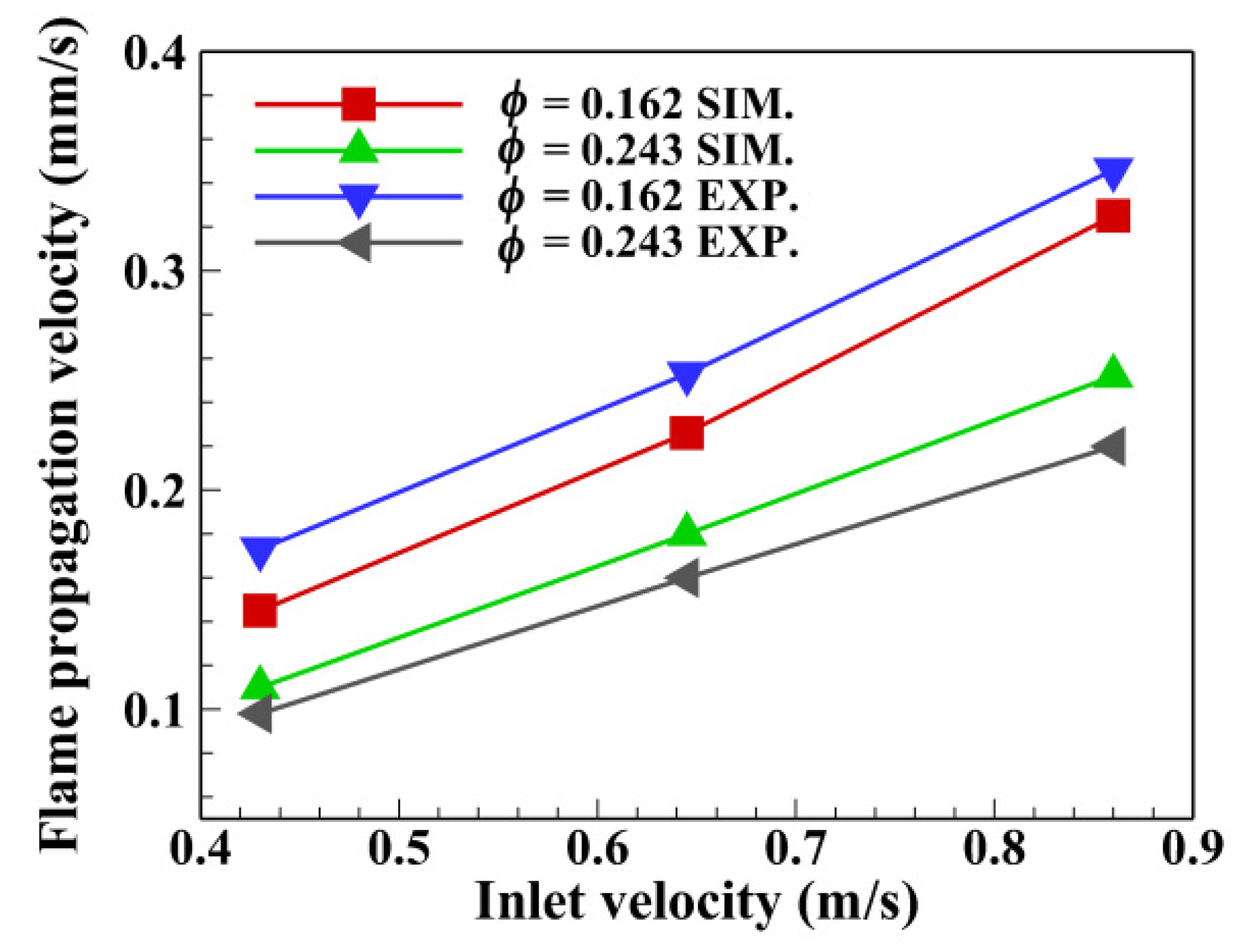
4.4. Effect of u0 on the Flame Propagation Velocity
5. Conclusions
- (1)
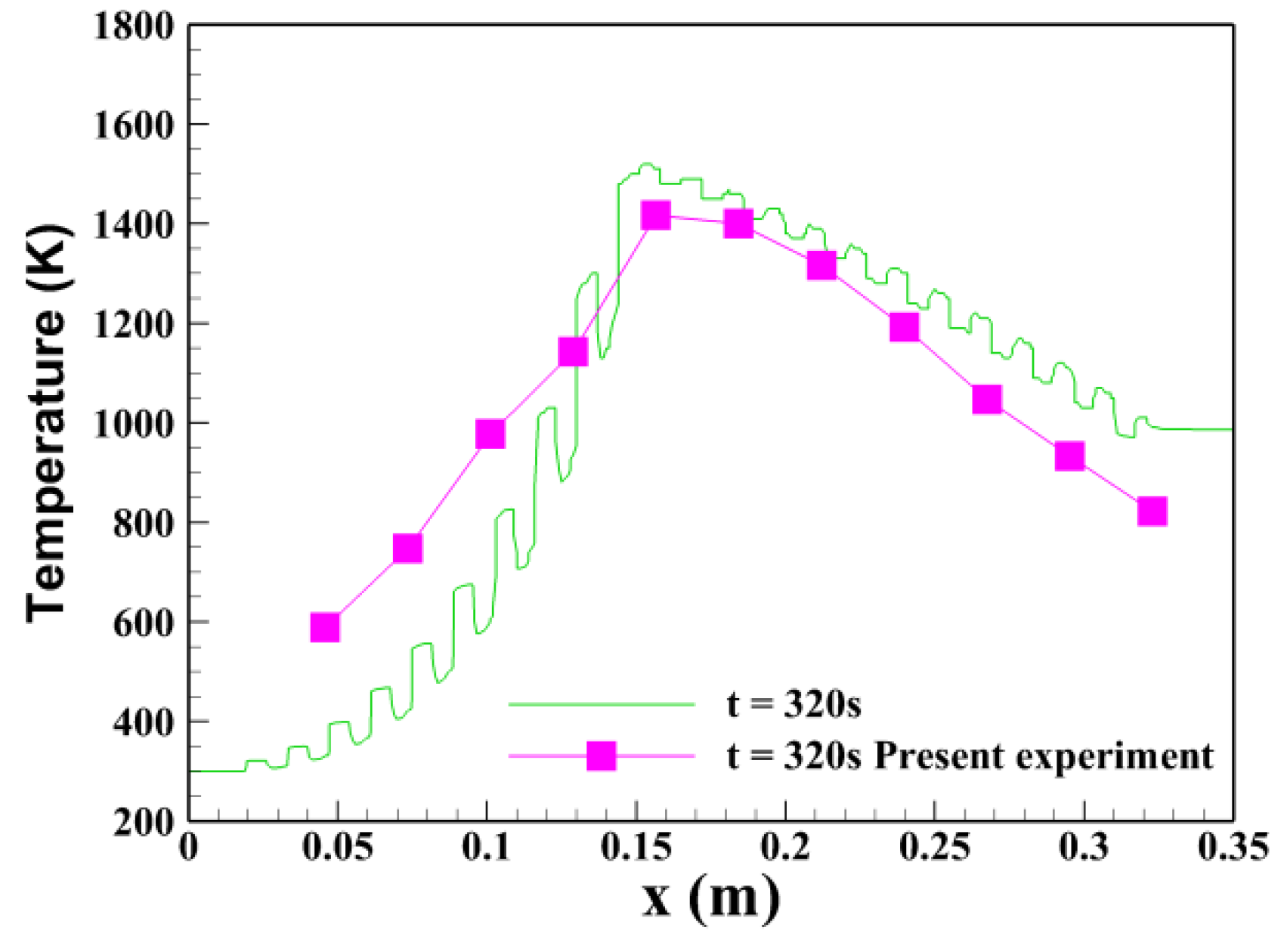
- At φ = 0.162 and u0 = 0.86 m/s, the trends of the temperature distributions towards the centerline of the burner between the simulation and experiment were similar. The peak temperature by numerical calculation was maintained at 1520 K, about 103 K greater than that by the experiment. The main reason of discrepancy between the predicted and experimental temperatures was that the global methane oxidation kinetics mechanism was used in the two-dimensional pore level simulation model.
