Chemical Impacts of Potential CO2 and Brine Leakage on Groundwater Quality with Quantitative Risk Assessment: A Case Study of the Farnsworth Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Site-Specific Water Chemistry and No-Impact Thresholds
2.3. Trace Metal Mobilization Due to CO2 Leakage
2.4. Quantification Risk Assessment of Groundwater Quality
2.4.1. Response Surface Methodology (RSM)
2.4.2. Reactive Transport Model
3. Results and Discussion
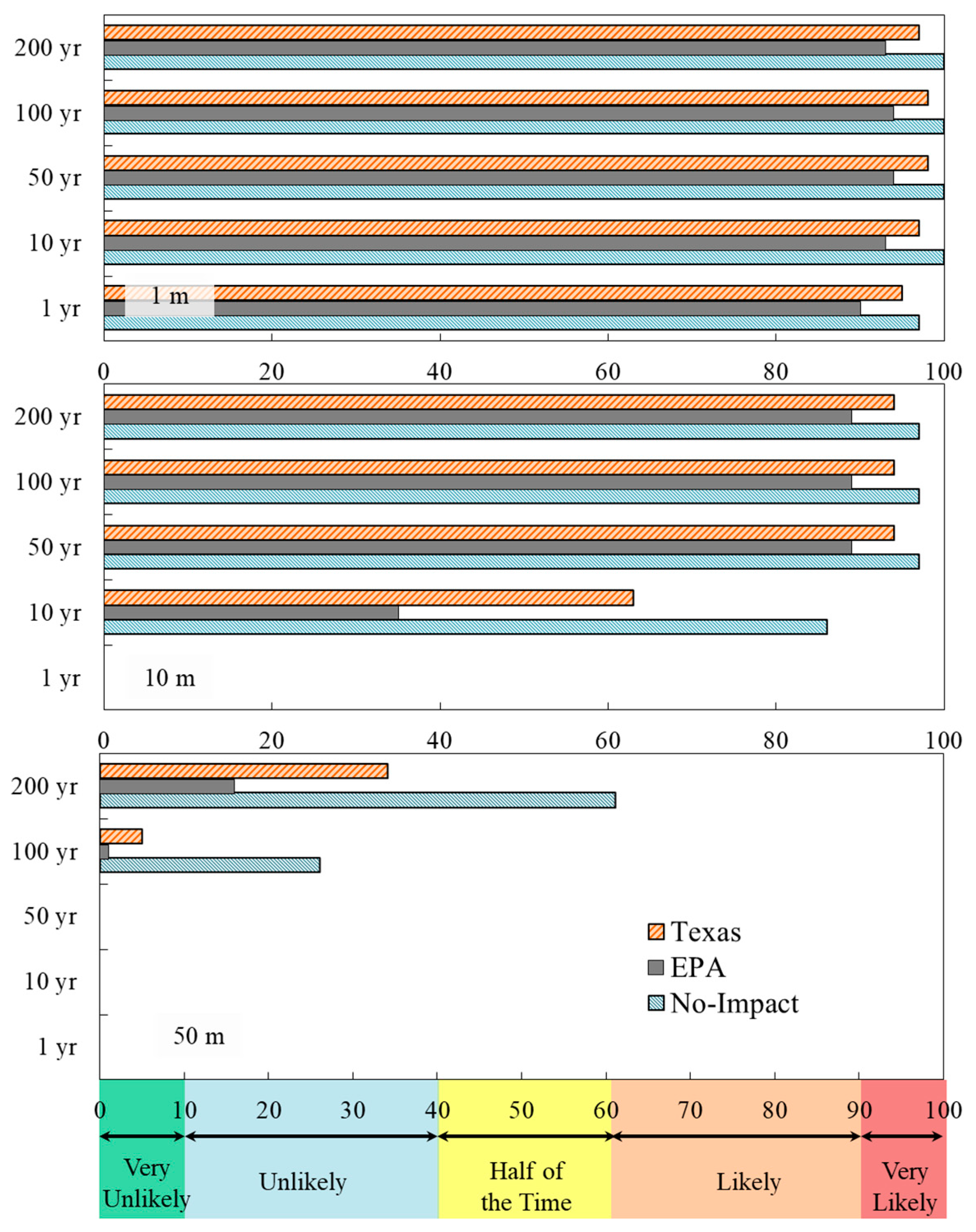
3.1. Impacts on Groundwater Quality
3.2. Thresholds and Indicators for Early Detection Criteria
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- McCulloch, S. 20 Years of Carbon Capture and Storage: Accelerating Future Deployment; International Energy Agency: Paris, France, 2016; ISBN 9789264267800. [Google Scholar]
- IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H., Loos, M., Meyer, L., Eds.; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.-Y.; McPherson, B.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50. [Google Scholar] [CrossRef] [PubMed]
- Bachu, S.; Watson, T.L. Review of failures for wells used for CO2 and acid gas injection in Alberta, Canada. Energy Procedia 2009, 1, 3531–3537. [Google Scholar] [CrossRef]
- Jia, W.; McPherson, B.; Dai, Z.; Irons, T.; Xiao, T. Evaluation of pressure management strategies and impact of simplifications for a post-EOR CO2 storage project. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 3, 281–292. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Little, M.G.; Jackson, R.B. Potential Impacts of Leakage from Deep CO2 Geosequestration on Overlying Freshwater Aquifers. Environ. Sci. Technol. 2010, 44, 9225–9232. [Google Scholar] [CrossRef]
- Qafoku, N.P.; Lawter, A.R.; Bacon, D.H.; Zheng, L.; Kyle, J.; Brown, C.F. Review of the impacts of leaking CO2 gas and brine on groundwater quality. Earth-Sci. Rev. 2017, 169, 69–84. [Google Scholar] [CrossRef]
- Liu, W.; Ramirez, A. State of the art review of the environmental assessment and risks of underground geo-energy resources exploitation. Renew. Sustain. Energy Rev. 2017, 76, 628–644. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.R.; Yang, C.; et al. Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Zheng, L.; Apps, J.A.; Zhang, Y.; Xu, T.; Birkholzer, J.T. On mobilization of lead and arsenic in groundwater in response to CO2 leakage from deep geological storage. Chem. Geol. 2009, 268, 281–297. [Google Scholar] [CrossRef]
