Abstract
Hydrogenation of succinic acid and maleic acid produces C4 value-added chemicals such as γ-butyrolactone and tetrahydrofuran. Here, unsupported ReOx nanoparticles transform succinic acid to γ-butyrolactone and tetrahydrofuran via catalytic transfer hydrogenation with isopropanol as a liquid phase hydrogen donor. This catalyst is also active for the sequential reaction of deoxydehydration and transfer hydrogenation in isopropanol, synthesizing renewable succinic acid and its esters from tartaric acid. One-step conversion of tartaric acid to γ-butyrolactone is achieved in a moderate yield and the possible reaction pathway is discussed.
1. Introduction
Biomass is a promising renewable energy resource which may provide a sustainable route to biofuels and chemicals [1]. Fine chemicals can be produced from biomass-derived building-block compounds [2]. In 2004, the U.S. Department of Energy selected succinic acid (SA), a C4-dicarboxylic acid, as one of the bio-based key platform chemicals [3]. Renewable SA can replace maleic anhydride to synthesize many valuable chemicals including γ-butyrolactone (GBL), tetrahydrofuran (THF), and 1,4-butanediol (1,4-BDO) [4,5,6,7]. GBL has been used as a starting material for the production of N-methyl-2-pyrrolidone and other pyrrolidone derivatives. THF is a monomer of poly(tetramethylene ether) glycol (PTMEG). Both GBL and THF are also utilized as solvents. The demand for GBL and THF is increasing annually and the global market size of GBL and THF in 2018 was approximately 0.6 and 3.2 billion USD, respectively [8,9]. Thus, the preparation of renewable SA and its transformation into GBL and THF are receiving much attention.
Several methods to synthesize SA from biomass-derived compounds have been reported. Renewable SA has been typically prepared through the bio-fermentation of glucose [10,11]. However, recent breakthroughs in the biological process, costly separation, and large quantities of waste byproducts make it difficult to realize at the industrial scale [12]. SA was synthesized from furfural through thermochemical processes [13,14,15]. However, the C5 starting compound formed C1 byproducts such as CO2 and formic acid. Tartaric acid (TA), a C4 sugar acid, has been proposed as an alternative resource to prepare SA without carbon loss. Tartaric acid is a naturally occurring organic acid and is generated in large quantities as a byproduct of wine-making [16,17]. MoOx-catalyzed hydrodeoxygenation converted TA to SA with a high yield (87%), but it required harsh conditions such as corrosive halogen, high pressure of H2, and acetic acid [18]. Moreover, the transformation of TA to SA was achieved through a combination of deoxydehydration (DODH) and hydrogenation in two steps without harsh conditions [19]. DODH is an attractive deoxygenation reaction to prepare olefins from vicinal diols [20,21]. DODH with oxorhenium complexes converted TA to maleic acid (MA) and the resulting MA was hydrogenated over a Pt/C catalyst, producing SA with a 97% yield [19]. Previously, we reported unsupported ReOx nanoparticles (ReOx NPs) as an active and reusable heterogeneous catalyst to synthesize olefins from polyols through DODH with a secondary alcohol reductant. High oxidation states of Re (Re5+ and Re7+) in the nanoparticles were involved in the DODH reaction [22]. We have found that the ReOx NPs are not only active for DODH but also for catalytic transfer hydrogenation (CTH) at high reaction temperature (T > 200 °C) with isopropanol as a hydrogen donor. The tandem reaction of DODH-CTH over the ReOx NPs can transform TA into SA and its ester in one step without the harsh conditions.
In addition, the ReOx NPs further convert the prepared SA and its ester to GBL and THF through cyclization and transfer hydrogenation. The formation of GBL or THF from SA has been achieved using direct hydrogenation with H2 gas over various monometallic or bimetallic catalysts including palladium, ruthenium, and rhenium-based catalysts [2,5,6,7,23]. CTH reaction using isopropanol as a hydrogen donor is a promising alternative to direct hydrogenation because the hydrogen donor is easy to handle and environmentally friendly, preventing possible hazards [24,25,26]. In this sense, CTH with a liquid phase hydrogen donor has been recently introduced to provide hydrogen for the hydrodeoxygenation of biomass-derived materials including 5-hydroxymethylfurfural, levulinic acid, and lignin-derived phenolics [27,28,29,30]. However, to the best of our knowledge, CTH has not been employed to prepare GBL or THF from SA or MA. Herein, we report new reaction pathways to prepare GBL and THF from renewable substrates over ReOx NPs. First, the conversion of SA to GBL or THF through cyclization and transfer hydrogenation with ReOx NPs is tested. Furthermore, a combination of deoxydehydration, transfer hydrogenation, and cyclization produces GBL from TA in one step.
2. Materials and Methods
2.1. Catalyst Preparation
Unsupported ReOx NPs were prepared from ammonium perrhenate (NH4ReO4, Strem Chemicals) in the presence of 3-octanol (Sigma-Aldrich, St. Louis, MO, USA), as described in detail elsewhere [22]. A mixture of ammonium perrhenate (268 mg, 1 mmol) and 3-octanol (20 mL, 126 mmol) was placed in a 100 mL round bottom flask and heated at 180 °C in a pre-heated oil bath. Black ReOx nanoparticles were synthesized after 12 h reflux in open air and isolated by centrifugation (11 000 rpm for 1.5 h). Unreacted ammonium perrhenate was removed by washing repeatedly with ethanol and hexane solution (1:1 wt %) and further centrifugation. The ReOx NPs were dried in 120 °C oven overnight and kept in powder form for future use.
2.2. Activity Test
All activity tests were performed in a Parr batch reactor vessel. The prepared unsupported ReOx nanoparticles (10 mg) were mixed with succinic acid (1 mmol, 118 mg, Alfa Aesar, Ward Hill, MA, USA) and isopropanol (10 mL) in the vessel. The vessel was pressurized with 15 bar of N2 and heated to the reaction temperature for a given amount of time. The reaction time was recorded after the temperature reached the desired value. After the reaction, the spent catalyst was separated from the reaction solution. The product solution was analyzed by NMR and GC with mesitylene as an internal standard. The conversion of different substrates including maleic acid and L–(+)–tartaric acid (Alfa Aesar , Ward Hill, MA, USA) was conducted under the same conditions. The yields of TA, MA, SA, and their esters were calculated by 1H NMR measured on an Agilent Technologies 400 MHz, 400-MR DD2 spectrometer. The yields of GBL and THF were quantified by gas chromatography with a flame ionized detector (GC-FID, Agilent Technologies, Wilmington, DE, USA, 6890N with a DB-5 capillary column).
