Real-Time Monitoring of the Thermal Effect for the Redox Flow Battery by an Infrared Thermal Imaging Technology
Abstract
:1. Introduction
2. Experimental
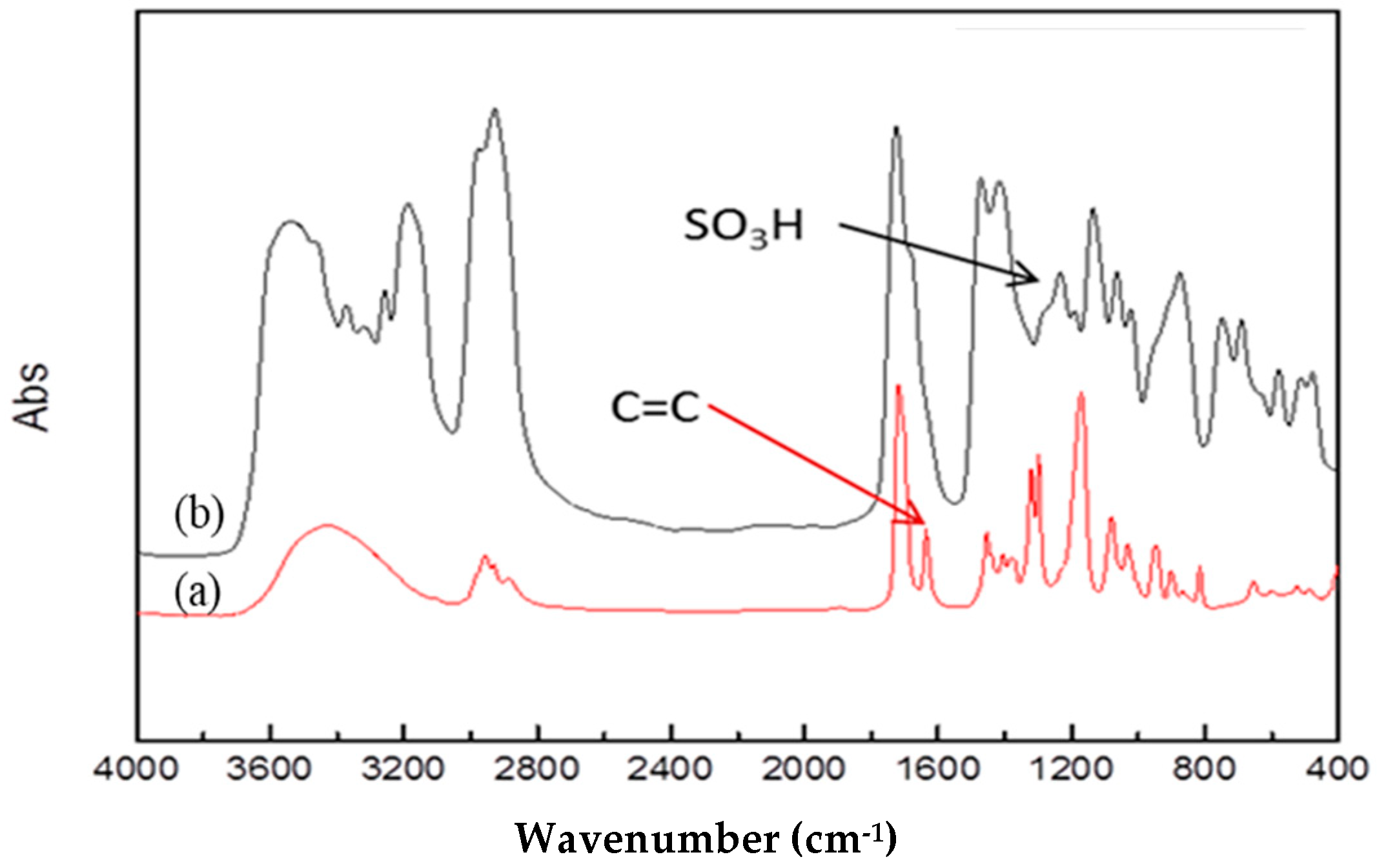
