Abstract
MgAl2-xGaxCl8 and MgGa2Cl7Br was synthesized from MgCl2, MgBr2, AlCl3, and GaCl3, and the physical properties were evaluated by XRD, AC conductivity, and X-ray fluorescence. MgAl2-xGaxCl8 has the same crystal structure as MgAl2Cl8, belongs to the space group C2/c, and forms a solid solution in all the synthesized compositions. We attempted to MgGa2Cl8-yBry synthesize in the same manner as MgAl2Cl8, but only obtained MgGa2Cl7Br as a single-phase compound. The AC conductivity of MgAl2-xGaxCl8 was lowest for MgAl2Cl8 and highest for MgGa2Cl8. The conductivity of MgGa2Cl8 at 400 K was 1.7 × 10−6 S/cm. The conductivity of MgGa2Cl7Br was higher than that of MgAl2-xGaxCl8 with a value of 1.6 × 10−5 S/cm at 400 K. The formation of Mg metal was observed at the surface of the cathode electrode in an experiment of flowing a direct current using an electrochemical cell, indicating that this compound is a magnesium ion conductor.
1. Introduction
Among commercially available secondary batteries, lithium-ion secondary batteries have excellent features such as high energy density and high cycle characteristics and are used as batteries for portable devices and electric vehicles [1,2]. In the future, the size and demand of batteries are expected to increase, and the safety and supply of lithium-based batteries are uncertain, so research of alternative secondary batteries is actively conducted. Magnesium ions are the charge carriers in Mg secondary batteries. Magnesium is more stable than alkali metal elements, abundant in nature, and expected to have a high capacity because of the two-electron reaction. Therefore, the Mg secondary batteries are expected as one of the post-lithium ion secondary batteries [3,4]. Acetonitrile and Grignard reagent have been reported as organic electrolytes of Mg secondary batteries, and magnesium metal formation and dissolution reactions on the working electrode have been observed using a magnesium metal sheet as a counter electrode [3,5,6,7,8]. For magnesium ion conductive solid electrolytes, some oxides and sulfide-based glasses have been reported. Solid oxide electrolytes have low ionic conductivity, and MgHf(WO4)3 and (Mg0.1Hf0.9)4/3.8Nb(PO4)3 have been reported to exhibit a conductivity of 2.5 × 10−4 S/cm at 873 K and 2.1 × 10−6 S/cm at 573 K, respectively [9,10]. Sulfide-based glass containing Mg2+ ion synthesized in a manner similar as lithium-ion conductive glass exhibits magnesium ion conductivity [11]. Some synthesized Mg(BH4)-based compounds also show relatively high ionic conductivity (1.3 × 10−5 S/cm at 303 K) [12,13]. Considering the increase in size of secondary batteries, it is essential to improve safety, so it is expected that the development of all-solid-state batteries for Mg secondary batteries will be required, similar to the current lithium-ion secondary batteries. MoS2 and MgMn2O4 have been reported as positive electrode active materials [14,15] and it is expected that many new related compounds will be synthesized in the future. The appropriate combinations of active materials and electrolytes are also required to sufficiently exhibit the characteristics of batteries, and the development of various solid electrolytes is very important for practical battery use.
Halo complexes of group 13 elements, such as MAlX4 and MGaX4(M = Li, Na, Cu, Ag; X = Cl, Br), have been reported as M+ ion conductors, and compounds with high conductivity have shown values of 10−5 S/cm at room temperature [16,17]. The crystal system of LiAlX4 is monoclinic and its space group is P21/c [18]. In these compounds, the self-diffusion rate of M+ ion increases with the reorientation motion of AlX4− anions, and the improvement of M+ ion conductivity is observed [16,19]. The electrolyte salt of the abovementioned acetonitrile solution, MgAl2Br8, is the double salt of MgBr2, and the halide of group 13 elements, AlBr3, similar to MAlX4. So far, only MgAl2Cl8 has been reported as the crystal structure of the compound of MgX2 and AlX3, and its crystal structure is mainly similar to LiAlX4 and NaAlX4 [20]. Compared to Li+ and Na+ ions, Mg2+ ion has a larger charge and becomes more difficult to diffuse, but the cation conduction is expected to be observable, similar to MAlX4. If Mg2+ ion conductivity is exhibited in MgX2-MIIIX3 (X = Cl, Br; MIII = Al, Ga, etc.) compounds, they may function as solid electrolytes and be applied as electrolyte materials for all-solid-state Mg ion secondary batteries. We researched the synthesis of these compounds and the application as electrolyte materials for all-solid-state batteries. In this study, MgAl2-xGaxCl8 and MgGa2Cl8-yBry were synthesized, the crystal structure was analyzed, and the Mg2+ ion conductivity was evaluated in order to investigate the potential of the Mg secondary battery as an electrolyte.
2. Materials and Methods
MgAl2-xGaxCl8 and MgGa2Cl8-yBry were synthesized from MgCl2, MgBr2, AlCl3, and GaCl3 as starting materials. MgCl2 and MgBr2 were used after vacuum-drying at 473 K and 573 K, respectively, for 24–48 h. AlCl3 and GaCl3 were purified by sublimation. The purified raw materials were mixed in a stoichiometric ratio using a mortar and then sealed in a Pyrex glass test tube. The mixed samples were melted in electric furnace at 573 K for 24 h and gradually cooled to room temperature to obtain the target compounds. Since each raw material and the synthesized compound showed hygroscopic properties, all samples were handled in a dry Ar atmosphere using a glovebox. The obtained compounds were evaluated by XRD and X-ray fluorescence, and the diffraction data was analyzed by the Rietveld method [21]. The electrical characteristics of the samples were evaluated by electric conductivity. Powder X-ray diffraction patterns were recorded by a Rigaku Rad-B system with Cu-Kα radiation (40 kV, 40 mA). A handmade cell was used to protect the samples from moisture. The conductivity was determined by a complex impedance method, measured at 35 frequencies between 42 Hz and 5 MHz (3532-80 LCR high tester, HIOKI) in a dry argon atmosphere. The conductivity was measured using a stainless-steel electrode and Mg metal electrode. A two-electrode cell made of stainless-steel was used to measure the conductivity. The cell was sealed with O-ring and measured in an Ar atmosphere. The thickness of the sample was about 1 mm, the diameter was 10 mm, and the conductivity was measured while compressing the sample with a spring (about 20 kgf/cm2).