2.3. Characterization
The physical and chemical properties of ReOx NPs were characterized using various characterization methods, as in our previous study [22]. In order to identify the acid type of catalyst samples, infrared spectra of catalysts were obtained with a Thermo Scientific Nicolet iS10 Fourier transform infrared spectroscopy (FT-IR) spectrometer. The catalysts were diluted with KBr, and then a baseline spectrum was obtained before pyridine introduction. Pyridine was introduced to the sample by flowing Ar through a pyridine bubbler for 10 min. To remove physisorbed pyridine, 100 sccm Ar was purged for 30 min. The spectra were obtained at room temperature.
3. Results and Discussion
3.1. Conversion of Succinic Acid (SA)
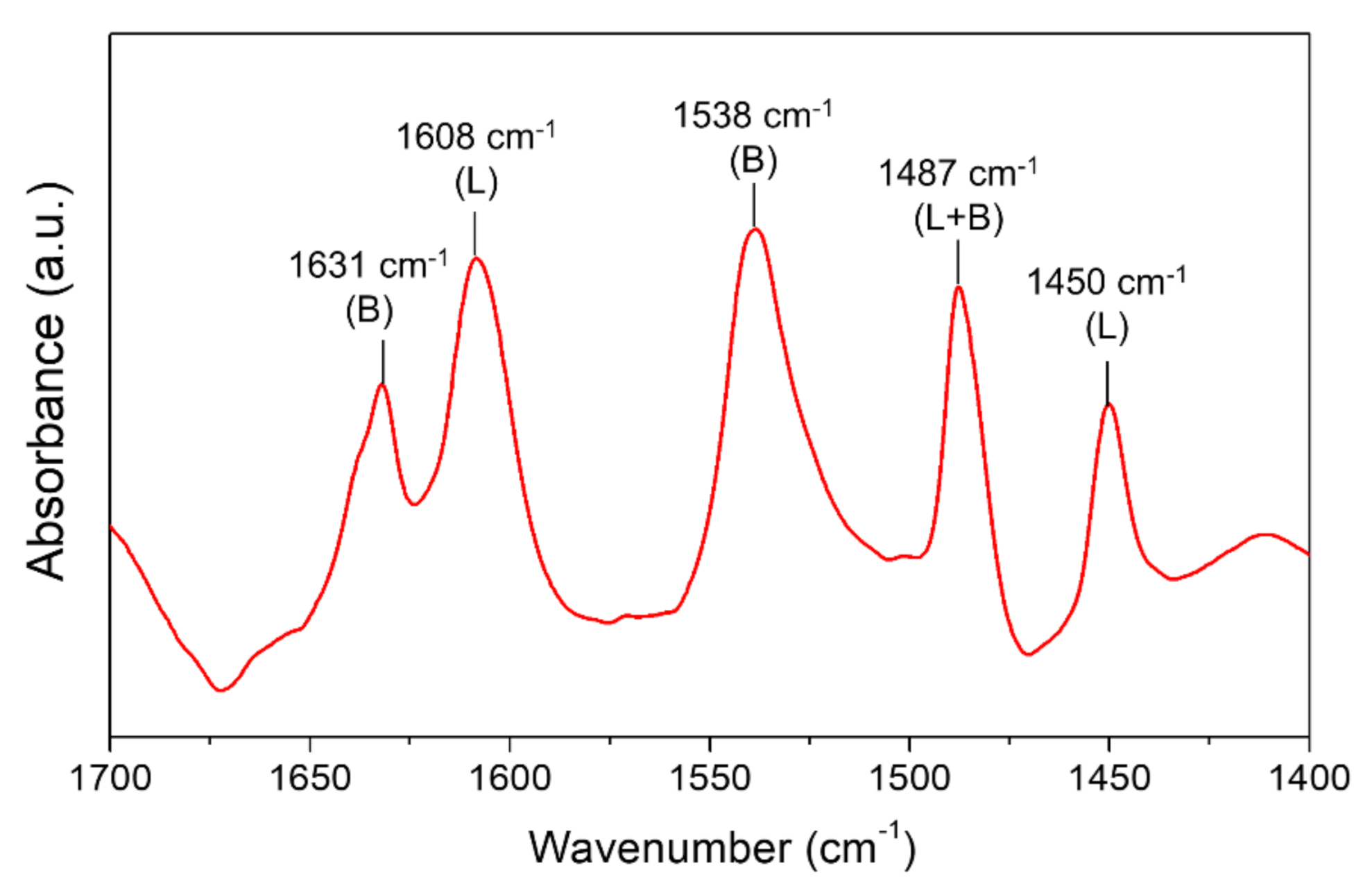
CTH reaction of succinic acid over ReOx NPs with isopropanol as a hydrogen donor was investigated at different reaction temperatures (170–250 °C). At lower temperatures (T < 200 °C), esterification of SA gave succinic acid monoisopropyl ester (SA-ME) as a major product and a small quantity of succinic acid diisopropyl ester (SA-DE) in 1 h (Figure 1a). The CH2 peak in the 1H NMR spectra of SA (δ = 2.43 ppm) in DMSO-d6 shifted downfield by 0.02 ppm (SA-ME) and 0.05 ppm (SA-DE) after esterification (Figure 2a). At 210 °C, SA conversion over ReOx produced not only ester compounds (SA-ME and SA-DE) but also GBL. Higher GBL yields of 16% and 43% were obtained in 1 h at 230 °C and 250 °C, respectively (Figure 1a). Previously, GBL was synthesized by direct hydrogenation of SA with molecular H2 [5,6,7]. The proposed mechanisms include dehydration of SA to succinic anhydride (SAN) over acid sites of the catalysts, followed by hydrogenation over metal sites [7,23]. ReOx NPs converted SAN to 47% yield of GBL and 40% of SA-ME/DE at 250 °C in 1 h (Figure 1d). This indicates SAN as a possible intermediate in the conversion of SA to GBL while it was not detected. At 250 °C, SAN in isopropanol is susceptible to alcoholysis to SA-ME, which is further esterified to SA-DE (Scheme 1). The alcoholization and further esterification also occurred without a catalyst, but no GBL was observed (Figure 1d). This demonstrates that CTH of SAN to GBL was catalyzed by ReOx NPs. SAN can be formed by dehydration of SA or dealcoholization of SA-ME and the resulting SAN is hydrogenated through CTH with isopropanol over ReOx NPs (Scheme 1). Pyridine probe FT-IR shows that ReOx NPs have both Brønsted and Lewis acid sites that can catalyze dehydration and dealcoholization (Figure 3). The bands at 1450 cm−1, 1487 cm−1, and 1608 cm−1 represent Lewis bonded pyridine. The bands at 1487 cm−1, 1538 cm−1, and 1631 cm−1 are assigned to Brønsted bonded pyridine. The previously suggested structural features of ReOx NPs, a combination of Re=O double bonds and Re-O single bonds, can provide Lewis acid sites [22]. Lewis acidity of rhenium-based catalysts including Re2O7, CH3ReO3, and HReO4 has been reported [19,31]. It