2.1. Synthesis and Basic Property Measurement of HS-SO3H Membrane
2.1.1. Ion Exchange Capacity
2.1.2. Water Uptake
2.1.3. Dynamic Permeability
2.2. Fabrication of C-TiO2-Pd Electrode
2.3. The Preparation of Iodine/Ascorbic Acid Electrolyte Solution
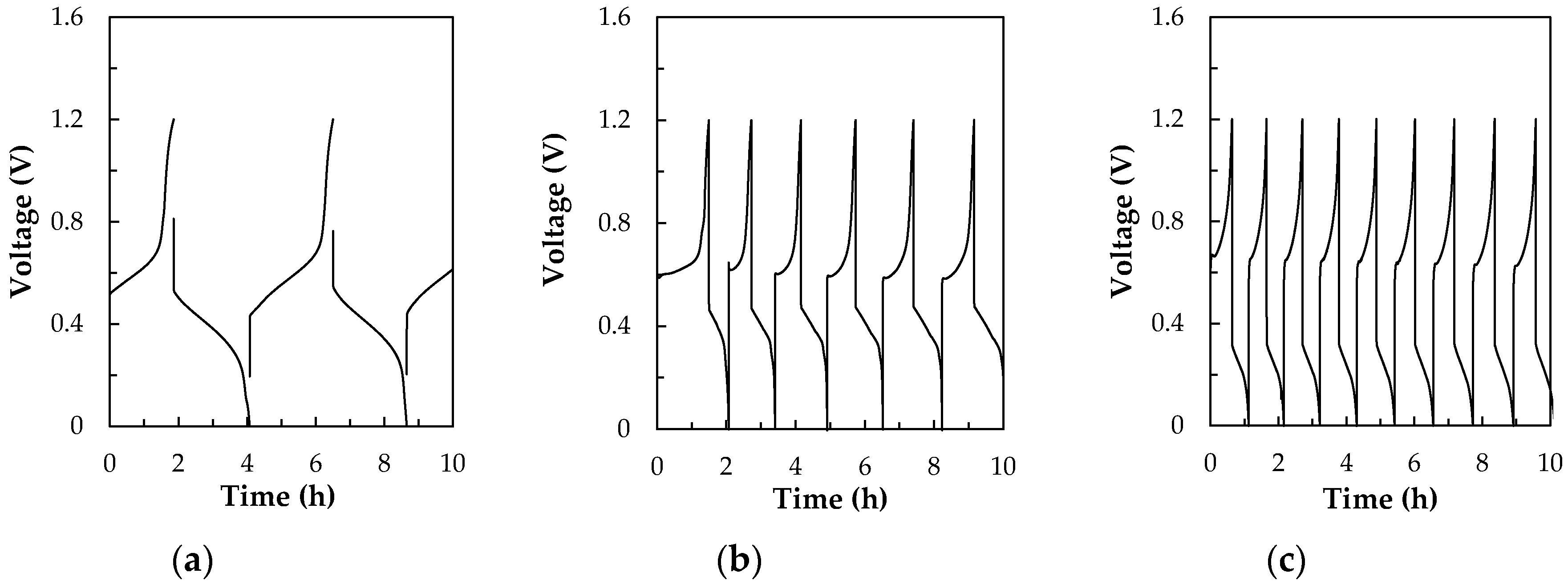
2.4. Charge-discharge Test Apparatus
3. Results and Discussion
3.1. Charge-discharge Test and Thermal Effect