The Influence of Freezing on the Course of Carbonization and Pyrolysis of a Bituminous High-Volatile Coal
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
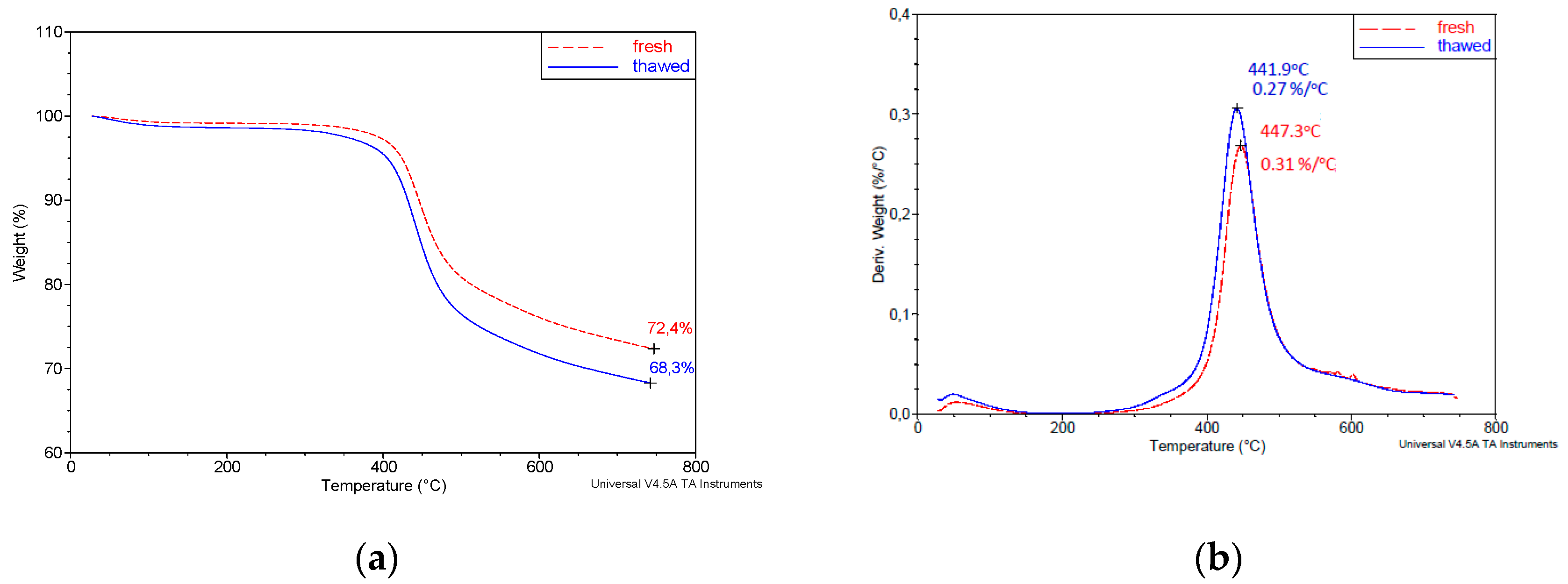
3.1. A Contrastive Analysis of the Course of Pyrolysis Process of Fresh and Thawed Samples
3.2. The Influence of Freezing on the Composition of Volatile Products of Destruction of Fresh and Thawed Coals
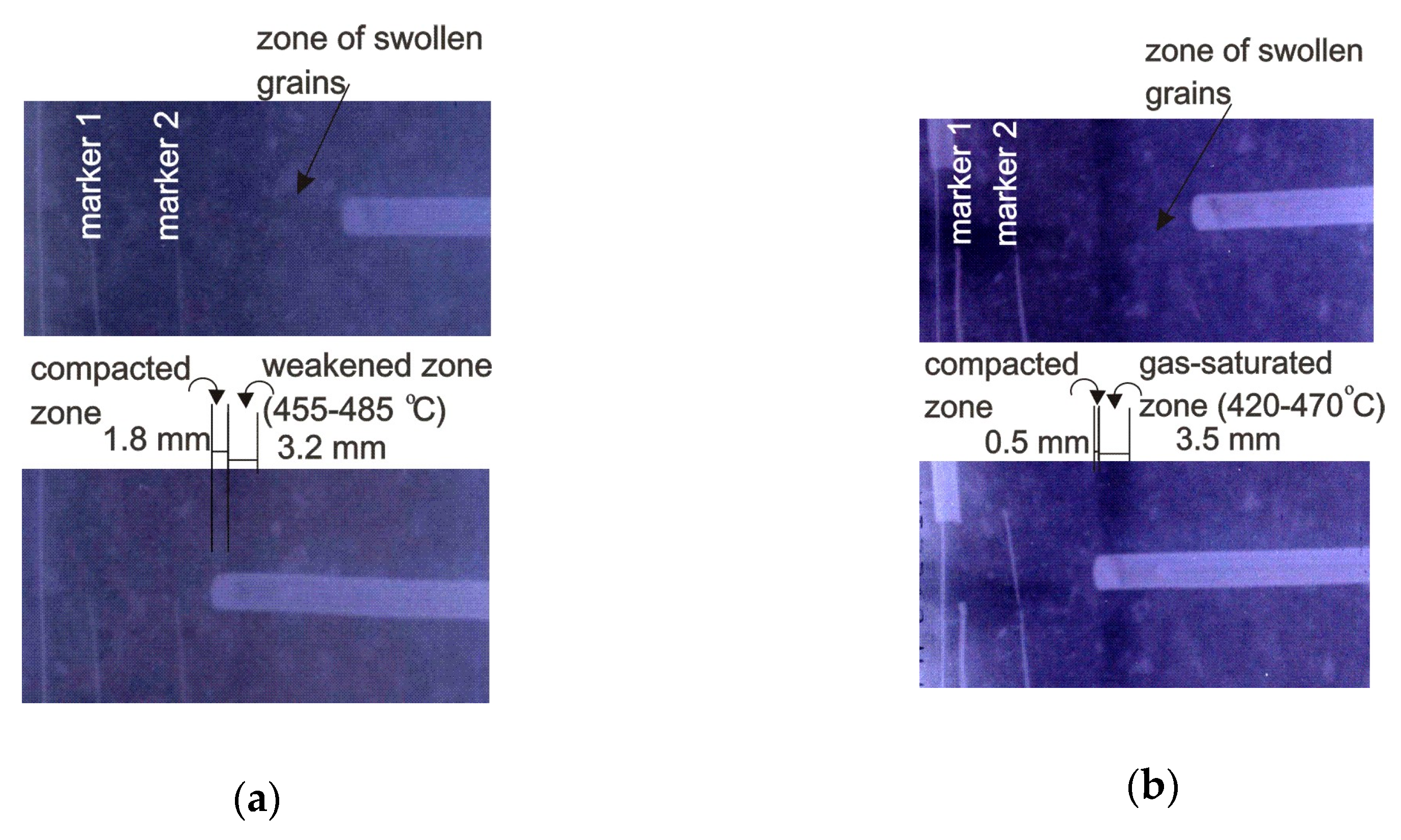
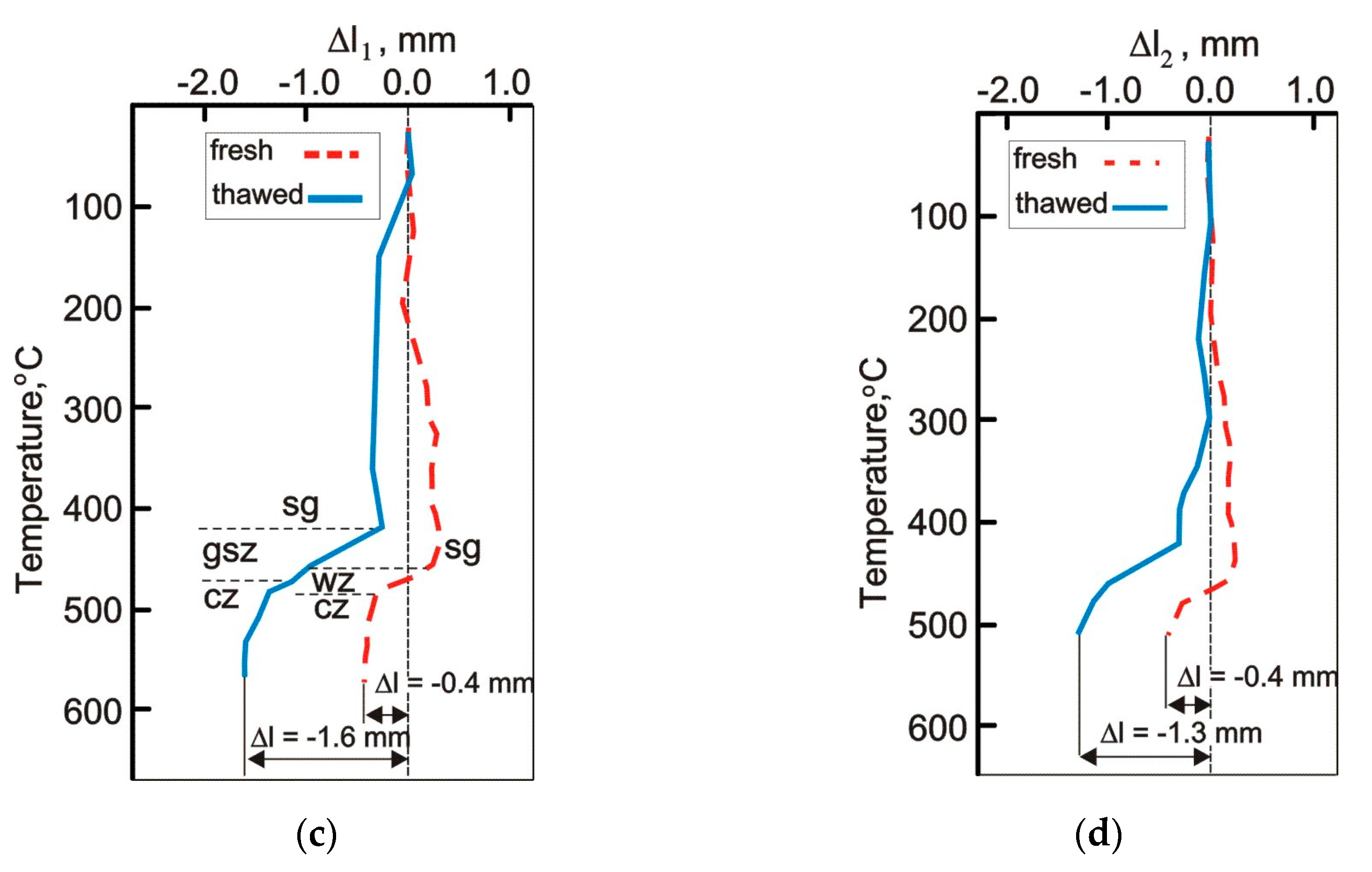
3.3. The Influence of Freezing on the Course of Carbonization of Studied Coal
3.4. The Yield of Material Soluble in a Methanol–Chloroform Mixture Obtained from the Material of the Zones of Plastic Layer along with Its Characteristics
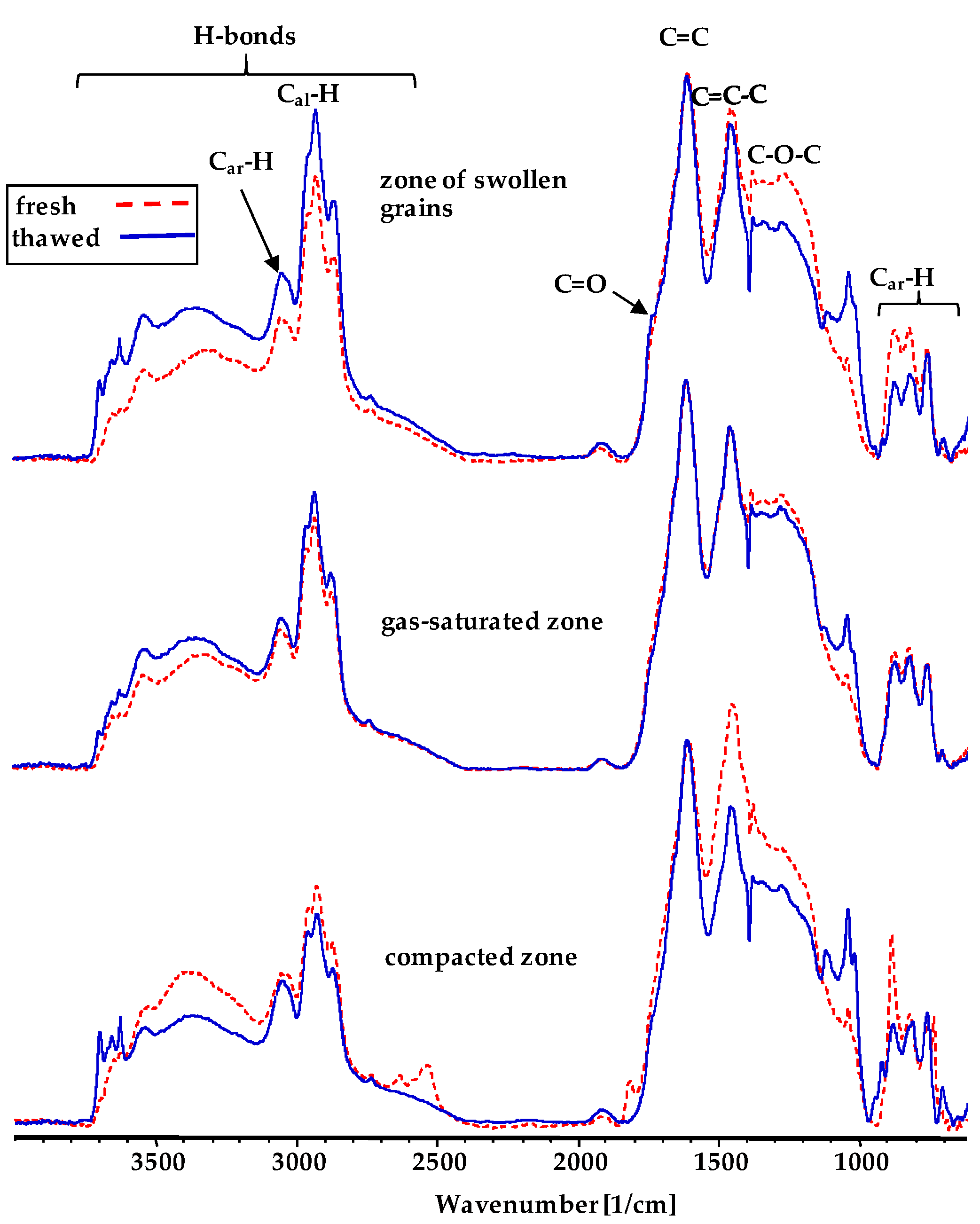
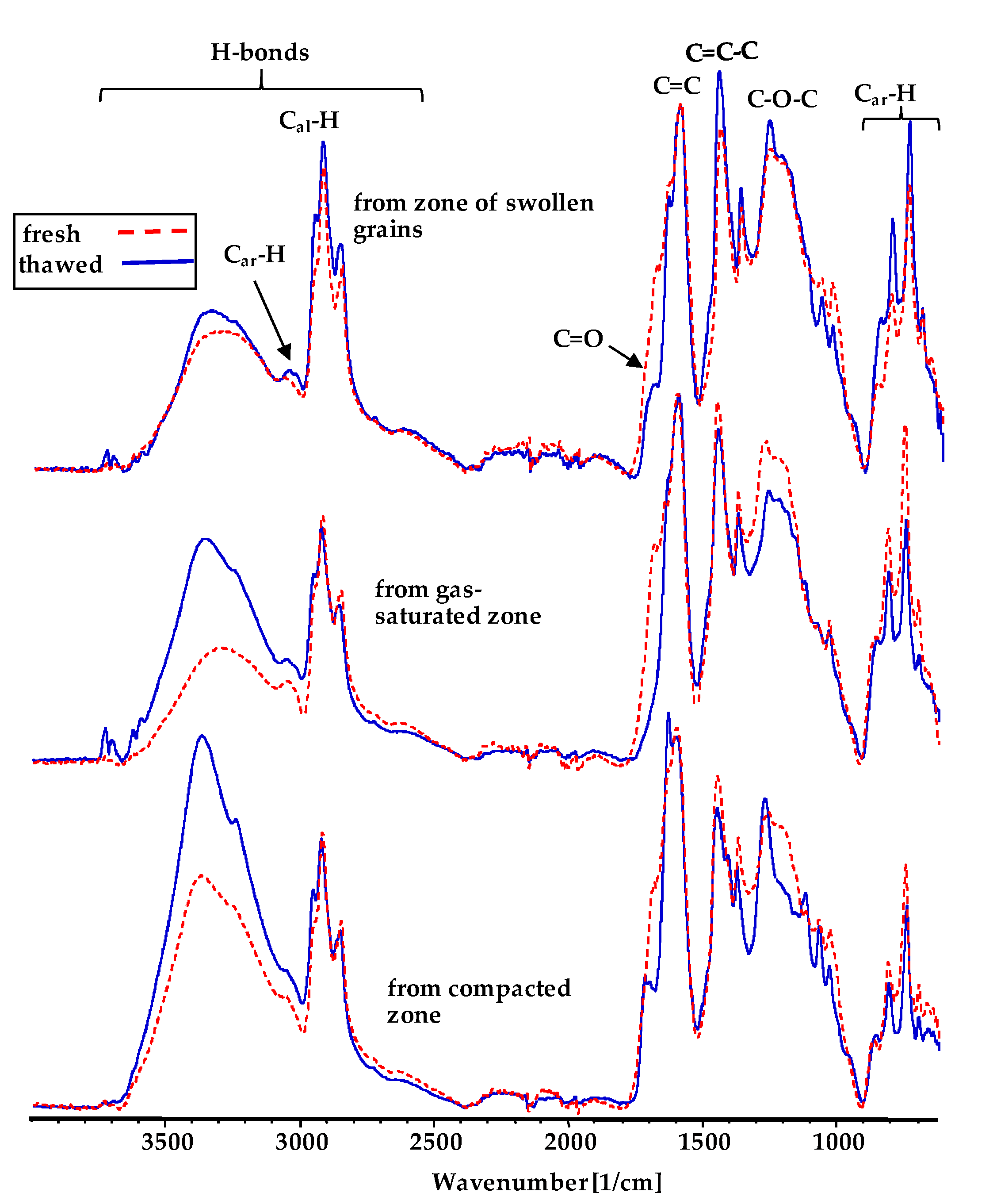
3.5. The Analysis of DRIFT Spectra of Plasticized Coals
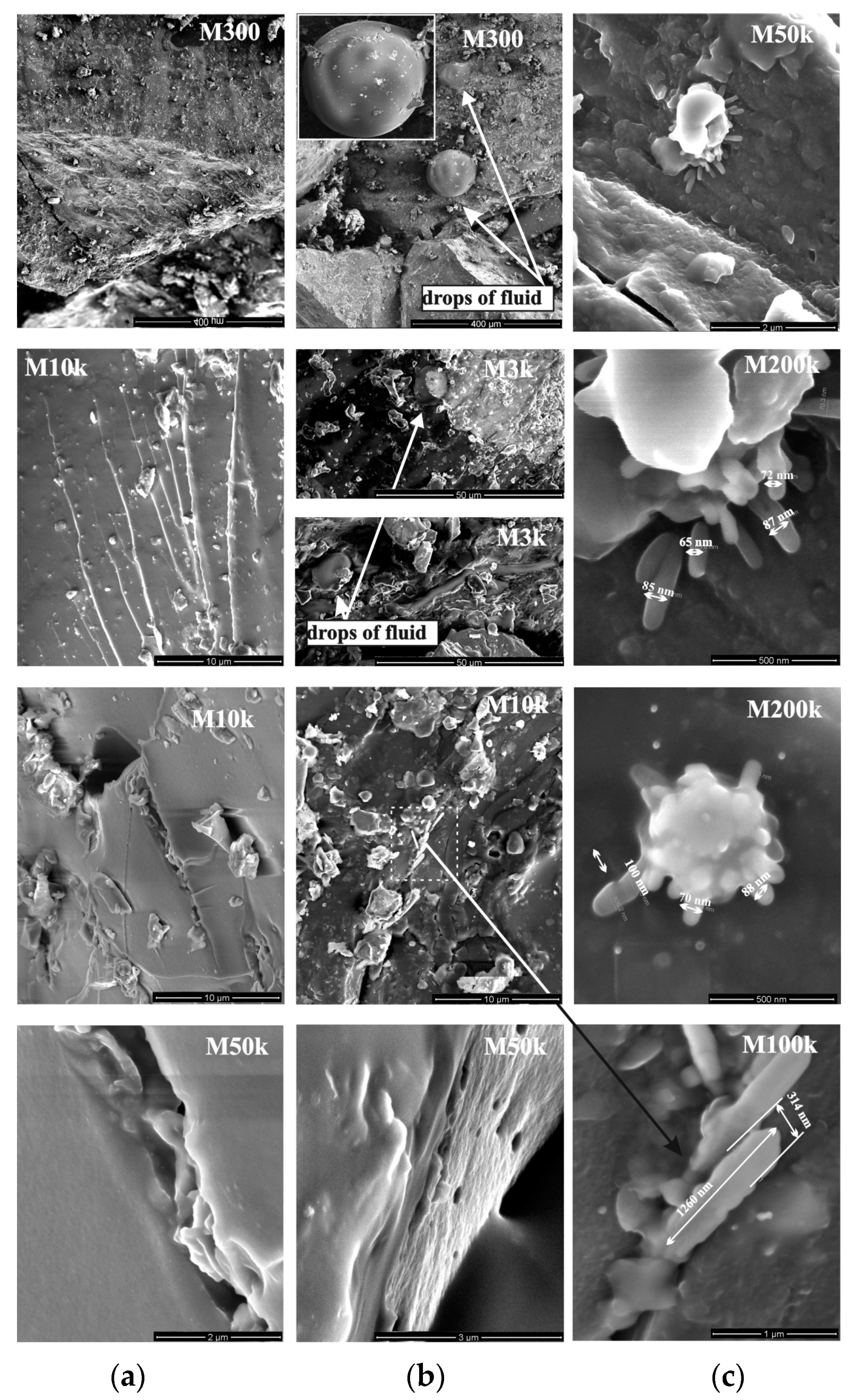
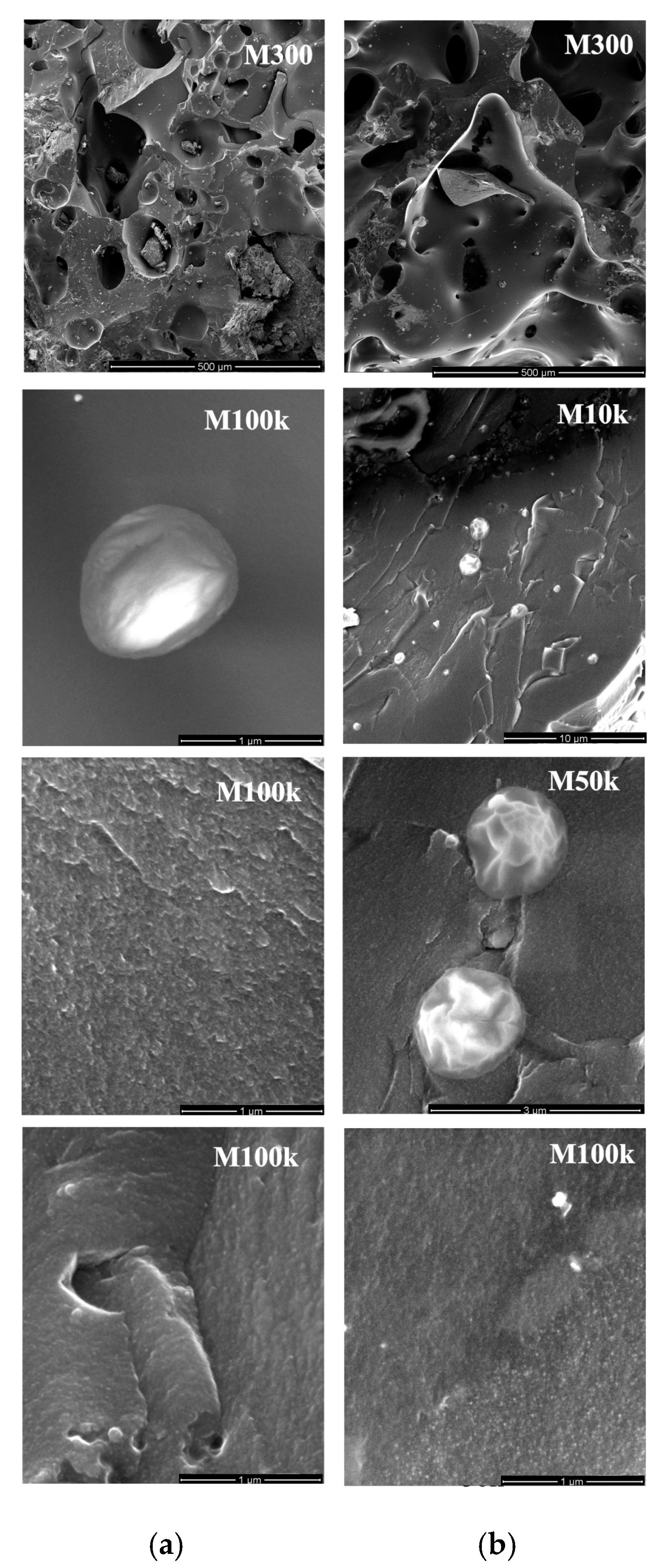
3.6. The SEM Images of Plasticized Samples from the Charges of Fresh and Thawed Coals
3.7. The Proposed Mechanism of Changes Taking Place during Carbonization of Thawed Coals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cimadevilla, J.L.G.; Alvarez, R.; Pis, J.J. Effect of coal weathering on technological properties of cokes produced at different scales. Fuel Process. Technol. 2005, 86, 809–830. [Google Scholar] [CrossRef]