is also reported that ReOx NPs contain ammonium ion, which is probably a Brønsted acid site in the catalyst [22]. In addition to dehydration, the acid sites on ReOx might catalyze C-O cleavage of SAN to GBL. Recently, Li et al. studied C-O cleavage of lignin model compounds over ReOx catalysts and acidic sites on ReOx were responsible for the cleavage of C-O bonds [32]. The control test without catalyst under the same conditions (250 °C, 1 h) yielded ester compounds (Figure 1a). Even though esterification occurred at 250 °C without catalyst, ReOx NPs facilitated esterification, yielding the higher SA-DE/SA-ME ratio.
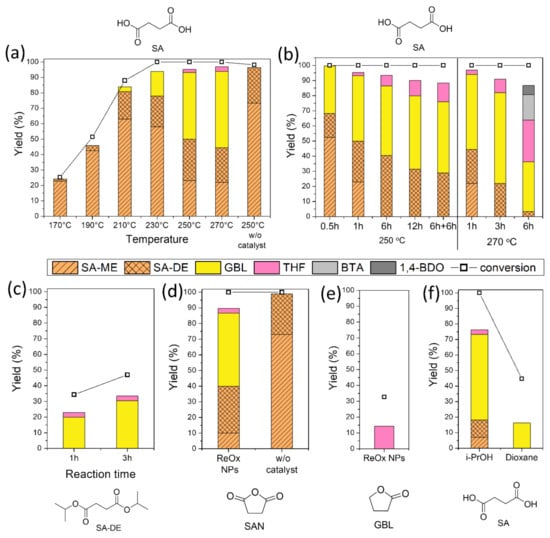
Figure 1.
Conversion of succinic acid (SA), succinic anhydride (SAN), succinic acid diisopropyl ester (SA-DE), and γ-butyrolactone (GBL) over ReOx/C in isopropanol. (a–e) Reaction conditions: batch reaction, ReOx NPs (10 mg, 4 mol% Re relative to substrate), substrate (1 mmol), i-PrOH (10 mL), and N2 (15 bar); (f) H2 (15 bar) and solvent (10 mL). Conversion and yield are calculated by 1H NMR and GC-FID.
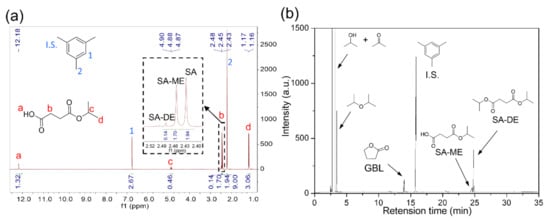
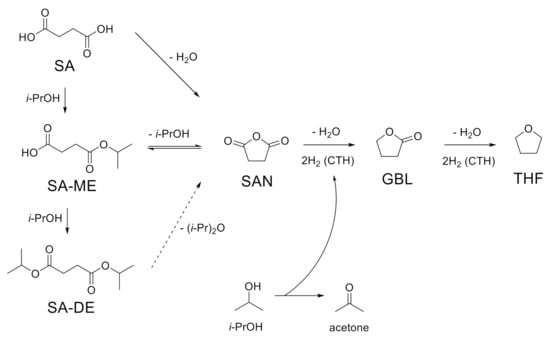
Scheme 1.
Possible reaction pathway for the conversion of SA.
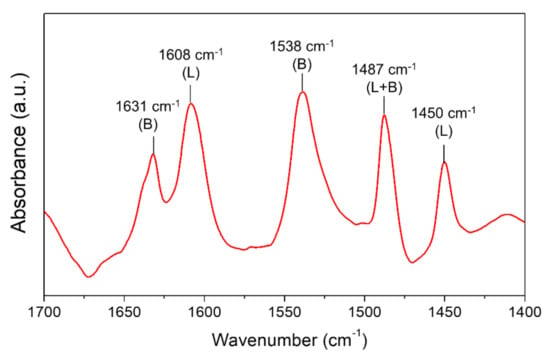
Figure 3.
FT-IR spectra of pyridine absorbed on ReOx NPs after pyridine absorption and desorption of physisorbed pyridine. L: Lewis bonded pyridine. B: Brønsted bonded pyridine.
The time profile of the conversion of SA over ReOx/C is shown in Figure 1b. In a half-hour at 250 °C, all SA were converted to SA-ME/DE or GBL. Over the course of the reaction up to 6 h, SA-ME disappeared, yielding more SA-DE, GBL, and THF. THF can be made by the further CTH of the synthesized GBL, evident by the formation of THF from GBL as a starting material (Figure 1e). The reaction between 6 and 12 h indicates that SA-DE was also transformed to GBL while the CTH rate of SA-DE seems slower compared to the rate of transformation of SA. This agrees with Figure 1c that shows that GBL was slowly produced from SA-DE as a starting material. To investigate if the slow conversion of SA-DE was not due to catalyst deactivation, the used catalyst after 6 h reaction was replaced with a new catalyst, followed by 6 h further reaction (6 h + 6 h in Figure 1b). The product composition of the 6 h + 6 h reaction was similar to the 12 h reaction without interruption, excluding the possibility of deactivation. A higher temperature was required to facilitate the conversion of SA-DE to GBL. At the elevated temperature of 270 °C, more GBL (50% yield) was produced from SA in 1 h. The 3 h reaction yielded 60% GBL and 9% THF, with 22% of remaining SA-DE. Most of the SA-DE were converted in 6 h and CTH of GBL also proceeded, making more THF (28% yield) and other C4 compounds, including 1,4-butanediol (1,4-BDO) and butyric acid (BTA). Unsupported ReOx NPs successfully catalyzed simultaneous CTH and dehydration of SA, yielding GBL and THF.