of All-V RFB and V/I RFB Systems
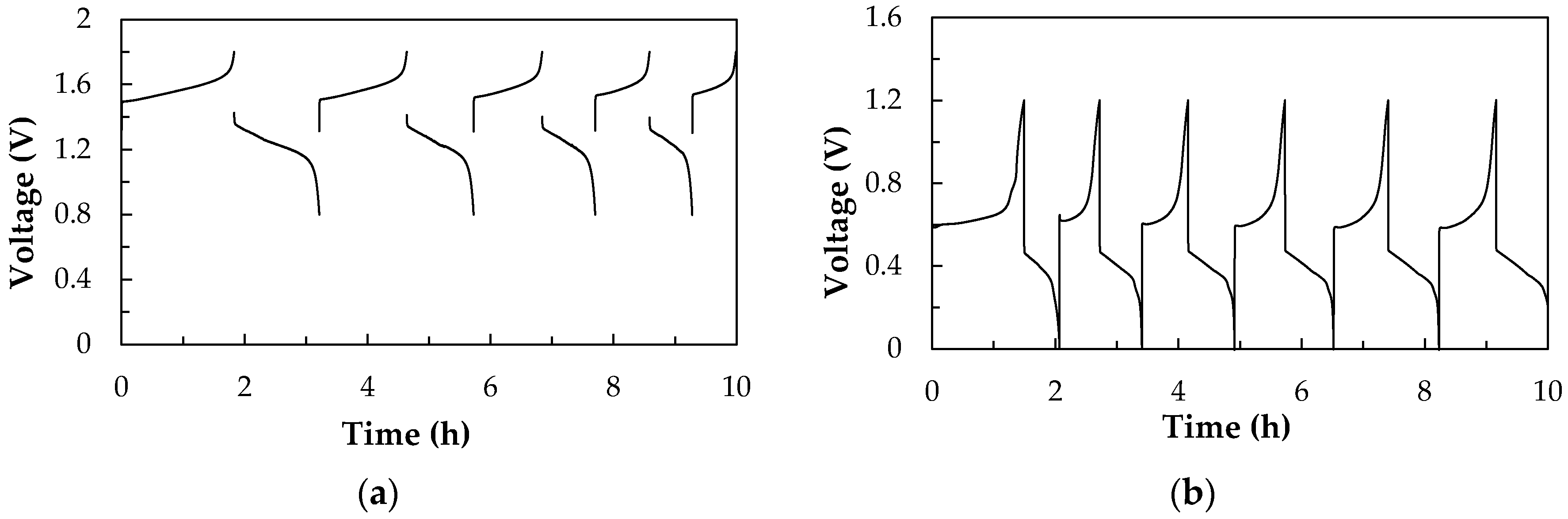
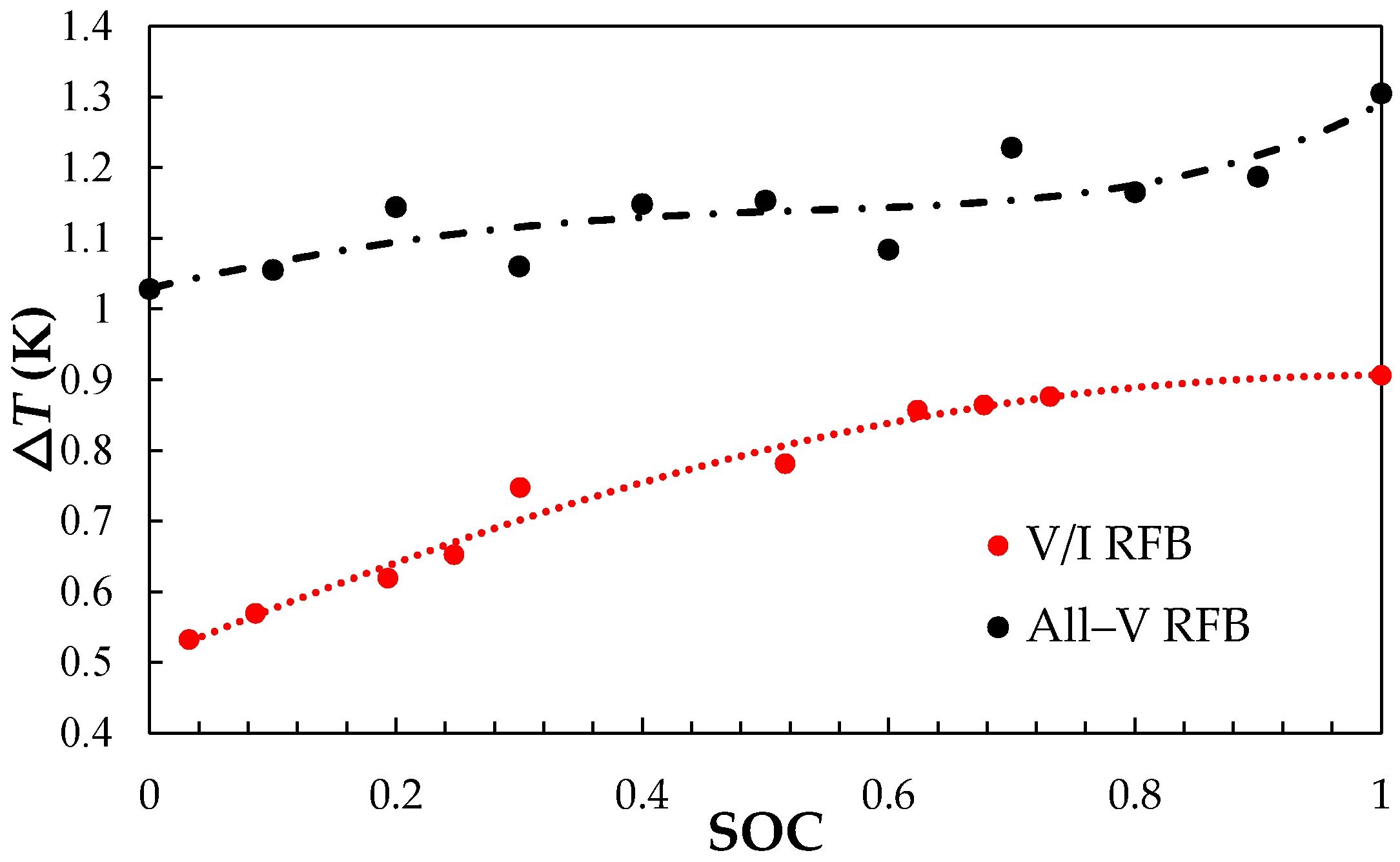
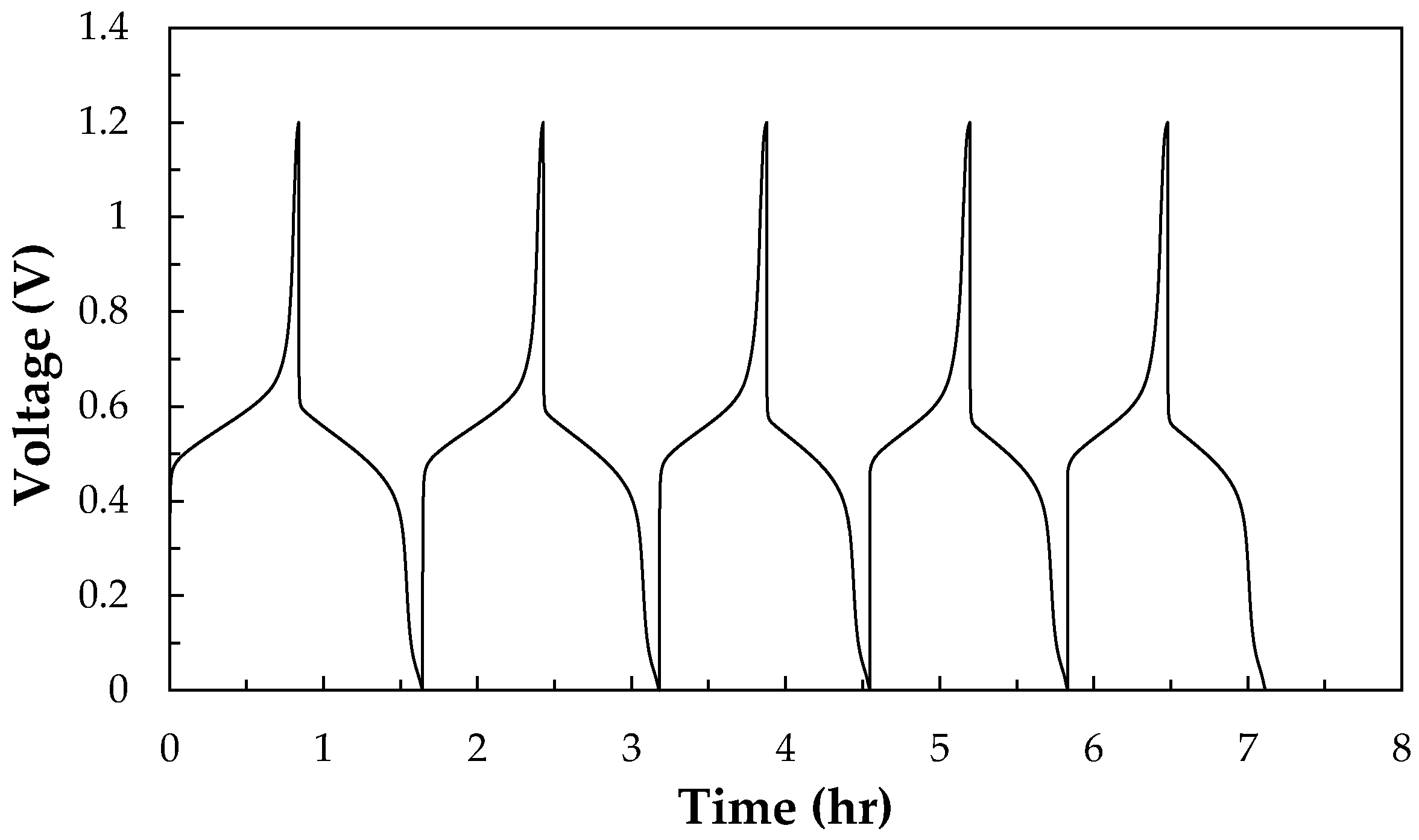
3.1.1. Charge-discharge Performances and