- Yang, C.; Hovorka, S.D.; Treviño, R.H.; Delgado-Alonso, J. Integrated Framework for Assessing Impacts of CO2 Leakage on Groundwater Quality and Monitoring-Network Efficiency: Case Study at a CO2 Enhanced Oil Recovery Site. Environ. Sci. Technol. 2015, 49, 8887–8898. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Viswanathan, H.; Xiao, T.; Hakala, A.; Lopano, C.; Guthrie, G.; McPherson, B. Reactive Transport Modeling of Geological Carbon Storage Associated with CO2 and Brine Leakage. In Science of Carbon Storage in Deep Saline Formations; Elsevier: Amsterdam, The Netherlands, 2019; pp. 89–116. [Google Scholar]
- Keating, E.H.; Fessenden, J.; Kanjorski, N.; Koning, D.J.; Pawar, R. The impact of CO2 on shallow groundwater chemistry: Observations at a natural analog site and implications for carbon sequestration. Environ. Earth Sci. 2010, 60, 521–536. [Google Scholar] [CrossRef]
- Xiao, T.; Dai, Z.; Viswanathan, H.; Hakala, A.; Cather, M.; Jia, W.; Zhang, Y.; McPherson, B. Arsenic mobilization in shallow aquifers due to CO2 and brine intrusion from storage reservoirs. Sci. Rep. 2017, 7, 2763. [Google Scholar] [CrossRef]
- Viswanathan, H.S.; Pawar, R.J.; Stauffer, P.H.; Kaszuba, J.P.; Carey, J.W.; Olsen, S.C.; Keating, G.N.; Kavetski, D.; Guthrie, G.D. Development of a Hybrid Process and System Model for the Assessment of Wellbore Leakage at a Geologic CO2 Sequestration Site. Environ. Sci. Technol. 2008, 42, 7280–7286. [Google Scholar] [CrossRef]
- Xiao, T.; McPherson, B.; Bordelon, A.; Viswanathan, H.; Dai, Z.; Tian, H.; Esser, R.; Jia, W.; Carey, W. Quantification of CO2-cement-rock interactions at the well-caprock-reservoir interface and implications for geological CO2 storage. Int. J. Greenh. Gas Control 2017, 63, 126–140. [Google Scholar] [CrossRef]
- Carroll, S.; Carey, J.W.; Dzombak, D.; Huerta, N.J.; Li, L.; Richard, T.; Um, W.; Walsh, S.D.C.; Zhang, L. Review: Role of chemistry, mechanics, and transport on well integrity in CO2 storage environments. Int. J. Greenh. Gas Control 2016, 49, 149–160. [Google Scholar] [CrossRef]
- Carey, J.W.; Wigand, M.; Chipera, S.J.; WoldeGabriel, G.; Pawar, R.; Lichtner, P.C.; Wehner, S.C.; Raines, M.A.; Guthrie, G.D. Analysis and performance of oil well cement with 30 years of CO2 exposure from the SACROC Unit, West Texas, USA. Int. J. Greenh. Gas Control 2007, 1, 75–85. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Lowry, G.V.; Dzombak, D.A.; Thaulow, N. Rate of CO2 Attack on Hydrated Class H Well Cement under Geologic Sequestration Conditions. Environ. Sci. Technol. 2008, 42, 6237–6242. [Google Scholar] [CrossRef]