3. Results and Discussion
3.1. XRD Patterns and Crystal Structure
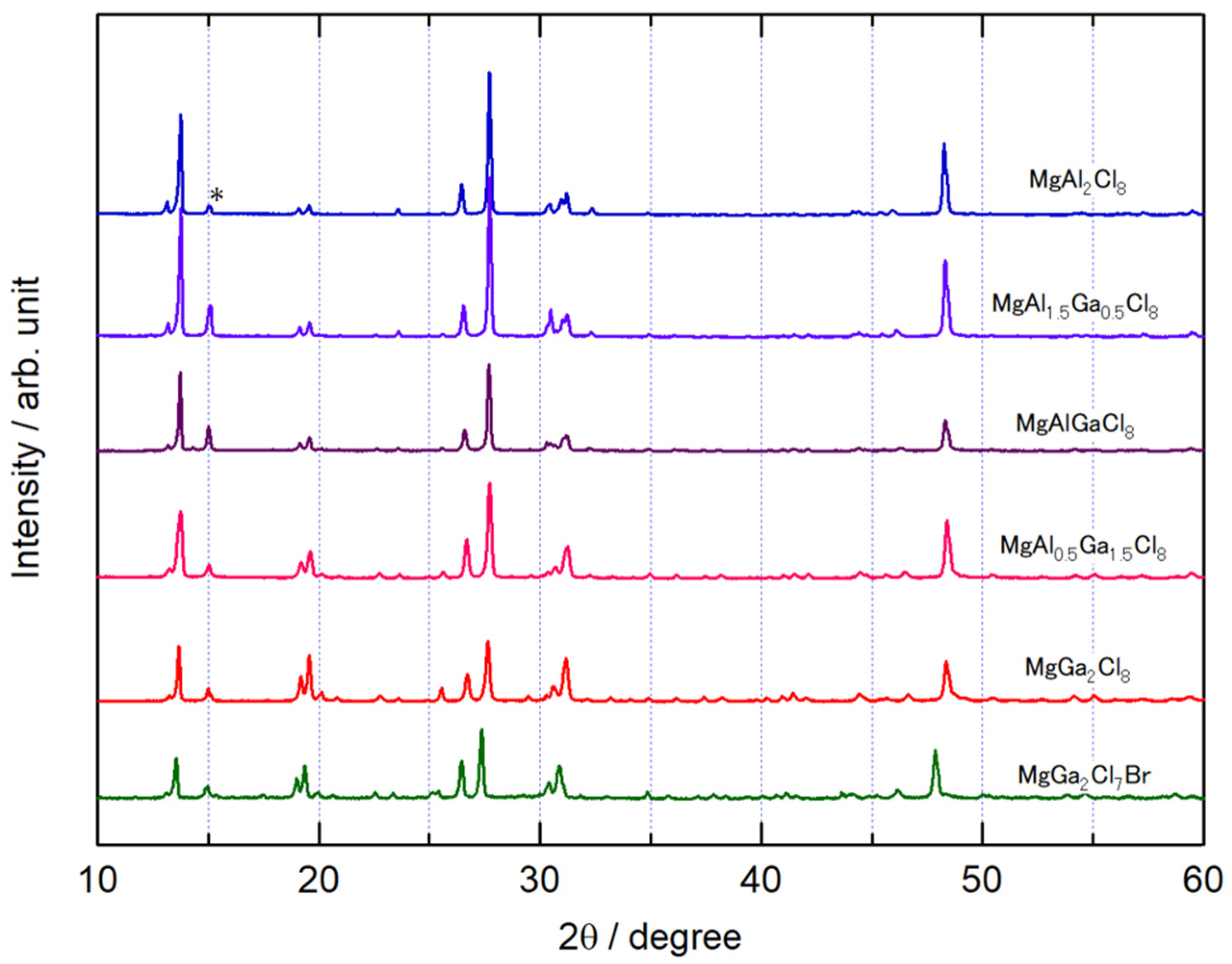
Figure 1 shows the XRD patterns of the synthesized MgAl2-xGaxCl8 and MgGa2Cl7Br. The XRD pattern of the MgAl2Cl8 is in good agreement with that of the previous report [20]. The crystal system was monoclinic and the space group was C2/c (No. 15). Although a slight peak of the source material MgCl2 was observed at 2θ = 15°, no other starting material peaks were observed, indicating that the target compound was obtained. The change of the peak positions among the XRD pattern of MgAl2-xGaxCl8 was small, and there was some difference in the intensity ratio of the peaks. The overall patterns of MgAl2-xGaxCl8 were very similar, and their compounds were presumed to have the same crystal structure. MgGa2Cl8-yBry (y = 1 to 8) was synthesized but only MgGa2Cl7Br (y = 1) was obtained. For samples with y > 1, the large diffraction peaks of GaBr3 were observed in the XRD measurement, and the peaks belonging to MgGa2Cl8-yBry were not observable or had low intensity. As shown in Figure 1, the diffraction peaks of MgGa2Cl7Br were shifted to lower angles compared with MgGa2Cl8, indicating that Cl− ion was partially substituted by Br− ion. On the other hand, even if Al3+ ion was replaced with Ga3+ ion as described above, there was almost no change in the angle of diffraction peaks. Comparing the volumes calculated using the lattice constants evaluated from the Rietveld analysis of the diffraction patterns, the volume change in MgAl2-xGaxCl8 was less than 0.4%.