Isopropanol offered hydrogen for CTH, producing acetone as a co-product. The amount of the produced acetone after 250 °C/1 h reaction (5.5 mmol) was much higher than the theoretically required amount of H2 (0.9 mmol) to produce 0.43 mmol of GBL and 0.02 mmol of THF. This indicates independent dehydrogenation of isopropanol over ReOx NPs and the possibility of the involvement of the produced H2 from isopropanol in the conversion of SA to GBL through direct dehydrogenation. To test this possibility, direct hydrogenation of SA was tested in dioxane with 15 bar of H2, which is approximately 39 mmol H2, calculated using the ideal gas law (Figure 1f). After 1 h at 250 °C, the amount of the synthesized GBL (16% yield) was much lower than that in the reaction with isopropanol as a reductant (43% yield). This demonstrates that the major pathway of the formation of GBL from SA is CTH reaction. In the presence of both isopropanol and molecular H2, a higher amount of GBL (55% yield) was gained through both CTH and direct hydrogenation. The reaction under H2 produced more unidentified products and showed lower mass recovery than the reaction in isopropanol without H2 gas. This might be because unexpected side reactions occurred under H2 atmosphere.
3.2. Conversion of Maleic Acid (MA)
MA is another versatile 1,4-dicarboxylic acid because it includes an unsaturated bond. Figure 4 shows the conversion of MA with ReOx NPs in isopropanol at different reaction temperatures. At all tested temperatures from 170 °C to 270 °C, esterification over ReOx NPs converted MA to maleic acid monoisopropyl ester (MA-ME) or maleic acid diisopropyl ester (MA-DE). Under the reaction conditions, MA and its esters were partially isomerized to fumaric acid (FA) and its esters (FA-ME and FA-DE). The resulting double bond compounds including MA, FA, and their esters were hydrogenated to SA, SA-ME, or SA-DE through CTH reaction with isopropanol as a hydrogen donor. At 170 °C, 13% yield of SA and its esters was obtained after 3 h reaction. At the elevated temperature of 250 °C, SA, SA-ME, and SA-DE are the major products, with a combined yield of 69% in 3 h. Similarly, CTH of MA to SA with noble metal catalysts and formic acid as a reductant was recently reported [33]. While CTH of C-C double bonds occurred without a catalyst, ReOx NPs facilitated the CTH reaction and achieved a higher yield of C-C single bond compounds. Further conversion of SA and SA-ME/DE to GBL, as discussed in the previous section, produced GBL with yields of 15% and 29% at 250 °C and 270 °C, respectively. Moreover, 43% yield of GBL and 5% yield of THF were gained in the 6 h reaction at 270 °C.
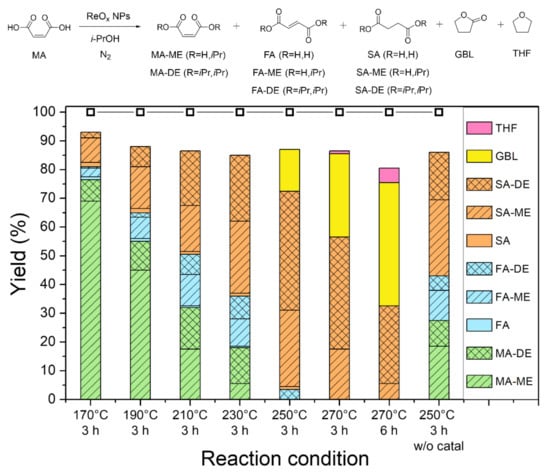
Figure 4.
Conversion of maleic acid (MA) over ReOx NPs in isopropanol: Reaction conditions: batch reaction, ReOx NPs (10 mg, 4 mol% Re relative to substrate), MA (1 mmol), i-PrOH (10 mL), and N2 (15 bar). Conversion and yield are calculated by 1H NMR and GC-FID.
3.3. Conversion of Tartaric Acid (TA)
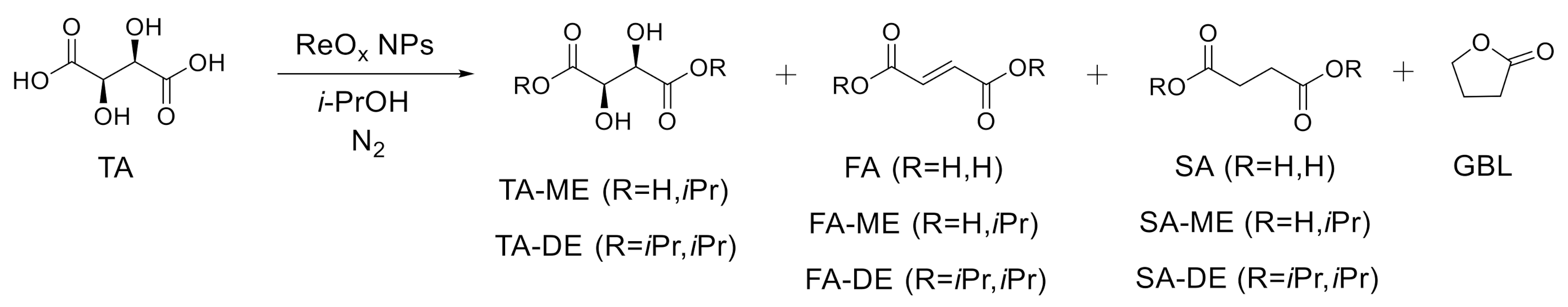
Unsupported ReOx NPs and isopropanol directly convert SA and MA into GBL and THF. We previously demonstrated that the ReOx NPs efficiently catalyzed the DODH reaction of glycerol with secondary alcohol as a reductant at 170 °C [22]. The possibility that DODH, combined with CTH and dehydration/dealcoholization, converts TA to GBL or THF in one step was investigated. First, transformation of TA to FA and its esters occurred on ReOx through DODH and esterification at 170 and 210 °C (entry 1 and 2 in Table 1). The presence of TA-ME/DE in the products indicates that esterification of TA can take place before DODH occurs. However, DODH of TA can proceed first, making FA (4% yield in entry 1). MA and its esters were not detected, indicating that trans-alkene is the more favored DODH product from (2R,3R)-tartaric acid. It was reported that MTO-catalyzed DODH converted (R,R)-1,2-diphenyl-1,2-ethanediol to trans-stilbene while cis-stilbene was formed from (R,S)-1,2-diphenyl-1,2-ethanediol [34]. The high selectivity of FA and its ester from TA agrees with previous reports. Rhenium-catalyzed DODH produced high yields (> 85%) of FA or its ester from TA or its ester in the presence of various reductants including alcohol [19,35,36], metal [37], H2 [38], and triphenylphosphine [39]. Similarly, DODH has been employed to convert C6 sugar acids and their derivatives to valuable bio-based products [35,40,41,42,43].