- Scherer, G.W.; Celia, M.A.; Prévost, J.-H.; Bachu, S.; Bruant, R.; Duguid, A.; Fuller, R.; Gasda, S.E.; Radonjic, M.; Vichit-Vadakan, W. Leakage of CO2 through Abandoned Wells: Role of Corrosion of Cement. In Carbon Dioxide Capture for Storage in Deep Geologic Formations; Elsevier: Amsterdam, The Netherlands, 2005; pp. 827–848. [Google Scholar]
- Carey, J.W.; Svec, R.; Grigg, R.; Zhang, J.; Crow, W. Experimental investigation of wellbore integrity and CO2–brine flow along the casing–cement microannulus. Int. J. Greenh. Gas Control 2010, 4, 272–282. [Google Scholar] [CrossRef]
- Miao, X.; Zhang, L.; Wang, Y.; Wang, L.; Fu, X.; Gan, M.; Li, X. Characterisation of wellbore cement microstructure alteration under geologic carbon storage using X-ray computed micro-tomography: A framework for fast CT image registration and carbonate shell morphology quantification. Cem. Concr. Compos. 2020, 108, 103524. [Google Scholar] [CrossRef]
- Xiao, T.; McPherson, B.; Pan, F.; Esser, R.; Jia, W.; Bordelon, A.; Bacon, D. Potential chemical impacts of CO2 leakage on underground source of drinking water assessed by quantitative risk analysis. Int. J. Greenh. Gas Control 2016, 50, 305–316. [Google Scholar] [CrossRef]
- Carroll, S.A.; Keating, E.; Mansoor, K.; Dai, Z.; Sun, Y.; Trainor-Guitton, W.; Brown, C.; Bacon, D. Key factors for determining groundwater impacts due to leakage from geologic carbon sequestration reservoirs. Int. J. Greenh. Gas Control 2014, 29, 153–168. [Google Scholar] [CrossRef]
- Onishi, T.; Nguyen, M.C.; Carey, J.W.; Will, B.; Zaluski, W.; Bowen, D.W.; Devault, B.C.; Duguid, A.; Zhou, Q.; Fairweather, S.H.; et al. Potential CO2 and brine leakage through wellbore pathways for geologic CO2 sequestration using the National Risk Assessment Partnership tools: Application to the Big Sky Regional Partnership. Int. J. Greenh. Gas Control 2019, 81, 44–65. [Google Scholar] [CrossRef]
- Bacon, D.H.; Qafoku, N.P.; Dai, Z.; Keating, E.H.; Brown, C.F. Modeling the impact of carbon dioxide leakage into an unconfined, oxidizing carbonate aquifer. Int. J. Greenh. Gas Control 2016, 44, 290–299. [Google Scholar] [CrossRef]
- Wilson, E.J.; Friedmann, S.J.; Pollak, M.F. Research for Deployment: Incorporating Risk, Regulation, and Liability for Carbon Capture and Sequestration. Environ. Sci. Technol. 2007, 41, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Dai, Z.; McPherson, B.; Viswanathan, H.; Jia, W. Reactive transport modeling of arsenic mobilization in shallow groundwater: Impacts of CO2 and brine leakage. Geomech. Geophys. Geo-Energy Geo-Resour. 2017, 3, 339–350. [Google Scholar] [CrossRef]