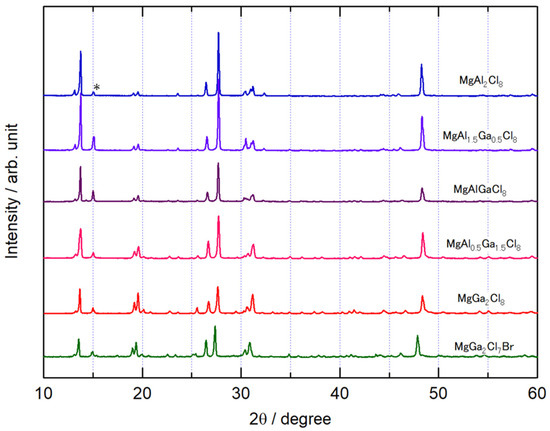
Figure 1.
XRD patterns of MgAl2-xGaxCl8 and MgGa2Cl7Br. The MgCl2 peak is indicated by “*”.
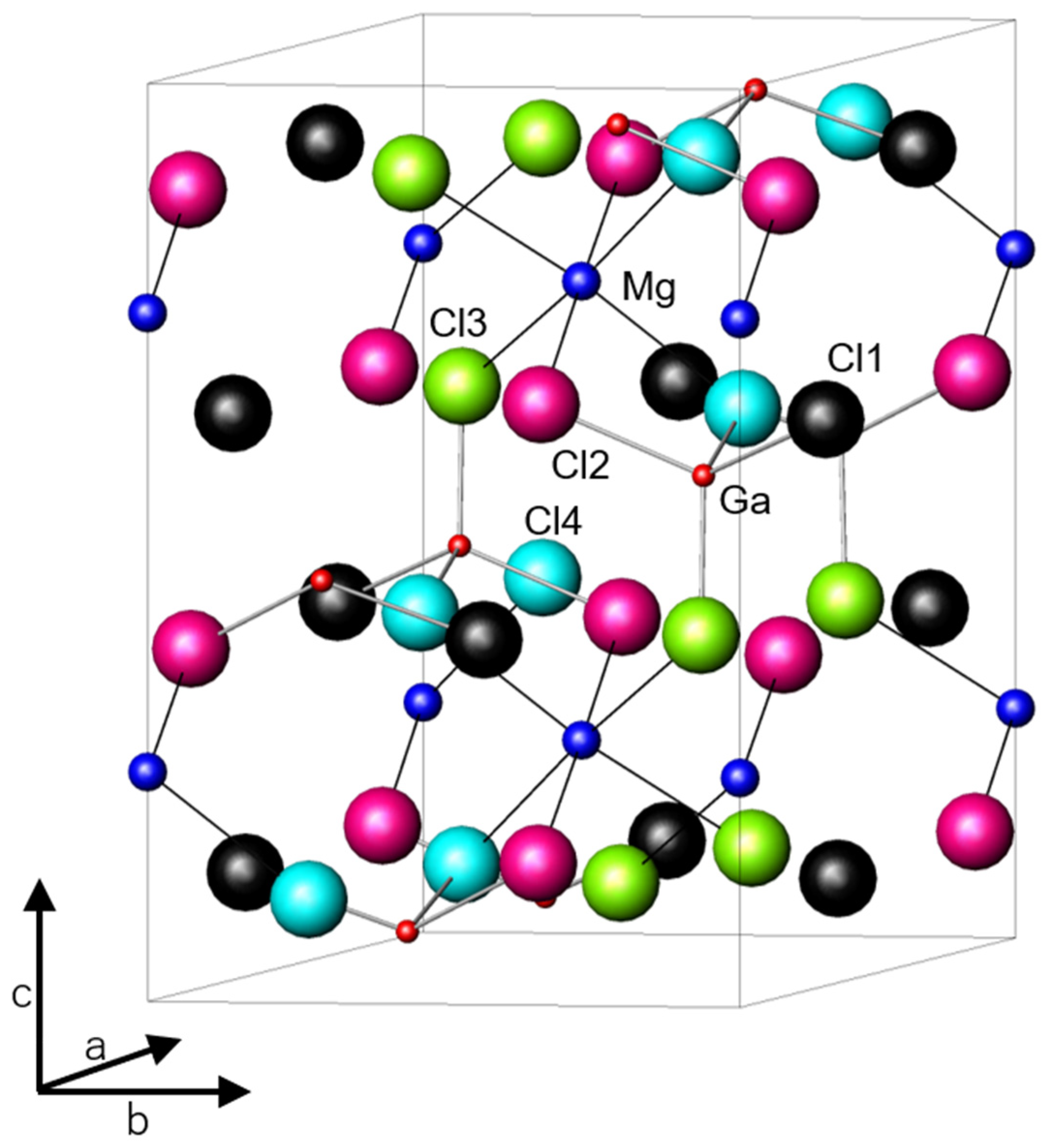
The Al3+ (Ga3+) ion occupies a tetrahedral site, and according to Shannon, the ionic radius of Ga3+ ion (0.47 Å) is 0.08 Å larger than that of Al3+ ion (0.39 Å) [22]. However, the lattice volumes of MgAl2-xGaxCl8 reflect no difference in the size of their ions. The crystal structure of MgGa2Cl8 is shown in Figure 2 and the results of the structure analysis performed by the Rietveld method in MgAl2-xGaxCl8 are summarized in Table 1. In the Rietveld analysis results of all compounds, Rwp was below 9.5 and S(=Rwp/Re) was below 1.8 (See Figure S1, Tables S1 and S2). Rwp and Re are reliability factors used for Rietveld refinement. The value of S is a scale indicating the reliability of the analysis.
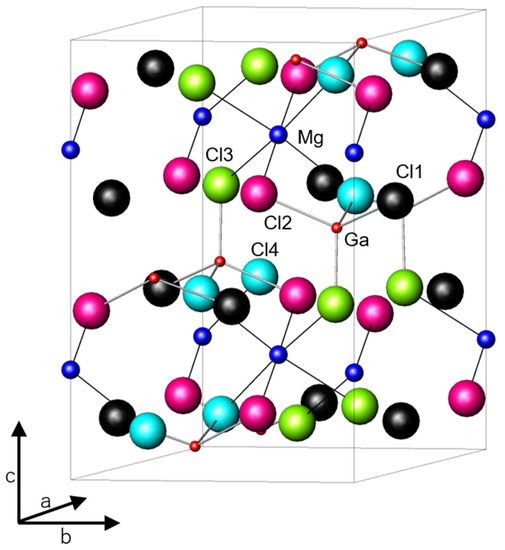
Figure 2.