- Wu, M.M.; Robbins, G.A.; Winschel, R.A.; Burke, F.P. Low-temperature coal weathering: Its chemical nature and effects on coal properties. Energy Fuels 1988, 2, 150–157. [Google Scholar] [CrossRef]
- Baris, K.; Kizgut, S.; Didari, V. Low-temperature oxidation of some Turkish coals. Fuel 2012, 93, 423–432. [Google Scholar] [CrossRef]
- Miroshnichenko, D.V.; Desna, N.A.; Kaftan, Y.S. Oxidation of coal in industrial conditions. 2. Modification of the plastic and viscous properties in oxidation. Coke Chem. 2014, 57, 375–380. [Google Scholar] [CrossRef]
- Ingram, G.R.; Rimstidt, J.D. Natural weathering of coal. Fuel 1984, 63, 292–296. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Zhang, T.; You, Z. Experimental study on mechanical characteristics and fracture patterns of unfrozen/freezing saturated coal and sandstone. Materials 2019, 12, 992. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Xie, X. An experimental study of the effect of injecting water and freezing on mechanical properties of outburst-prone coal seam. Procedia Earth Plan. Sci. 2009, 1, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J.; Tang, Z.; Yu., G. Failure mechanizm of coal after cryogenic freezing with cyclic liquid nitrtogen and its influences on coalbed methane exploitation. Energy Fuels 2016, 30, 8567–8578. [Google Scholar] [CrossRef]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J. Factors controlling the mechanical properties degradation and permeability of coal subjected to liquid nitrogen freeze-thaw. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Li, S.; Zhai, C.; Lin, H.; Zhao, P.; Shi, Y.; Bai, Y. Changes in the pore structure of lignite after repeated cycles of liquid nitrogen freezing as determined by nitrogen adsorption and mercury intrusion. Fuel 2020, 267, 117214. [Google Scholar] [CrossRef]
- Cai, C.; Gao, F.; Yang, Y. The effect of liquid nitrogen cooling on coal cracking and mechanical properties. Energy Explor. Exploit. 2018, 36, 1609–1628. [Google Scholar] [CrossRef] [Green Version]
- Lynch, L.J.; Webster, D.S. Effect of thermal treatment on the interaction of brown coal and water: A nuclear magnetic resonance study. Fuel 1982, 61, 271–275. [Google Scholar] [CrossRef]