- Yang, C.; Mickler, P.J.; Reedy, R.; Scanlon, B.R.; Romanak, K.D.; Nicot, J.-P.; Hovorka, S.D.; Trevino, R.H.; Larson, T. Single-well push–pull test for assessing potential impacts of CO2 leakage on groundwater quality in a shallow Gulf Coast aquifer in Cranfield, Mississippi. Int. J. Greenh. Gas Control 2013, 18, 375–387. [Google Scholar] [CrossRef]
- Yang, C.; Treviño, R.H.; Hovorka, S.D.; Delgado-Alonso, J. Semi-analytical approach to reactive transport of CO2 leakage into aquifers at carbon sequestration sites. Greenh. Gases Sci. Technol. 2015, 5, 786–801. [Google Scholar] [CrossRef]
- Dai, Z.; Middleton, R.; Viswanathan, H.; Fessenden-Rahn, J.; Bauman, J.; Pawar, R.; Lee, S.-Y.; McPherson, B. An Integrated Framework for Optimizing CO2 Sequestration and Enhanced Oil Recovery. Environ. Sci. Technol. Lett. 2014, 1, 49–54. [Google Scholar] [CrossRef]
- Jia, W.; McPherson, B.J.; Pan, F.; Xiao, T.; Bromhal, G. Probabilistic analysis of CO2 storage mechanisms in a CO2 -EOR field using polynomial chaos expansion. Int. J. Greenh. Gas Control 2016, 51, 218–229. [Google Scholar] [CrossRef]
- Rohmer, J.; Bouc, O. A response surface methodology to address uncertainties in cap rock failure assessment for CO2 geological storage in deep aquifers. Int. J. Greenh. Gas Control 2010, 4, 198–208. [Google Scholar] [CrossRef][Green Version]
- Ampomah, W.; Balch, R.S.; Cather, M.; Will, R.; Gunda, D.; Dai, Z.; Soltanian, M.R. Optimum design of CO2 storage and oil recovery under geological uncertainty. Appl. Energy 2017, 195, 80–92. [Google Scholar] [CrossRef]
- Balch, R.; Mcpherson, B. Integrating Enhanced Oil Recovery and Carbon Capture and Storage Projects: A Case Study at Farnsworth Field, Texas. In Proceedings of the SPE Western Regional Meeting, Anchorage, AK, USA, 23–26 May 2016; Society of Petroleum Engineers: Houston, TX, USA, 2016. [Google Scholar]
- Balch, R.; McPherson, B.; Grigg, R. Overview of a Large Scale Carbon Capture, Utilization, and Storage Demonstration Project in an Active Oil Field in Texas, USA. Energy Procedia 2017, 114, 5874–5887. [Google Scholar] [CrossRef]
- Xiao, T.; Xu, H.; Moodie, N.; Esser, R.; Jia, W.; Zheng, L.; Rutqvist, J.; McPherson, B. Chemical-Mechanical Impacts of CO2 Intrusion into Heterogeneous Caprock. Water Resour. Res. 2020. [Google Scholar] [CrossRef]
- Nakao, S.; Tosha, T. Progress Report of AIST’s Research Programs for CO2 Geological Storage. Energy Procedia 2013, 37, 4990–4996. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1985.