Crystal structure of MgGa2Cl8 obtained by Rietveld analysis of the XRD pattern. Cl1-4 indicate halogen sites.

Table 1.
Results of the Rietveld analysis of XRD data for MgAl2-xGaxCl8.
Cl− ions occupy four types of sites crystallographically. The Mg2+ ion is octahedrally coordinated by six halogen ions, and the Ga3+ ion is tetrahedrally coordinated by four Cl− ions. Among the four halogen ions, three halogen ions at the sites labeled Cl2, Cl3, and Cl4 in Figure 2 bridge between the Mg2+ and Ga3+ ions. The remaining halogen ion labeled Cl1 is bound only to the Ga3+ ion. Both the bond lengths of Al (Ga) -Cl and Mg-Cl increased as the amount of Ga3+ ion increased in MgAl2-xGaxCl8 as shown in Table 1. Although the change in lattice volume due to the substitution was small, it is considered that the bond length changes depending on the arrangement of cations and anions. In addition, the bond length of Al (Ga) -Cl increased as expected because the ionic radius of the central cation increased in the substitution from Al to Ga. It is interesting that the bond length of Mg-Cl also increased accordingly. As shown in Table 1, β approached 90° gradually due to the Ga substitution. Although the expansion of the lattice was small, it is considered that the lattice distortion was reduced by increasing the size of the MCl4− anion. Rietveld analysis was also performed on MgGa2Cl7Br. The occupancy of Cl− and Br− at each of the halogen sites were refined in the analysis. The total occupancy of each site was limited to 1, and the ratio of the total amount of each of Cl− and Br− was limited to 7:1 to be equal to the composition ratio of Cl− and Br−. In MgGa2Cl7Br, the lattice constant increased and the bond length of Mg-X (X = Cl− or Br−) increased due to the partial substitution from Cl− to Br−. The change in Mg-X bond length due to partial substitution of Cl− was similar to the change due to substitution of all Al3+ to Ga3+ in MgAl2Cl8, indicating that the effects of both on Mg-X bond length were similar. In MgGa2Cl7Br, no large deviation was confirmed in the occupancy of Cl− and Br− at the Cl1, Cl2, and Cl4 site, and the occupancy of Br− at the Cl3 site was slightly higher than that of the other sites (See Table S3).
3.2. AC Conductivity
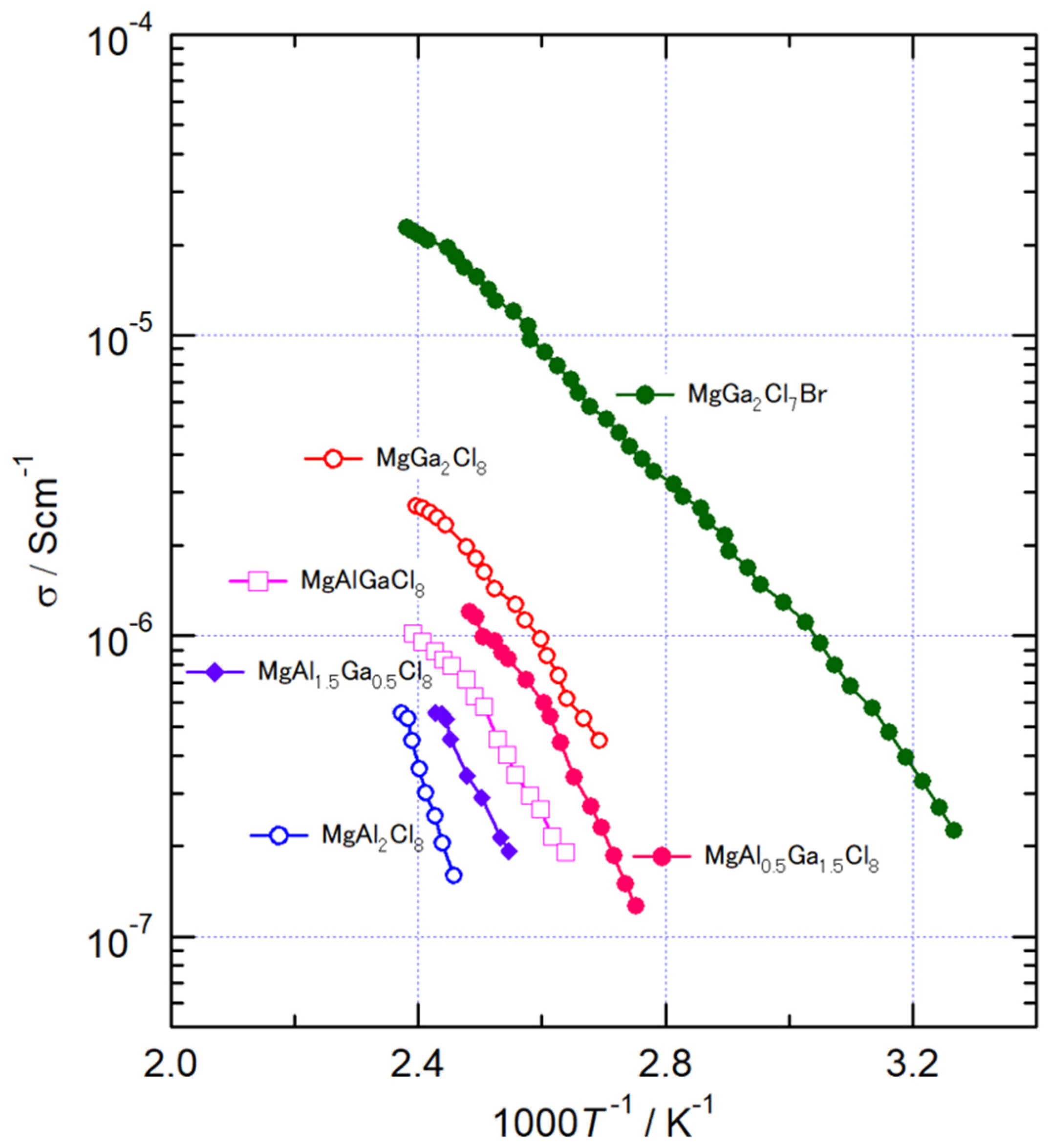