- Norinaga, K.; Hayashi, J.; Kudo, N.; Chiba, T. Evaluation effect of predrying on the porous structure of water-swollen coal based on the freezing property of pore condensed water. Energy Fuels 1999, 13, 1058–1066. [Google Scholar] [CrossRef]
- Nwaka, D.; Tahmasebi, A.; Tian, L.; Yu, J. The effects of pore structure on the behavior of water in lignite coal and activated carbon. J. Colloid Interface Sci. 2016, 477, 138–147. [Google Scholar] [CrossRef]
- Kleinberg, R.L.; Griffin, D.D. NMR measurements of permafrost: Unfrozen water assay, pore-scale distribution of ice, and hydraulic permeability of sediments. Cold Reg. Sci. Technol. 2005, 42, 63–77. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Li, B.; Wen, Z. Non-uniformity of coal damage caused by liquid nitrogen freeze-thaw. J. Nat. Gas Sci. Eng. 2019, 69, 102946. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, C.; Liu, S.; Qin, L.; Dong, R. Investigation of temperature effects from LCO2 with different cycle parameters on the coal pore variation based on infrared thermal imagery and low-field nuclear magnetic resonance. Fuel 2018, 215, 528–540. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, C.; Liu, S.; Qin, L.; Wu, S. Pore variation of three different metamorphic coals by multiple freezing-thawing cycles of liquid CO2 injection for coalbed methane recovery. Fuel 2017, 208, 41–51. [Google Scholar] [CrossRef]