- (2)
- The high temperature zone was located at the range of 130–140 mm, and the peak temperature was 1526 K at φ = 0.162 and u0 = 0.43 m/s. The flame thickness can be regards as the length of cylinder diameter under the test condition.
- (3)
- The peak temperatures along the flame propagation under various equivalence ratios and inlet velocities increased with inlet velocity at the same equivalence ratio. The magnitudes of the maximum temperature were greater than adiabatic temperature at the corresponding test conditions. It was demonstrated that all the test conditions were under the situation of the sub-adiabatic combustion. The maximum temperature was 2.16 times higher than the flame adiabatic temperature at φ = 0.162 and u0 = 0.86 m/s.
- (4)
- The flame propagation velocity had a positive relationship with the inlet velocity under the same equivalence ratio. The magnitude of the flame propagation velocity was in the order of 0.1 mm/s, which was typical low-velocity filtration combustion reported by Babkin et al. [36]. The predictions of flame propagation velocities were in good agreement with the experimental data. The peak relative error of flame propagation velocity between the numerical and experimental results was 16% at ϕ = 0.162 and u0 = 0.43 m/s.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| special heat (kJ/kg·K) | |
| length of the burner (m) | |
| width of the burner (m) | |
| diameter of cylinder (m) | |
| pressure (Pa) | |
| time (s) | |
| temperature (K) | |
| velocity (m/s) | |
| vertical velocity (m/s) | |
| magnitude of local velocity (m/s) | |
| velocity vector of gas mixture (m/s) | |
| molecular weight of species (kg/kmol) | |
| vertical coordinate axis (m) | |
| horizontal coordinate axis (m) | |
| transversal coordinate aixs (m) | |
| Greek Symbols | |
| porosity | |
| transversal coordinate axis (m) | |
| density (kg/m3) | |
| thermal conductivity (W/m·K) | |
| dynamic viscosity (Pa·s) | |
| reaction rate (kmol/m3·s) | |
| equivalence ratio | |
| Subscripts | |
| gas phase | |
| solid phase | |
| inlet zone of burner | |
| outlet zone of burner | |
| species in the gas mixture | |
References
- Zhdanok, S.; Kennedy, L.A.; Kosster, G. Superadiabatic combustion of methane air mixture under filtration in packed bed. Combust. Flame 1995, 100, 221–231. [Google Scholar] [CrossRef]
- Foutko, S.I.; Shabunya, S.I.; Zhdanok, S.A.; Kennedy, L.A. Superadiabatic combustion wave in a diluted methane-air mixture under filtration in a packed bed. Symp. Combust. 1996, 26, 3377–3382. [Google Scholar] [CrossRef]