The temperature dependence of AC conductivity of MgAl2-xGaxCl8 (x = 0, 0.5, 1, 1.5, 2) and MgGa2Cl7Br. MgAl2Cl8 (x = 0) had the lowest conductivity of the synthesized compounds, approximately 10−7 S/cm even above 400 K. The conductivity increased with the substitution of the Al3+ ion to Ga3+ ion. In MgAl2-xGaxCl8, the highest conductivity was observed at MgGa2Cl8 with a value of 1.7 × 10−6 S/cm at 400 K. In all compositions, the conductivity σ changed monotonously with respect to the reciprocal of temperature. The activation energy, Ea, for the conduction was evaluated by the following equation:
where σ and A are the conductivity and the pre-exponential parameter, respectively [23]. The values of Ea of MgAl2-xGaxCl8 were 126, 75.4, 67.6, 69.1, and 59.1 kJ/mol at x = 0, 0.5, 1, 1.5, and 2, respectively (See Figure S2). MgGa2Cl8 had the lowest Ea of MgAl2-xGaxCl8, indicating that Li-ion conduction becomes easier in this compound. In the XRD measurement, the Mg-Cl bond length of MgAl2-xGaxCl8 increased with increasing x, suggesting that the diffusion of Mg2+ ion also becomes easier with the amount of substitution with Ga3+. Therefore, it is considered that the Ea of MgGa2Cl8 was the smallest among MgAl2-xGaxCl8 in the conductivity measurement. The conductivity of MgGa2Cl7Br was 1.6 × 10−5 S/cm, which was about an order of magnitude higher than that of MgGa2Cl8 as shown in Figure 3. Its Ea was 47.7 kJ/mol, which was also lower than MgGa2Cl8, and it was found that the diffusion of Mg ions became easier. It is considered that longer Mg-X distance due to the partial substitution of Cl− ion improved the Mg2+ ion conductivity.
σT = A exp (−Ea/RT),
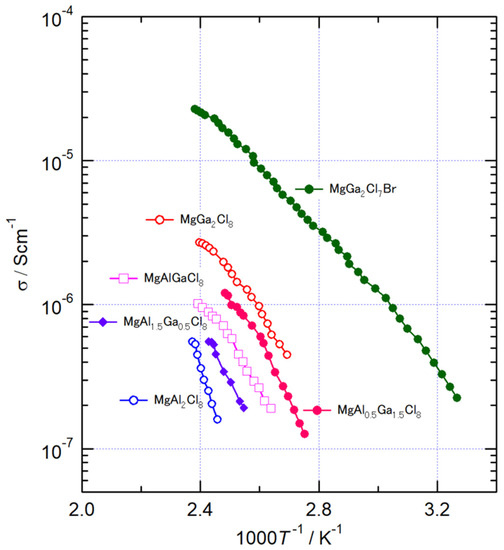
Figure 3.
Temperature dependence of AC conductivity of MgAl2-xGaxCl8 and MgGa2Cl7Br.
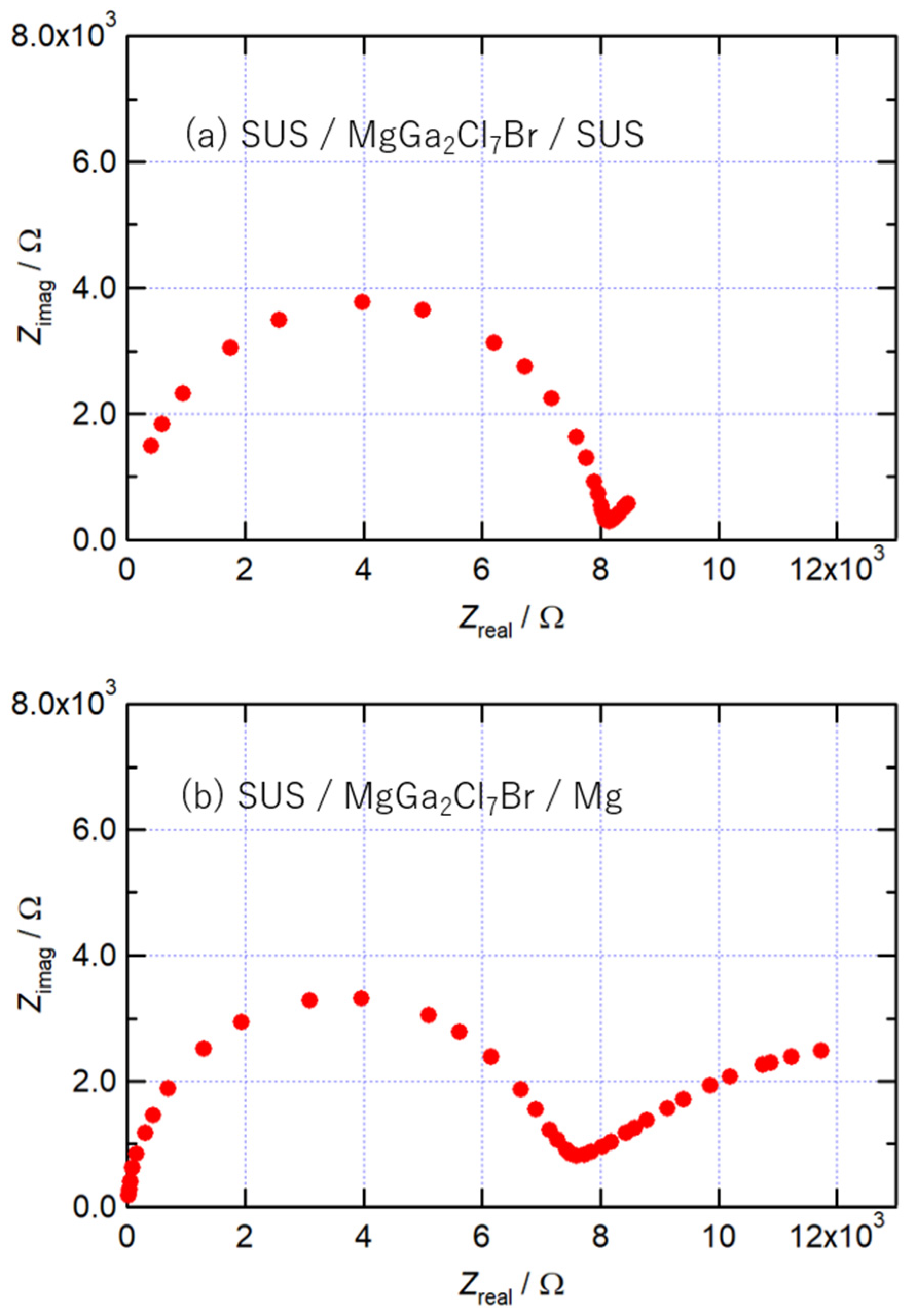
The Nyquist plots of MgGa2Cl7Br at 400 K, obtained using cells with different working electrodes, are shown in Figure 4. When a stainless-steel sheet was used as the working electrode, one arc was observed as shown in Figure 4a. On the other hand, when the working electrode was Mg sheet, another arc appeared on the low-frequency side as shown in Figure 4b, in addition to the arc on the high-frequency side observed in the cell with the stainless working electrode. This arc appeared due to the charge transfer resistance with the Mg electrode, suggesting that MgGa2Cl7Br is an Mg2+ ion conductor. The new arc shown in Figure 4b was slightly smaller than the arc on the high-frequency side, and the charge transfer resistance was confirmed not to be much different from the bulk resistance.
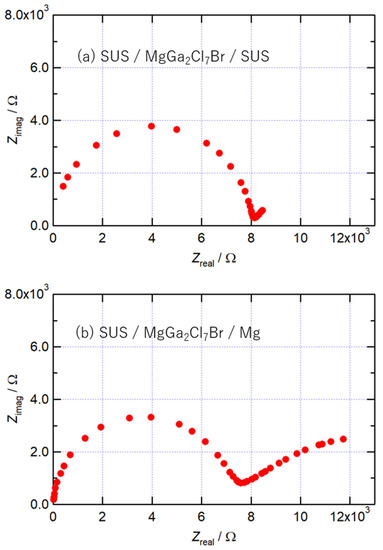
Figure 4.