- Berlin, A.A. Mechanochemical transformation and synthesis of polymers. Rubber Chem. Technol. 1961, 34, 215–227. [Google Scholar] [CrossRef]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J.; Yu, G.; Sun, Y. Changes in the petrophysical properties of coal subjected to liquid nitrogen freeze-thaw—A nuclear magnetic resonance investigation. Fuel 2017, 194, 102–114. [Google Scholar] [CrossRef]
- Zhai, C.; Qin, L.; Liu, S.; Xu, J.; Tang, Z.; Wu, S. Pore structure in coal: Pore evolution after cryogenic freezing with cycling liquid nitrogen injection and its implication on coalbed methane extraction. Energy Fuels 2016, 30, 6009–6020. [Google Scholar] [CrossRef]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J.; Wu, S.; Dong, R. Fractal dimensions of low rank coal subjected to liquid nitrogen freeze-thaw based on nuclear magnetic resonance applied for coalbed methane recovery. Powder Technol. 2018, 325, 11–20. [Google Scholar] [CrossRef]
- Epshtein, S.A.; Shkuratnik, V.L.; Kossovich, E.L.; Agarkov, K.V.; Nesterova, V.G.; Gavrilova, D.I. Effects of cyclic freezing and thawing of coals at their behavior at low- and high-temperature oxidation. Fuel 2020, 267, 117191. [Google Scholar] [CrossRef]
- Zubkova, V.; Strojwas, A.; Kaniewski, M.; Ziomer, S.; Indyka, P. Some aspects of influence of the composition of volatile products and extracted material on grain swelling processes and volume changes of commercial coals of different rank. Fuel 2019, 243, 554–568. [Google Scholar] [CrossRef]
- Zubkova, V. Some peculiarities of formation mechanism of metallurgical coke from Polish coals. Fuel 2004, 83, 1205–1214. [Google Scholar] [CrossRef]
- Kidena, K.; Murata, S.; Nomura, M. Studies on the chemical structural change during carbonization process. Energy Fuels 1996, 10, 672–678. [Google Scholar] [CrossRef]
- Stanger, R.; Borrowdale, J.; Smith, N.; Xei, W.; Tran, Q.A.; Lucas, J.; Wall, T. Changes in solvent-extracted matter for heated coal during metaplast formation using high-range mass spectrometry. Energy Fuels 2015, 29, 7101–7113. [Google Scholar] [CrossRef]
- Shui, H.; Zheng, M.; Wang, Z.; Li, X. Effect of coal soluble constituents on caking property of coal. Fuel 2007, 86, 1396–1401. [Google Scholar] [CrossRef]