- Bubnovich, V.I.; Zhdanok, S.A.; Dobrego, K.V. Analytical study of the combustion waves propagation under filtration of methane-air mixture in a packed bed. Int. J. Heat Mass Transfer 2006, 49, 2578. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, X.; Liu, M. Characteristic analysis of low-velocity gas flirtation combustion in an inert packed bed. Combust. Theory Model. 2006, 10, 683–700. [Google Scholar] [CrossRef]
- Shi, J.R.; Xie, M.Z.; Liu, H.; Li, G.; Zhou, L. Numerical simulation and theoretical analysis of low-velocity filtration combustion of lean mixture. Int. J. Heat Mass Transfer 2008, 51, 1818–1829. [Google Scholar] [CrossRef]
- Mital, R.; Gore, J.P.; Viskanta, R. A study of the structure of submerged reaction zone in porous ceramic radiant burners. Combust. Flame 1997, 111, 175–184. [Google Scholar] [CrossRef]
- Barra, A.J.; Diepvens, G.; Ellzey, J.L.; Henneke, M.R. Numerical study of the effects of material properties on flame stabilization in porous burner. Combust. Flame 2003, 134, 369–379. [Google Scholar] [CrossRef]
- Liu, H.; Dong, S.; Li, B.W.; Chen, H.G. Parametric investigations of premixed methane-air combustion in two-section porous media by numerical simulation. Fuel 2010, 8, 1736–1742. [Google Scholar] [CrossRef]
- Gao, H.B.; Qu, Z.G.; He, Y.L.; Tao, W.Q. Methane/air premixed combustion in a two-layor porous media burner with different foam materials. Fuel 2014, 115, 154–161. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, A.W.; Yao, H.; Liu, W. Numerical investigation of filtration gas combustion in a mesoscale combustor filled with inert fibrous porous media. Int. J. Heat Mass Transfer 2015, 91, 18–26. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.T.; Chen, J.X.; Shi, J.R.; Liu, X.L. Experimental study on standing wave regimes of premixed H2-air combustion in planar micro-combustors partially filled with porous media. Fuel 2016, 167, 98–105. [Google Scholar] [CrossRef]
- Hoffmann, J.G.; Echigo, R.; Yoshida, H.; Tada, S. Experimental study on combustion in porous media with a reciprocating flow system. Combust. Flame 1997, 111, 32–46. [Google Scholar] [CrossRef]
- Keramiotis, C.; Stelzner, B.; Trimis, D.; Founti, M. Porous burners for low emission combustion: An experimental investigation. Energy 2012, 45, 213–219. [Google Scholar] [CrossRef]
- Toledo, M.; Bubnoich, V.; Saveliev, A.; Kennedy, L. Hydrogen production in ultrarich combustion of hydrocarbon fuels in porous media. Int. J. Hydrogen Energy 2009, 34, 1818–1827. [Google Scholar] [CrossRef]