Nyquist plots of MgGa2Cl7Br at 400 K obtained using (a) stainless and (b) Mg as a working electrode.
The activation energy of conductivity in MgGa2Cl7Br was lower than the reported activation energy of Mg2+ ion conductors at 63.9 kJ/mol of (Mg0.1Hf0.9)4/3.8Nb(PO4)3, 92.0 kJ/mol of Mg0.7(Zr0.85Nb0.15)4(PO4)6, and 80.6kJ/mol of MgHf(WO4)3, indicating that the diffusion of Mg ions is easier [9,10,24]. Higher electric conductivity was obtained at a lower temperature in MgGa2Cl7Br compared with those of the previous compounds, such as 2.1 × 10−6 S/cm (573 K) of (Mg0.1Hf0.9)4/3.8Nb(PO4)3, 1.1 × 10−7 S/cm of Mg0.7(Zr0.85Nb0.15)4(PO4)6, and 2 × 10−6 S/cm (573 K; extrapolated) of MgHf(WO4)3, indicating that MgGa2Cl7Br is a good Mg2+ ion conductor.
3.3. X-ray Fluorescence
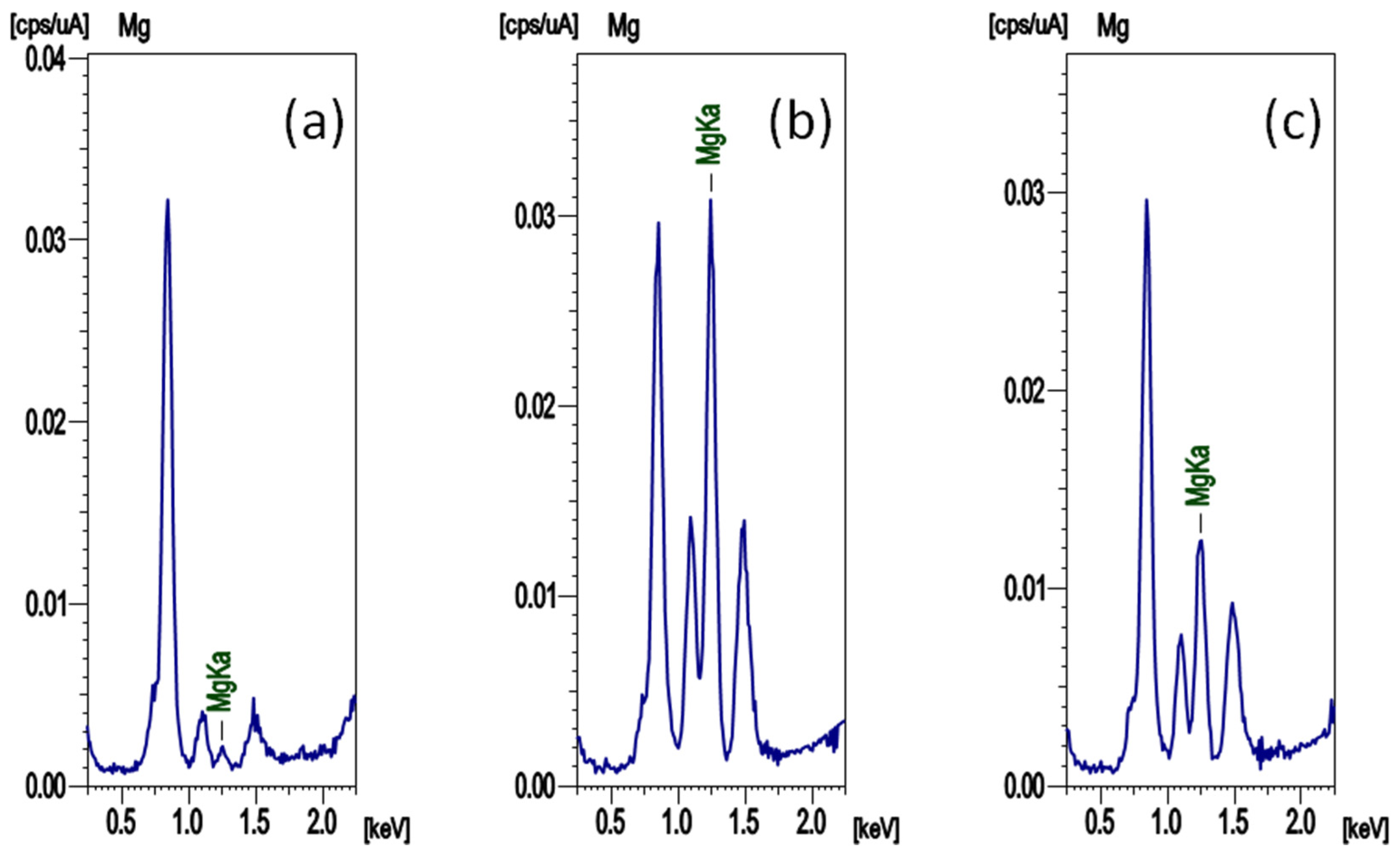
The results of fluorescent X-ray analysis on the surface of the working electrode when a constant current was applied to the fabricated cell are shown in Figure 5. The measured cell was composed of a Ni metal sheet as the working electrode and an Mg metal sheet as the counter electrode, and MgGa2Cl7Br was used as the electrolyte. The spectrum of Figure 5a is that of the working electrode before the current was passed, and the spectrum of Figure 5b is that of the electrode after the current of −50 μA was applied for 36 h. The spectrum of Figure 5c shows the results of the working electrode after a current of −50 μA was applied for 36 h and a current of +50 μA was applied for 36 h. A large peak attributed to Mg Kα was observed in Figure 5b, clarifying that Mg was precipitated on the working electrode. In the spectrum of Figure 5c, after applying the positive current, the peak of Mg in Figure 5b became small, and it was found that the Mg deposited by negative current on the working electrode was consumed in the oxidation reaction. Considering the applied electric quantity, the Mg peak must disappear in Figure 5c, but the peak was observed, indicating that the current was consumed by other reactions. When a negative current was applied the cell, Mg was precipitated by a reduction reaction on the working electrode, and when a positive current was applied, Mg on the working electrode was oxidized. Therefore, it could be reconfirmed that MgGa2Cl7Br is an Mg ion conductor.
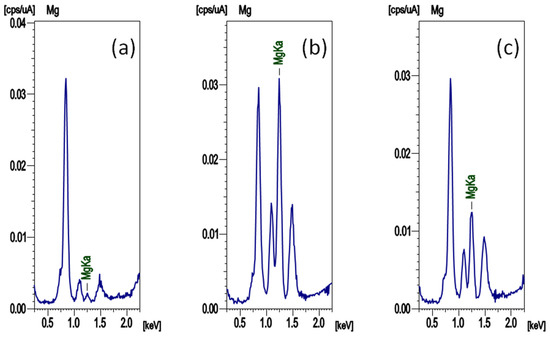
Figure 5.
X-ray fluorescence spectra of the surface of the working electrode of the cell (a) before applying a current, (b) after applying a current of −50 μA for 36 h, and (c) applying a current of +50 μA for 36 h after (b).