Table 1.
Conversion of tartaric acid over ReOx NPs 1.
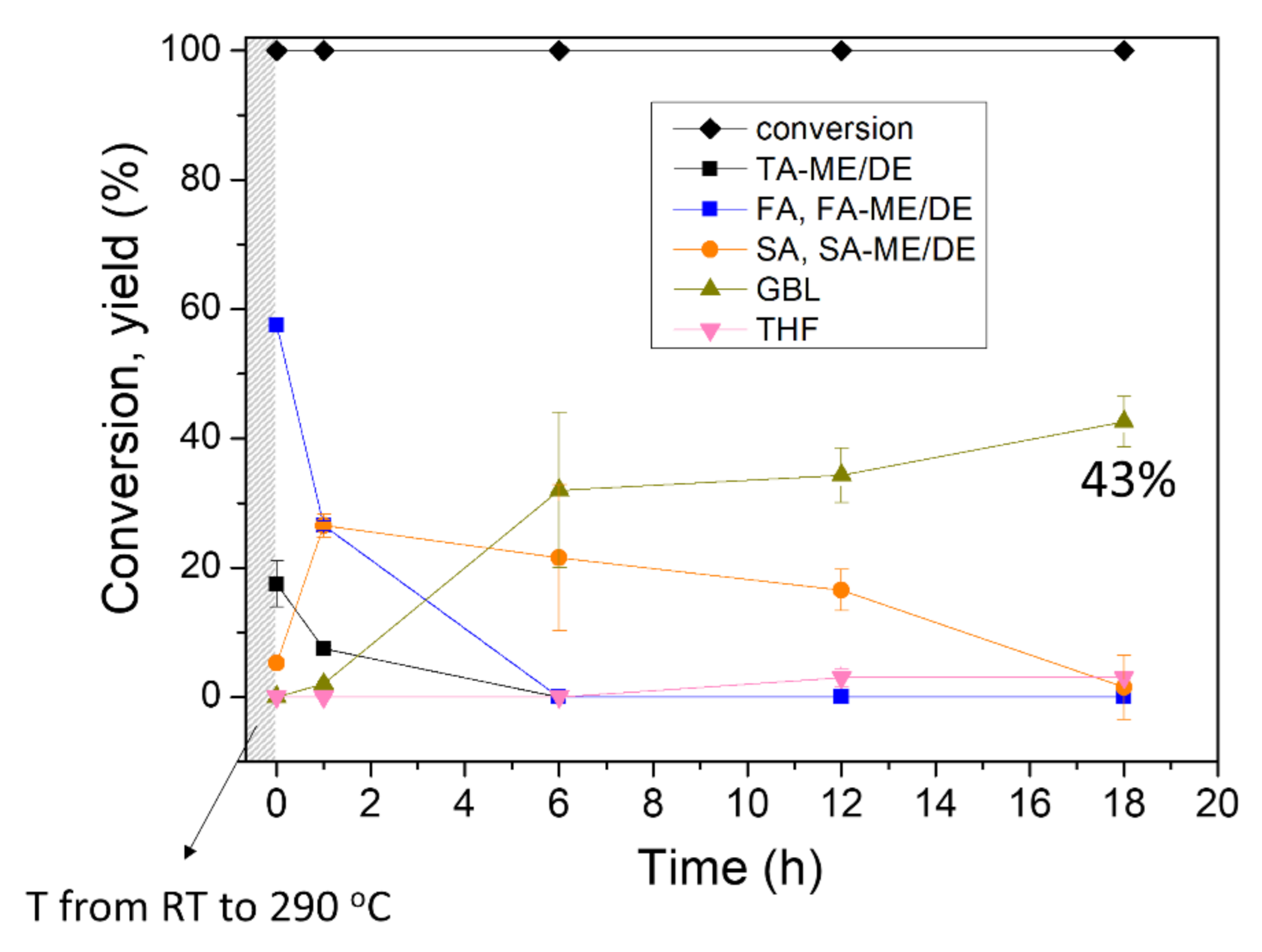
At the reaction temperature above 250 °C, CTH reaction of C-C double bonds also proceeded as well as DODH, making SA and its esters (entry 3–5 in Table 1). In addition to the saturated products, GBL was also formed by simultaneous CTH and dehydration/dealcoholization, with a yield of 18% and 20% at 250 and 270 °C, respectively. In order to achieve a higher yield of GBL, the reaction was performed at 290 °C. After 12 h reaction, 34% yield of GBL was produced from tartaric acid and 18 h reaction produced more GBL, with a yield of 43%. Over the course of the reaction from tartaric acid, only a small amount of THF was formed (entry 5 and 6). A control experiment without catalyst showed small conversion, demonstrating that the conversion of TA was indeed catalyzed by ReOx NPs (entry 7). The time profile of the TA conversion over ReOx NPs at 290 °C is shown in Figure 5. Before the temperature reached 290 °C, all TA was already converted to TA-ME/DE or FA and its esters. The resulting FA and its esters were transformed to SA and its esters in 6 h. Over the course of 18 h, GBL was slowly produced from SA and its esters and 43% yield of GBL was attained. The high reaction temperature of 290 °C led to many side reactions and byproducts, including BTA and isopropyl branched GBL and succinate.