- Dhamrat, R.S.; Ellzey, J.L. Numerical and experimental study of the conversion of methane to hydrogen in a porous media reactor. Combust. Flame 2006, 144, 698–709. [Google Scholar] [CrossRef]
- Trimis, D.; Durst, F. Combustion in a porous medium-advances and applications. Combust. Sci. Technol. 1996, 121, 153–168. [Google Scholar] [CrossRef]
- Chou, S.K.; Yang, W.M.; Li, J.; Li, Z.W. Porous media combustion for micro thermophotovoltaic system applications. Appl. Energy 2010, 87, 2862–2867. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, D.G.; Fan, A.W.; Yao, H. Experimental and numerical investigations on flame stability of methane/air mixtures in mesoscale combustors filled with fibrous porous media. Energy Convers. Manag. 2016, 123, 402–409. [Google Scholar] [CrossRef]
- Bani, S.; Pan, J.F.; Tang, A.; Lu, Q.; Zhang, Y. Micro combustion in a porous media for thermophotovoltaic power generation. Appl. Therm. Eng. 2018, 129, 596–605. [Google Scholar] [CrossRef]
- Kang, X.; Deng, Y.C.; Fan, A.W. Impact of wall thermal properties on flame stability of small-scale combustors. J. Combust. Sci. Technol. 2019, 25, 11–15. [Google Scholar]
- Howell, J.R.; Hall, M.J.; Ellzey, J.L. Combustion of hydrocarbon fuels within porous inert media. Prog. Energy Combust. Sci. 1996, 127, 121–145. [Google Scholar] [CrossRef]
- Kamal, M.M.; Mohamad, A.A. Combustion in porous media. Proc. Inst. Mech. Eng. Part A 2006, 220, 487–508. [Google Scholar] [CrossRef]
- Mujeebu, M.A.; Abdullah, M.Z.; AbuBakar, M.Z.; Mohamad, A.A.; Abdullah, M.K. Applications of porous media combustion technology—A review. Appl. Energy 2009, 86, 1365–1375. [Google Scholar] [CrossRef]
- Mujeebu, M.A.; Abdullah, M.Z.; Mohamad, A.A.; AbuBakar, M.Z. Trends in modeling of porous media combustion. Prog. Energy Combust. Sci. 2010, 36, 627–650. [Google Scholar] [CrossRef]
- Mujeebu, M.A. Hydrogen and syngas production by superadiabatic combustion—A review. Appl. Energy 2016, 173, 210–224. [Google Scholar] [CrossRef]
- Wood, S.; Harris, A.T. Porous burners for lean-burn applications. Prog. Energy Combust. Sci. 2008, 34, 667–684. [Google Scholar] [CrossRef]
- Zheng, C.H.; Cheng, L.M.; Saveliev, A.; Luo, Z.Y.; Cen, K.F. Gas and solid phase temperatures measurements of porous media combustion. Proc. Combust. Inst. 2011, 33, 3301–3308. [Google Scholar] [CrossRef]