- Rao, C.N.R. Ultra-Violet and Visible Spectroscopy. Chemical Applications; Copyright for the Polish edition by PWN; Butterworth and Co (Publishers) LTD: Warsaw, Poland, 1982; pp. 99–106. First published 1975, pp. 58–62. [Google Scholar]
- Jasienko, S.; Gierus-Piasecka, I. Properties and structure of group components separated from vitrites of coking coals by selective thermosolvolysis. Fuel 1982, 61, 557–564. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Andresen, J.M.; Snape, C.E. Quantification by in situ n.m.r. of the contributions from pyridine-extractables and metaplast to the generation of coal plasticity. Fuel 1997, 76, 1301–1308. [Google Scholar] [CrossRef]
- Lynch, L.J.; Webster, D.S.; Sakurovs, R.; Barton, W.A.; Maher, T.P. The molecular basis of coal thermoplasticity. Fuel 1988, 67, 579–583. [Google Scholar] [CrossRef]
- Lee, S.; Mahoney, M.; Yu, J. Advances in the understanding of the formation and chemistry of the plastic layer during coke-making: A comprehensive review. Fuel 2020, 263, 116655. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, S.; Wu, Y.; Qiu, G.; Sun, C.; Zhang, Q.; Dang, J.; Wen, L.; Hu, M.; Xu, J.; et al. Structural transformation of fluid phase extracted from coal matrix during thermoplastic stage of coal pyrolysis. Fuel 2018, 232, 374–383. [Google Scholar] [CrossRef]