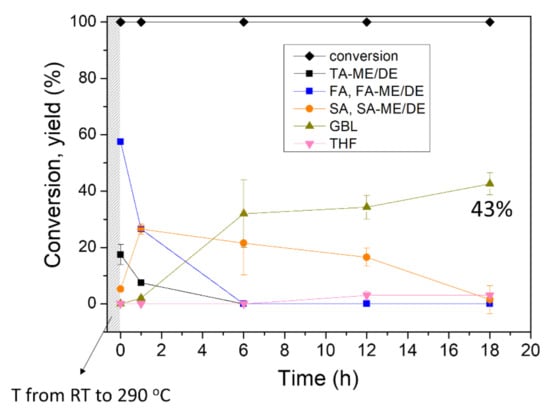
Figure 5.
Reaction profile of the conversion of tartaric acid (TA) at 290 °C. Reaction conditions: batch reaction, ReOx NPs (10 mg, 4 mol% Re relative to substrate), TA (1 mmol), i-PrOH (10 mL), and N2 (15 bar). Conversion and yield are calculated by 1H NMR and GC-FID.
3.4. Reaction Pathway
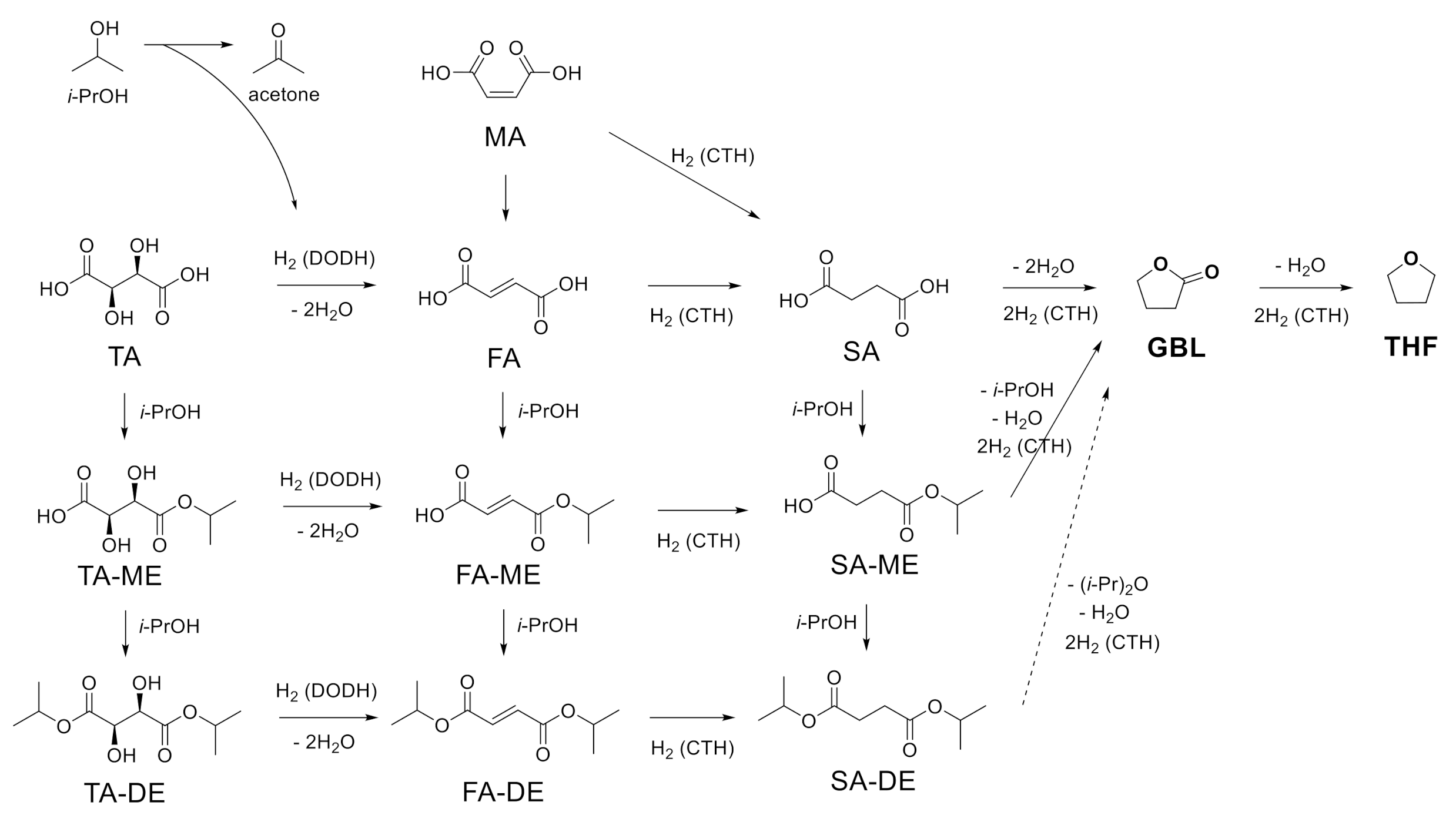
Based on the results of the conversion of SA, MA, and TA with unsupported ReOx NPs, the possible reaction scheme is illustrated in Scheme 2. In isopropanol, the carboxylic acid group in TA is esterified, making TA-ME or TA-DE. TA and its esters are converted to FA and its esters through DODH, removing hydroxyl groups and making a trans-double bond. Hydrogen is provided from isopropanol for DODH reaction. This step produces acetone as a byproduct. CTH reaction over ReOx in isopropanol saturates the resulting double bonds in FA, FA-ME, and FA-DE to SA, SA-ME, and SA-DE, respectively. SA undergoes intramolecular dehydration and CTH, affording GBL. Dealcoholization and CTH also produce GBL from SA-ME/DE. Further CTH of GBL yields THF. MA, another starting material, is isomerized to FA. Both MA and FA can be hydrogenated to SA and the resulting SA and SA-ME/DE follow the same pathway, giving GBL and THF.