- Henneke, M.R.; Ellzey, J.L. Modeling of filtration combustion in a packed bed. Combust. Flame 1991, 117, 832–840. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.W.; Zeng, M.; Nakayama, A. Computational study of forced convective heat transfer in structured packed beds with spherical or ellipsoidal particles. Chem. Eng. Sci. 2010, 65, 726–738. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Bu, S.S.; Zeng, M.; Wang, Q.W.; Nakayama, A. Experimental analysis of forced convective heat transfer in novel structured packed beds of particles. Chem. Eng. Sci. 2012, 71, 126–137. [Google Scholar] [CrossRef]
- Min, D.K.; Shin, H.D. Laminar premixed flame stabilized inside a honeycomb ceramic. Int. J. Heat Mass Transfer 1991, 34, 341–356. [Google Scholar]
- Sahraoui, M.; Kaviany, M. Direct simulation vs volume-averaged treatment of adiabatic, premixed flame in a porous medium. Int. J. Heat Mass Transfer 1994, 37, 2817–2834. [Google Scholar] [CrossRef] [Green Version]
- Jouybari, N.F.; Maerefat, M.; Nimvari, M.E. Pore scale simulation vs volume averaged treatment of turbulent reacting and nonreacting flow in a porous medium. J. Porous Media 2014, 17, 103–116. [Google Scholar] [CrossRef]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.F.; Matthews, R.D. The necessity of using detailed kinetics in models for premixed combustion within porous media. Combust. Flame 1993, 93, 457–466. [Google Scholar] [CrossRef]
- Babkin, V.S.; Korzhavin, A.A.; Bunev, V.A. Propagation of premixed gaseous explosion flames in porous media. Combust. Flame 1991, 87, 182–190. [Google Scholar] [CrossRef]


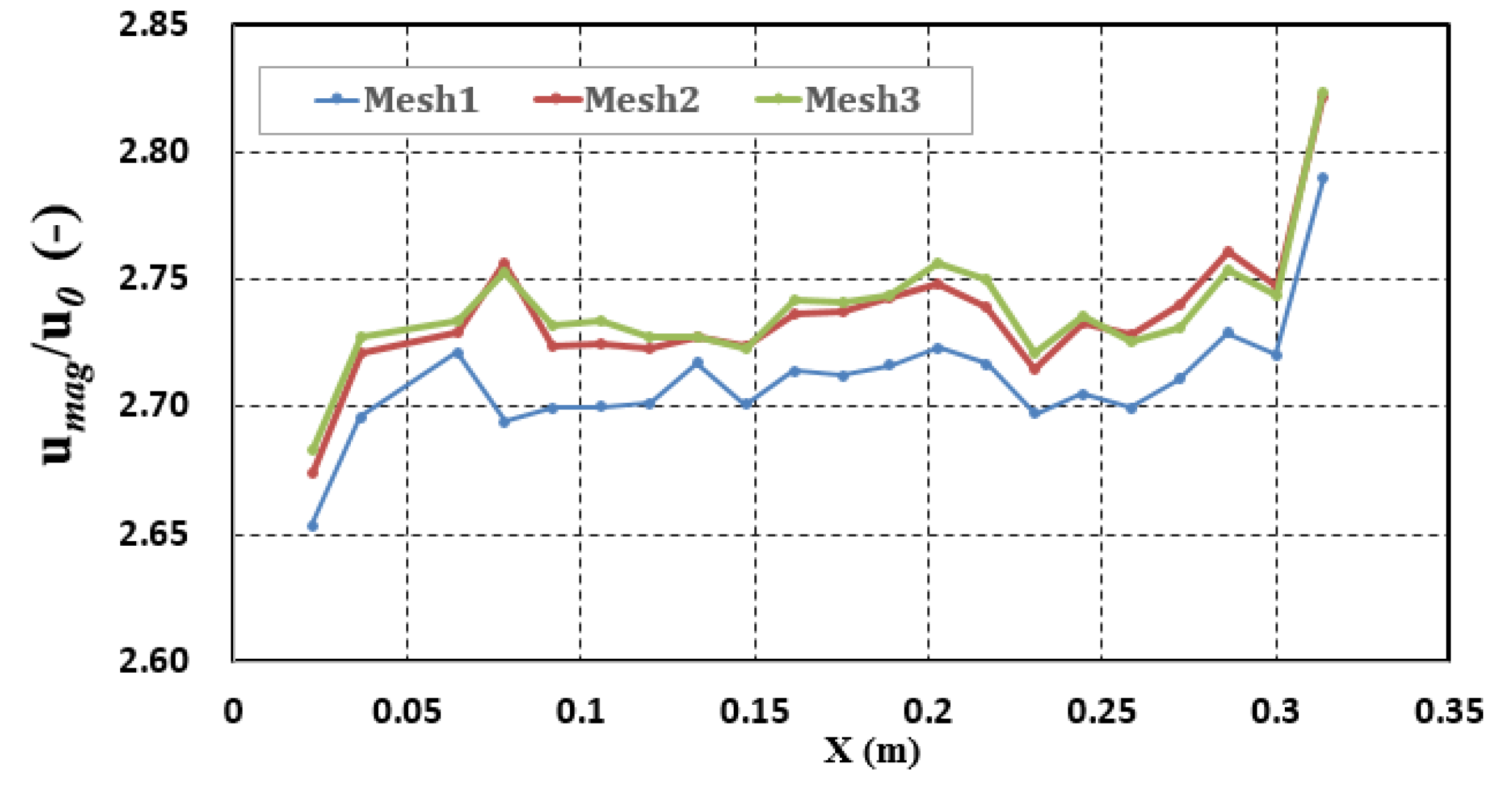

| Mesh Properties | Mesh 1 | Mesh 2 | Mesh 3 |
|---|---|---|---|
| Minimum Cell Volume | 3.98 × 10−8 | 9.62 × 10−9 | 2.34 × 10−9 |
| Cell total number | 27,800 | 37,451 | 61,394 |
| Equivalence ratio φ (-) | 0.243, 0.324 |
| Inlet velocity u0 (m/s) | 0.43, 0.645, 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, M.; Xie, M.-Z.; Shi, J.-R.; Liu, H.-S.; Chen, Z.-S.; Chang, Y.-C. Numerical and Experimental Investigations on Combustion Characteristics of Premixed Lean Methane–Air in a Staggered Arrangement Burner with Discrete Cylinders. Energies 2020, 13, 6397. https://doi.org/10.3390/en13236397
Yue M, Xie M-Z, Shi J-R, Liu H-S, Chen Z-S, Chang Y-C. Numerical and Experimental Investigations on Combustion Characteristics of Premixed Lean Methane–Air in a Staggered Arrangement Burner with Discrete Cylinders. Energies. 2020; 13(23):6397. https://doi.org/10.3390/en13236397
Chicago/Turabian StyleYue, Meng, Mao-Zhao Xie, Jun-Rui Shi, Hong-Sheng Liu, Zhong-Shan Chen, and Ya-Chao Chang. 2020. "Numerical and Experimental Investigations on Combustion Characteristics of Premixed Lean Methane–Air in a Staggered Arrangement Burner with Discrete Cylinders" Energies 13, no. 23: 6397. https://doi.org/10.3390/en13236397