- Yang, C.; Dai, Z.; Romanak, K.D.; Hovorka, S.D.; Treviño, R.H. Inverse Modeling of Water-Rock-CO2 Batch Experiments: Potential Impacts on Groundwater Resources at Carbon Sequestration Sites. Environ. Sci. Technol. 2014, 48, 2798–2806. [Google Scholar] [CrossRef]
- Choi, B.-Y. Potential impact of leaking CO2 gas and CO2-rich fluids on shallow groundwater quality in the Chungcheong region (South Korea): A hydrogeochemical approach. Int. J. Greenh. Gas Control 2019, 84, 13–28. [Google Scholar] [CrossRef]
- Last, G.; Murray, C.; Brown, C.; Jordan, P.; Sharma, M. No-Impact Threshold Values for NRAP’s Reduced Order Models; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2013. [Google Scholar]
- Frye, E.; Bao, C.; Li, L.; Blumsack, S. Environmental Controls of Cadmium Desorption during CO2 Leakage. Environ. Sci. Technol. 2012, 46, 4388–4395. [Google Scholar] [CrossRef]
- Shao, H.; Qafoku, N.P.; Lawter, A.R.; Bowden, M.E.; Brown, C.F. Coupled Geochemical Impacts of Leaking CO2 and Contaminants from Subsurface Storage Reservoirs on Groundwater Quality. Environ. Sci. Technol. 2015, 49, 8202–8209. [Google Scholar] [CrossRef]
- Lawter, A.R.; Qafoku, N.P.; Asmussen, R.M.; Kukkadapu, R.K.; Qafoku, O.; Bacon, D.H.; Brown, C.F. Element mobilization and immobilization from carbonate rocks between CO2 storage reservoirs and the overlying aquifers during a potential CO2 leakage. Chemosphere 2018, 197, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Johnston, C.T. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, H.; Dai, Z.; Lopano, C.; Keating, E.; Hakala, J.A.; Scheckel, K.G.; Zheng, L.; Guthrie, G.D.; Pawar, R. Developing a robust geochemical and reactive transport model to evaluate possible sources of arsenic at the CO2 sequestration natural analog site in Chimayo, New Mexico. Int. J. Greenh. Gas Control 2012, 10, 199–214. [Google Scholar] [CrossRef][Green Version]
- Zheng, L.; Spycher, N. Modeling the potential impacts of CO2 sequestration on shallow groundwater: The fate of trace metals and organic compounds before and after leakage stops. Greenh. Gases Sci. Technol. 2018, 8, 161–184. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Goldberg, S. Modeling Selenite Adsorption Envelopes on Oxides, Clay Minerals, and Soils using the Triple Layer Model. Soil Sci. Soc. Am. J. 2013, 77, 64. [Google Scholar] [CrossRef]
- Zheng, L.; Apps, J.A.; Spycher, N.; Birkholzer, J.T.; Kharaka, Y.K.; Thordsen, J.; Beers, S.R.; Herkelrath, W.N.; Kakouros, E.; Trautz, R.C. Geochemical modeling of changes in shallow groundwater chemistry observed during the MSU-ZERT CO2 injection experiment. Int. J. Greenh. Gas Control 2012, 7, 202–217. [Google Scholar] [CrossRef]
- Pan, F.; McPherson, B.J.; Esser, R.; Xiao, T.; Appold, M.S.; Jia, W.; Moodie, N. Forecasting evolution of formation water chemistry and long-term mineral alteration for GCS in a typical clastic reservoir of the Southwestern United States. Int. J. Greenh. Gas Control 2016, 54, 524–537. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Wolery, T.J. EQ3/6, a Software Package for Geochemical Modeling of Aqueous Systems: Package Overview and Installation Guide (Version 7.0); Lawrence Livermore National Lab.: Livermore, CA, USA, 1992. [Google Scholar]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling; National Energy Technology Laboratory–United States Department of Energy: Menlo Park, CA, USA, 2004.