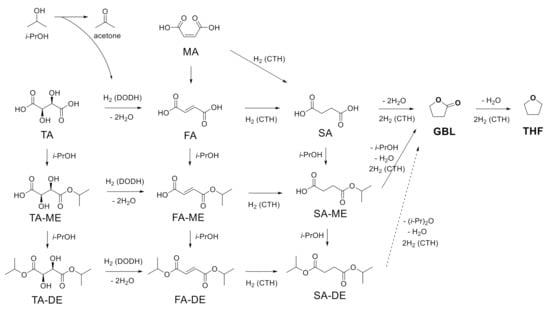
Scheme 2.
Possible reaction pathway for the conversion of succinic acid (SA), maleic acid (MA), and tartaric acid (TA).
4. Conclusions
In this study, valuable C4 chemicals including GBL and THF were synthesized from SA, MA, and TA through ReOx-catalyzed DODH, CTH, and cyclization reactions. Isopropanol provided hydrogen for both DODH and CTH reactions without needing the addition of H2 gas. GBL was produced from SA at a temperature above 200 °C with a possible intermediate of SAN. Intramolecular dehydration of SA or dealcoholization of SA-ME/DE was catalyzed by acidic sites on ReOx, evident by the pyridine probe FT-IR result. CTH-assisted C-O bond cleavage proceeded with ReOx NPs, producing GBL from SAN. Further CTH of GBL yielded THF. The highest yield of GBL from SA was 60%, with 9% yield of THF at 270 °C. SA and its esters can be prepared from MA by CTH of C-C double bond and from TA by DODH-CTH of a diol. Under the optimized conditions, ReOx NPs afforded 43% yield of GBL from TA with isopropanol as a hydrogen donor in one step.
Author Contributions
Designed and conducted experiments, analyzed data, and wrote manuscript, J.H.J.; designed experiments, interpreted data/results, and edited manuscript, M.M.A.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US Department of Energy, Office of Science, Basic Energy Science, award no. DE-SC0019161.
Acknowledgments
The authors are grateful for all the financial support for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; No. DOE/GO-102004-1992; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Delhomme, C.; Weuster-Botz, D.; Kühn, F.E. Succinic acid from renewable resources as a C4 building-block chemical—A review of the catalytic possibilities in aqueous media. Green Chem. 2009, 11, 13–26. [Google Scholar] [CrossRef]
- Di, X.; Shao, Z.; Li, C.; Li, W.; Liang, C. Hydrogenation of succinic acid over supported rhenium catalysts prepared by the microwave-assisted thermolytic method. Catal. Sci. Technol. 2015, 5, 2441–2448. [Google Scholar] [CrossRef]
- Shao, Z.; Li, C.; Di, X.; Xiao, Z.; Liang, C. Aqueous-Phase Hydrogenation of Succinic Acid to γ-Butyrolactone and Tetrahydrofuran over Pd/C, Re/C, and Pd–Re/C Catalysts. Ind. Eng. Chem. Res. 2014, 53, 9638–9645. [Google Scholar] [CrossRef]
- Patankar, S.C.; Sharma, A.G.; Yadav, G.D. Biobased process intensification in selective synthesis of γ-butyrolactone from succinic acid via synergistic palladium–copper bimetallic catalyst supported on alumina xerogel. Clean Technol. Environ. Policy 2018, 20, 683–693. [Google Scholar] [CrossRef]
- Global Gamma-Butyrolactone Market Report 2019; Garner Insights: Pune, India, 2019.
- Global Tetrahydrofuran Market by Technology, by Application, by Geographic Scope and Forecast to 2026; Verified Market Research: Pune, India, 2019.
- Nghiem, N.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Cao, D.; Cai, W.; Tao, W.; Zhang, S.; Wang, D.; Huang, D. Lactic Acid Production from Glucose Over a Novel Nb2O5 Nanorod Catalyst. Catal. Lett. 2017, 147, 926–933. [Google Scholar] [CrossRef]
- Tachibana, Y.; Masuda, T.; Funabashi, M.; Kunioka, M. Chemical Synthesis of Fully Biomass-Based Poly(butylene succinate) from Inedible-Biomass-Based Furfural and Evaluation of Its Biomass Carbon Ratio. Biomacromolecules 2010, 11, 2760–2765. [Google Scholar] [CrossRef]
- Choudhary, H.; Nishimura, S.; Ebitani, K. Metal-free oxidative synthesis of succinic acid from biomass-derived furan compounds using a solid acid catalyst with hydrogen peroxide. Appl. Catal. A 2013, 458, 55–62. [Google Scholar] [CrossRef]
- Zhu, W.; Tao, F.; Chen, S.; Li, M.; Yang, Y.; Lv, G. Efficient Oxidative Transformation of Furfural into Succinic Acid over Acidic Metal-Free Graphene Oxide. ACS Sustain. Chem. Eng. 2019, 7, 296–305. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased Plasticizers from Tartaric Acid, an Abundantly Available, Renewable Material. Ind. Eng. Chem. Res. 2018, 57, 15234–15242. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased flame retardants from tartaric acid and derivatives. Poly. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Fu, J.; Vasiliadou, E.S.; Goulas, K.A.; Saha, B.; Vlachos, D.G. Selective hydrodeoxygenation of tartaric acid to succinic acid. Catal. Sci. Technol. 2017, 7, 4944–4954. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y. Highly Selective Deoxydehydration of Tartaric Acid over Supported and Unsupported Rhenium Catalysts with Modified Acidities. ChemSusChem 2016, 9, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Tshibalonza, N.N.; Monbaliu, J.-C.M. The deoxydehydration (DODH) reaction: A versatile technology for accessing olefins from bio-based polyols. Green Chem. 2020, 22, 4801–4848. [Google Scholar] [CrossRef]
- Yi, J.; Liu, S.; Abu-Omar, M.M. Rhenium-Catalyzed Transfer Hydrogenation and Deoxygenation of Biomass-Derived Polyols to Small and Useful Organics. ChemSusChem 2012, 5, 1401–1404. [Google Scholar] [CrossRef]