4. Conclusions
We attempted to synthesize MgAl2-xGaxCl8 and MgGa2Cl8-yBry and obtained MgAl2-xGaxCl8 (x = 0 to 2) and MgGa2Cl7Br. Their crystal system was monoclinic and the space group was C2/c (No. 15). In XRD measurement of MgAl2-xGaxCl8, as the amount of Ga increased, the diffraction peaks did not shift significantly but the Mg-Cl bond length increased. The lattice constant and volume increased with the partial substitution of Cl with Br between MgGa2Cl8 and MgGa2Cl7Br. The Mg-X bond length also increased with substitution. Rietveld analysis clarified that four kinds of halogen sites in the MgGa2Cl7Br crystal were occupied by both a Br− ion and Cl− ion. There was a site with a relatively high Br− occupancy, but no particular ordering was observed. MgGa2Cl7Br showed the highest conductivity, which was 1.6 × 10−5 S/cm at 400 K in the measurement of AC conductivity. The behavior of the Nyquist plots and the results of fluorescent X-ray analysis, observed in the cell using Mg as the electrode, suggest that these compounds are magnesium ion conductors. The conductivity value of MgGa2Cl7Br was high as an Mg ion conductor but was slightly insufficient for use as a solid electrolyte in Mg secondary batteries. With this conductivity value, there is concern that voltage drop and energy loss will occur when MgAl2Cl7Br is used as the electrolyte for solid-state batteries and a further increase in conductivity is required. In this study, it was shown that the anion substitution effect can improve Mg2+ ion conductivity. Further studies are expected to improve the ion conductivity.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/13/24/6687/s1, Figure S1: Result of Rietveld analysis of XRD pattern of MgGa2Cl8. The red dotted line, the black line, and the blue line are the observed XRD patterns, the calculated values, and the differences between the observed and the calculated value, respectively. The marks indicate the position of the XRD peaks, Figure S2: Plots of σT against 1/T of MgAl2-xGaxCl8 and MgGa2Cl7Br. The activation energy of the conductivity was evaluated from the linear part over a wide temperature range including the median of the plot of each compound, Table S1: Results of the Rietveld analysis of XRD data for MgAl2-xGaxCl8, Table S2: Crystallographic data for MgGa2Cl8 and MgGa2Cl7Br, Table S3: Coordinates and occupancy refined by Rietveld analysis for MgGa2Cl8 and MgGa2Cl7Br.