- Manning, B.A.; Goldberg, S. Adsorption and Stability of Arsenic(III) at the Clay Mineral−Water Interface. Environ. Sci. Technol. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Appelo, C.J.A.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Xu, T.; Spycher, N.; Sonnenthal, E.; Zhang, G.; Zheng, L.; Pruess, K. TOUGHREACT Version 2.0: A simulator for subsurface reactive transport under non-isothermal multiphase flow conditions. Comput. Geosci. 2011, 37, 763–774. [Google Scholar] [CrossRef]
- Pruess, K.; Spycher, N. ECO2N—A fluid property module for the TOUGH2 code for studies of CO2 storage in saline aquifers. Energy Convers. Manag. 2007, 48, 1761–1767. [Google Scholar] [CrossRef]
- Bacon, D.H.; Dai, Z.; Zheng, L. Geochemical Impacts of Carbon Dioxide, Brine, Trace Metal and Organic Leakage into an Unconfined, Oxidizing Limestone Aquifer. Energy Procedia 2014, 63, 4684–4707. [Google Scholar] [CrossRef]





| Name | Ogallala | PW | EPA MCL | Texas MCL | NIT |
|---|---|---|---|---|---|
| pH | 7.7 | 7.2 | 6.5 | 7.0 | 7.5 |
| Total dissolved solids (TDS) | 380 | 4064 | 500 | 1000 | 508 |
| Mn | 0.008 | 0.27 | 0.05 | 0.05 | 0.05 |
| As | 0.003 | 0.005 | 0.01 | 0.01 | 0.005 |
| Se | 0.004 | 0.07 | 0.05 | 0.002 | 0.007 |
| Parameter Name | Low (−1) | Mid (0) | High (+1) | Distribution |
|---|---|---|---|---|
| CO2 leakage rate: g/s | 0 | 0.5 | 1.0 | Uniform |
| Brine leakage rate: g/s | 0 | 0.25 | 0.5 | Uniform |
| Aquifer thickness: m | 40 | 120 | 200 | Normal |
| Adsorbent SSA: m2/g | 1 | 50.5 | 100 | Uniform |
| Simulation | Values of Independent Parameters | Simulation | Values of Independent Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 14 | 0 | −1 | 1 | 0 |
| 2 | −1 | 1 | 0 | 0 | 15 | 0 | 1 | −1 | 0 |
| 3 | 1 | −1 | 0 | 0 | 16 | 0 | 1 | 1 | 0 |
| 4 | 1 | 1 | 0 | 0 | 17 | −1 | 0 | −1 | 0 |
| 5 | 0 | 0 | −1 | −1 | 18 | −1 | 0 | 1 | 0 |
| 6 | 0 | 0 | −1 | 1 | 19 | 1 | 0 | −1 | 0 |
| 7 | 0 | 0 | 1 | −1 | 20 | 1 | 0 | 1 | 0 |
| 8 | 0 | 0 | 1 | 1 | 21 | 0 | −1 | 0 | −1 |
| 9 | −1 | 0 | 0 | −1 | 22 | 0 | −1 | 0 | 1 |
| 10 | −1 | 0 | 0 | 1 | 23 | 0 | 1 | 0 | −1 |
| 11 | 1 | 0 | 0 | −1 | 24 | 0 | 1 | 0 | 1 |
| 12 | 1 | 0 | 0 | 1 | 25 | 0 | 0 | 0 | 0 |
| 13 | 0 | −1 | −1 | 0 | |||||
| Name | Ogallala | PW | Name | Ogallala | PW |
|---|---|---|---|---|---|