- Jang, J.H.; Sohn, H.; Camacho-Bunquin, J.; Yang, D.; Park, C.Y.; Delferro, M.; Abu-Omar, M.M. Deoxydehydration of Biomass-Derived Polyols with a Reusable Unsupported Rhenium Nanoparticles Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 11438–11447. [Google Scholar] [CrossRef]
- Hong, U.G.; Hwang, S.; Seo, J.G.; Lee, J.; Song, I.K. Hydrogenation of succinic acid to γ-butyrolactone (GBL) over palladium catalyst supported on alumina xerogel: Effect of acid density of the catalyst. J. Ind. Eng. Chem. 2011, 17, 316–320. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Leng, X.; Zhu, H.; Liu, G.; Huang, Z. Transfer Hydrogenation of Alkenes Using Ethanol Catalyzed by a NCP Pincer Iridium Complex: Scope and Mechanism. J. Am. Chem. Soc. 2018, 140, 4417–4429. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Riente, P.; Rodríguez-Reinoso, F.; Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Yus, M. Platinum nanoparticles supported on titania as an efficient hydrogen-transfer catalyst. J. Catal. 2008, 260, 113–118. [Google Scholar] [CrossRef]
- Johnstone, R.A.W.; Wilby, A.H.; Entwistle, I.D. Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds. Chem. Rev. 1985, 85, 129–170. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from Fructose Using Formic Acid as a Reagent. Angew. Chem. Int. Ed. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.-B.; Guo, Q.-X.; Fu, Y. RANEY® Ni catalyzed transfer hydrogenation of levulinate esters to γ-valerolactone at room temperature. Chem. Comm. 2013, 49, 5328. [Google Scholar] [CrossRef]
- Kim, M.; Ha, J.-M.; Lee, K.-Y.; Jae, J. Catalytic transfer hydrogenation/hydrogenolysis of guaiacol to cyclohexane over bimetallic RuRe/C catalysts. Catal. Comm. 2016, 86, 113–118. [Google Scholar] [CrossRef]
- Korstanje, T.J.; de Waard, E.F.; Jastrzebski, J.T.B.H.; Klein Gebbink, R.J.M. Rhenium-Catalyzed Dehydration of Nonbenzylic and Terpene Alcohols to Olefins. ACS Catal. 2012, 2, 2173–2181. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Pan, X.; Ji, J.; Ren, Y.; Wang, H.; Ji, N.; Liu, Q.; Li, C. One-Pot Conversion of Lignin into Naphthenes Catalyzed by a Heterogeneous Rhenium Oxide-Modified Iridium Compound. ChemSusChem 2020, 13, 4409–4419. [Google Scholar] [CrossRef]
- López Granados, M.; Moreno, J.; Alba-Rubio, A.C.; Iglesias, J.; Martín Alonso, D.; Mariscal, R. Catalytic transfer hydrogenation of maleic acid with stoichiometric amounts of formic acid in aqueous phase: Paving the way for more sustainable succinic acid production. Green Chem. 2020, 22, 1859–1872. [Google Scholar] [CrossRef]
- Liu, S.; Senocak, A.; Smeltz, J.L.; Yang, L.; Wegenhart, B.; Yi, J.; Kenttämaa, H.I.; Ison, E.A.; Abu-Omar, M.M. Mechanism of MTO-Catalyzed Deoxydehydration of Diols to Alkenes Using Sacrificial Alcohols. Organometallics 2013, 32, 3210–3219. [Google Scholar] [CrossRef]
- Shiramizu, M.; Toste, F.D. Expanding the Scope of Biomass-Derived Chemicals through Tandem Reactions Based on Oxorhenium-Catalyzed Deoxydehydration. Angew. Chem. Int. Ed. 2013, 52, 12905–12909. [Google Scholar] [CrossRef] [PubMed]
- Boucher-Jacobs, C.; Nicholas, K.M. Catalytic Deoxydehydration of Glycols with Alcohol Reductants. ChemSusChem 2013, 6, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Michael McClain, J.; Nicholas, K.M. Elemental Reductants for the Deoxydehydration of Glycols. ACS Catal. 2014, 4, 2109–2112. [Google Scholar] [CrossRef]
- Denning, A.L.; Dang, H.; Liu, Z.; Nicholas, K.M.; Jentoft, F.C. Deoxydehydration of Glycols Catalyzed by Carbon-Supported Perrhenate. ChemCatChem 2013, 5, 3567–3570. [Google Scholar] [CrossRef]
- Sharkey, B.E.; Denning, A.L.; Jentoft, F.C.; Gangadhara, R.; Gopaladasu, T.V.; Nicholas, K.M. New solid oxo-rhenium and oxo-molybdenum catalysts for the deoxydehydration of glycols to olefins. Catal. Today 2018, 310, 86–93. [Google Scholar] [CrossRef]
- Larson, R.T.; Samant, A.; Chen, J.; Lee, W.; Bohn, M.A.; Ohlmann, D.M.; Zuend, S.J.; Toste, F.D. Hydrogen Gas-Mediated Deoxydehydration/Hydrogenation of Sugar Acids: Catalytic Conversion of Glucarates to Adipates. J. Am. Chem. Soc. 2017, 139, 14001–14004. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Lu, T.; Yi, G.; Su, H.; Zhang, Y. Highly Efficient Chemical Process to Convert Mucic Acid into Adipic Acid and DFT Studies of the Mechanism of the Rhenium-Catalyzed Deoxydehydration. Angew. Chem. Int. Ed. 2014, 53, 4200–4204. [Google Scholar] [CrossRef]
- Lin, J.; Song, H.; Shen, X.; Wang, B.; Xie, S.; Deng, W.; Wu, D.; Zhang, Q.; Wang, Y. Zirconia-supported rhenium oxide as an efficient catalyst for the synthesis of biomass-based adipic acid ester. Chem. Commun. 2019, 55, 11017–11020. [Google Scholar] [CrossRef]
- Hočevar, B.; Prašnikar, A.; Huš, M.; Grilc, M.; Likozar, B. H2-Free Re-Based Catalytic Dehydroxylation of Aldaric Acid to Muconic and Adipic Acid Esters. Angew. Chem. Int. Ed. 2020, 59, 2–12. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).