| Proximate Analysis | Ultimate Analysis | ||
|---|---|---|---|
| Wa, [%] | 8.5 | Ca, [%] | 82.2 |
| Vdaf, [%] | 33.0 | Ha, [%] | 4.82 |
| Ad, [%] | 7.0 | Na, [%] | 1.36 |
| FSI | 7.0 | Sa, [%] | 0.47 |
| RI | 77 | Odiff *), [%] | 4.15 |
| Sample. | Zone of Swollen Grains | Weakened/Gas-Saturated Zone | Compacted Zone |
|---|---|---|---|
| fresh | 0.62 ± 0.03 | 0.72 ± 0.05 | 0.41 ± 0.04 |
| thawed | 2.69 ± 0.02 | 1.46 ± 0.05 | 0.83 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubkova, V.; Strojwas, A. The Influence of Freezing on the Course of Carbonization and Pyrolysis of a Bituminous High-Volatile Coal. Energies 2020, 13, 6476. https://doi.org/10.3390/en13246476
Zubkova V, Strojwas A. The Influence of Freezing on the Course of Carbonization and Pyrolysis of a Bituminous High-Volatile Coal. Energies. 2020; 13(24):6476. https://doi.org/10.3390/en13246476
Chicago/Turabian StyleZubkova, Valentina, and Andrzej Strojwas. 2020. "The Influence of Freezing on the Course of Carbonization and Pyrolysis of a Bituminous High-Volatile Coal" Energies 13, no. 24: 6476. https://doi.org/10.3390/en13246476