Author Contributions
Conceptualization, Y.T.; methodology, Y.T. and R.S.; validation, Y.T., and Y.Y.; formal analysis, R.S. and A.N.; investigation, Y.T.; data curation, Y.T., A.N. and Y.Y.; writing—original draft preparation, Y.T.; writing—review and editing, Y.T. and Y.K.; supervision, Y.T.; project administration, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724. [Google Scholar] [CrossRef]
- Saha, P.; Datta, M.K.; Velikokhatnyi, O.I.; Manivannan, A.; Alman, D.; Kumta, P.N. Rechargeable magnesium battery: Current status and key challenges for the future. Prog. Mater. Sci. 2014, 66, 1–86. [Google Scholar] [CrossRef]
- Matsui, M. Study on electrochemically deposited Mg metal. J. Power Sources 2011, 196, 7048–7055. [Google Scholar] [CrossRef]
- Gregory, T.; Hoffman, R.J.; Winterton, R.C. Nonaqueous Electrochemistry of Magnesium: Applications to Energy Storage. J. Electrochem. Soc. 1990, 137, 775–780. [Google Scholar] [CrossRef]
- Orikasa, Y.; Masese, T.; Koyama, Y.; Mori, T.; Hattori, M.; Yamamoto, K.; Okado, T.; Huang, Z.D.; Minato, T.; Tassel, C.; et al. High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 2014, 4, 5622. [Google Scholar] [CrossRef] [PubMed]
- Sagane, F.; Ogi, K.; Konno, A.; Egashira, M.; Kanamura, K. The Effect of the Cyclic Ether Additives to the Ethereal Electrolyte Solutions for Mg Secondary Battery. Electrochemistry 2016, 84, 76–78. [Google Scholar] [CrossRef]
- Omote, A.; Yotsuhashi, S.; Zenitani, Y.; Yamada, Y. High Ion Conductivity in MgHf (WO4)3 Solids with Ordered Structure: 1-D Alignments of Mg2+ and Hf4+ Ions. J. Am. Ceram. Soc. 2011, 94, 2285–2288. [Google Scholar] [CrossRef]
- Tamura, S.; Yamane, M.; Hoshino, Y.; Imanaka, N. Highly conducting divalent Mg2þ cation solid electrolytes with well-ordered three-dimensional network structure. J. Solid State Chem. 2016, 235, 7–11. [Google Scholar] [CrossRef]
- Yamanaka, T.; Hayashi, A.; Yamauchi, A.; Tatsumisago, M. Preparation of magnesium ion conducting MgS–P2S5–MgI2 glasses by a mechanochemical technique. Solid State Ion. 2014, 262, 601–603. [Google Scholar] [CrossRef]
- Ruyet, R.L.; Berthelot, R.; Salager, E.; Florian, P.; Fleutot, B.; Janot, R. Investigation of Mg(BH4)(NH2)-Based Composite Materials with Enhanced Mg2+ Ionic Conductivity. J Phys. Chem. C 2019, 123, 10756–10763. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Inukai, M.; Oguchi, H.; Takagi, S.; Orimo, S. Magnesium Borohydride Ammonia Borane as a Magnesium Ionic Conductor. ACS Appl. Energy Mater. 2020, 3, 3174–3179. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg Batteries with Graphene-like MoS2 Cathode and Ultrasmall Mg Nanoparticle Anode. Adv. Mater. 2011, 23, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.D.; Devaraju, M.K.; Tran, P.D.; Gambe, Y.; Nayuki, K.; Sasaki, Y.; Honma, I. Unravelling the Surface Structure of MgMn2O4 Cathode Materials for Rechargeable Magnesium-Ion Battery. Chem. Mater. 2017, 29, 6245–6251. [Google Scholar] [CrossRef]
- Tomita, Y.; Ohki, H.; Yamada, K.; Okuda, T. Ionic conductivity and structure of halocomplex salts of group 13 elements. Solid State Ion. 2000, 136–137, 351–355. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R.A. Ionic conductivity of alkali metal chloroaluminates. Phys. Lett. A 1976, 58, 245–248. [Google Scholar] [CrossRef]
- Mairesse, G.; Barbier, P.; Wignacourt, J.P. Comparison of the crystal structures of alkaline (M = Li,Na,K,Rb,Cs) and pseudo-alkaline (M = NO,NH4) tetrachloroaluminates, MAlCl4. Acta. Cryst. B 1979, 35, 1573–1580. [Google Scholar] [CrossRef]
- Tomita, Y.; Yamada, K.; Ohki, H.; Okuda, T. Structure and Dynamics of Li3InBr6 and NaInBr4 by Means of Nuclear Magnetic Resonance. Bull. Chem. Soc. Jpn. 1997, 70, 2405–2410. [Google Scholar] [CrossRef]
- Einarsrud, M.A.; Justnes, H.; Rytter, E.; Øye, H.A. Structure and stability of solid and molten complexes in the MgCl2-AlCl3 system. Polyhedron 1987, 6, 975–986. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Study of Inter Atomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chandra, S. Superionic Solid, Principles and Applications; North-Holland Publishing Company: Amsterdam, The Netherlands, 1981; p. 225. [Google Scholar]
- Imanaka, N.; Okazaki, Y.; Adachi, G. Divalent Magnesium Ionic Conduction in Mg1−2x(Zr1−xNbx)4P6O24(x = 0–0.4) Solid Solutions. Electrochem. Solid-State Lett. 2000, 3, 327. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).