| pH (unitless) | 7.7 | 7.2 | SiO2 (aq) | 6.613 × 10−4 | 6.667 × 10−4 |
| Ca2+ | 1.189 × 10−3 | 1.937 × 10−3 | Cl− | 1.582 × 10−3 | 5.619 × 10−2 |
| Mg2+ | 7.935 × 10−5 | 1.286 × 10−4 | HCO3− | 2.355 × 10−3 | 4.267 × 10−3 |
| Na+ | 2.068 × 10−3 | 6.407 × 10−2 | SO42− | 5.105 × 10−4 | 2.735 × 10−4 |
| K+ | 2.649 × 10−4 | 4.534 × 10−4 | NO3− | 2.399 × 10−4 | 3.898 × 10−6 |
| Fe2+ | 8.880 × 10−9 | 6.517 × 10−9 | H2AsO4− | 5.290 × 10−9 | 6.775 × 10−8 |
| AlO2− | 2.311 × 10−10 | 1.732 × 10−10 | HSeO3− | 1.733 × 10−8 | 8.887 × 10−6 |
| Mn2+ | 1.457 × 10−7 | 4.568 × 10−6 |
| Mineral Name | Formula | Volume Fraction | Surface Area (cm2/g) |
|---|---|---|---|
| Primary | |||
| Quartz | SiO2 | 0.780 | 23.29 |
| Calcite | CaCO3 | 0.110 | 53.96 |
| K-Feldspar | KAlSi3O8 | 0.092 | 222.42 |
| Dolomite | CaMg(CO3)2 | 0.004 | 9.80 |
| Smectite | Na0.29Mg0.26Al1.77Si3.97O10(OH)2 | 0.005 | 151.60 |
| Albite | NaAlSi3O8 | 0.005 | 11.40 |
| Secondary | |||
| Illite | K0.6Mg0.25Al1.8(Al0.5Si3.5O10)(OH)2 | 0 | 272.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, T.; McPherson, B.; Esser, R.; Jia, W.; Dai, Z.; Chu, S.; Pan, F.; Viswanathan, H. Chemical Impacts of Potential CO2 and Brine Leakage on Groundwater Quality with Quantitative Risk Assessment: A Case Study of the Farnsworth Unit. Energies 2020, 13, 6574. https://doi.org/10.3390/en13246574
Xiao T, McPherson B, Esser R, Jia W, Dai Z, Chu S, Pan F, Viswanathan H. Chemical Impacts of Potential CO2 and Brine Leakage on Groundwater Quality with Quantitative Risk Assessment: A Case Study of the Farnsworth Unit. Energies. 2020; 13(24):6574. https://doi.org/10.3390/en13246574
Chicago/Turabian StyleXiao, Ting, Brian McPherson, Richard Esser, Wei Jia, Zhenxue Dai, Shaoping Chu, Feng Pan, and Hari Viswanathan. 2020. "Chemical Impacts of Potential CO2 and Brine Leakage on Groundwater Quality with Quantitative Risk Assessment: A Case Study of the Farnsworth Unit" Energies 13, no. 24: 6574. https://doi.org/10.3390/en13246574
APA StyleXiao, T., McPherson, B., Esser, R., Jia, W., Dai, Z., Chu, S., Pan, F., & Viswanathan, H. (2020). Chemical Impacts of Potential CO2 and Brine Leakage on Groundwater Quality with Quantitative Risk Assessment: A Case Study of the Farnsworth Unit. Energies, 13(24), 6574. https://doi.org/10.3390/en13246574