Thermal Effect of All-V RFB and V/I RFB Systems
For All-V RFB System
- Positive reaction
- Negative reaction
- Overall reaction= 155.6 KJ/mole.
For V/I RFB System
- Positive reaction
- Negative reaction
- Overall reaction= 67.4 KJ/mole.
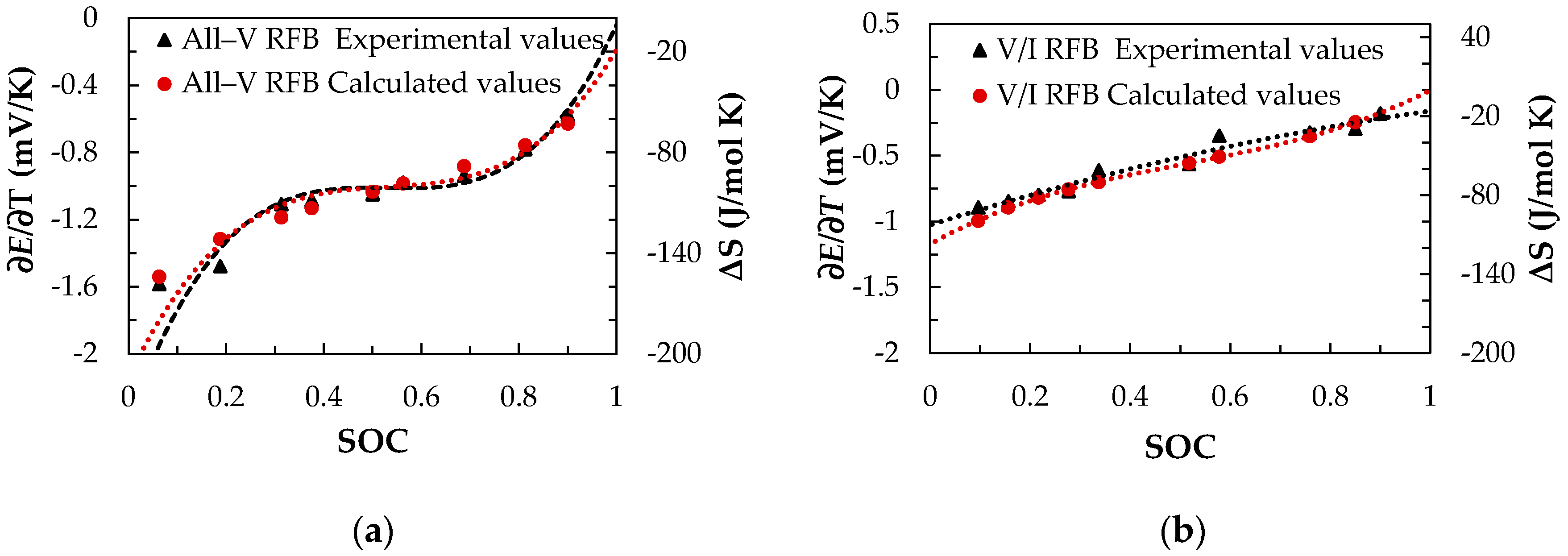
3.1.2. Measurement of the Temperature Coefficient (∂E/∂T) and Entropy Values
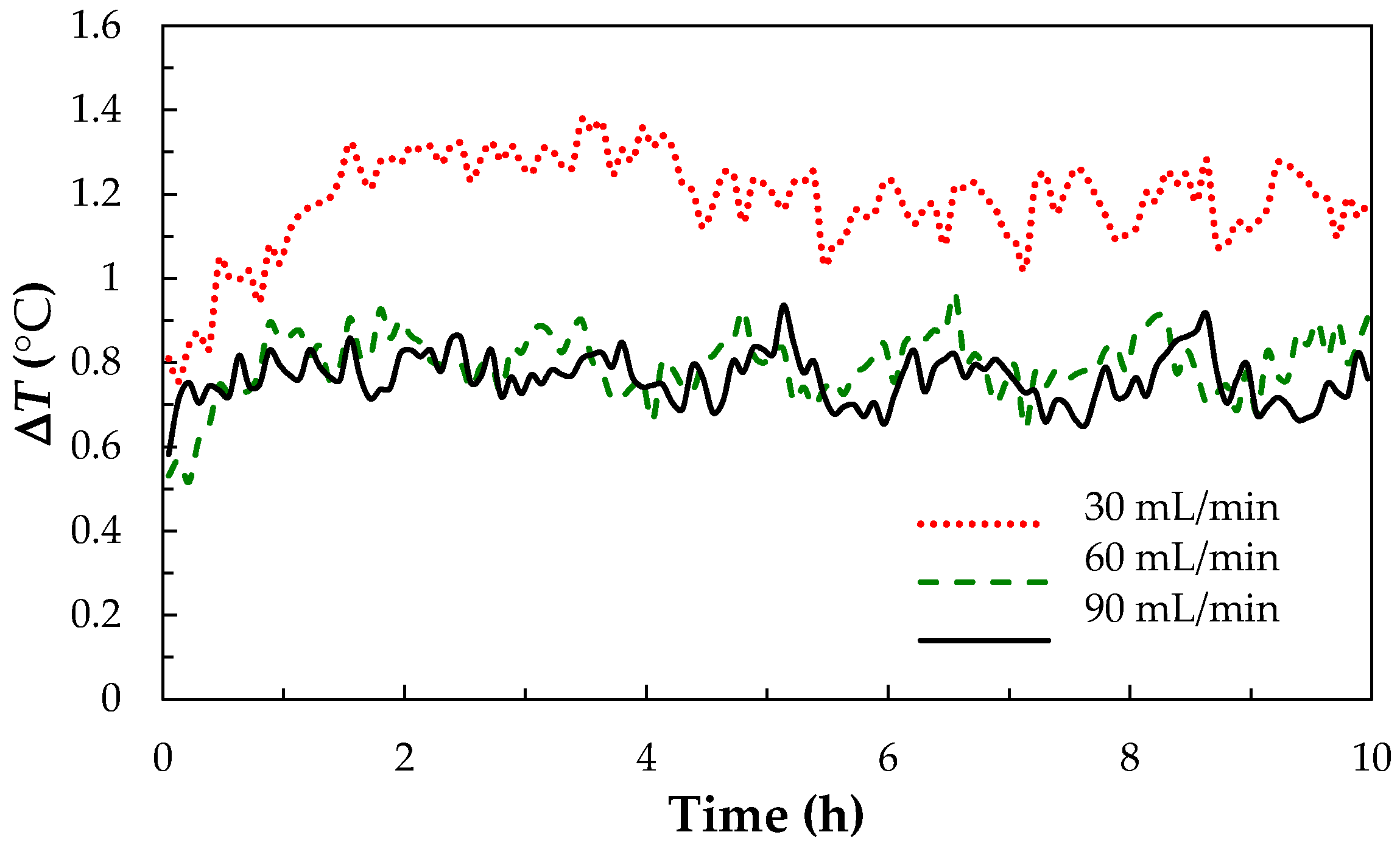
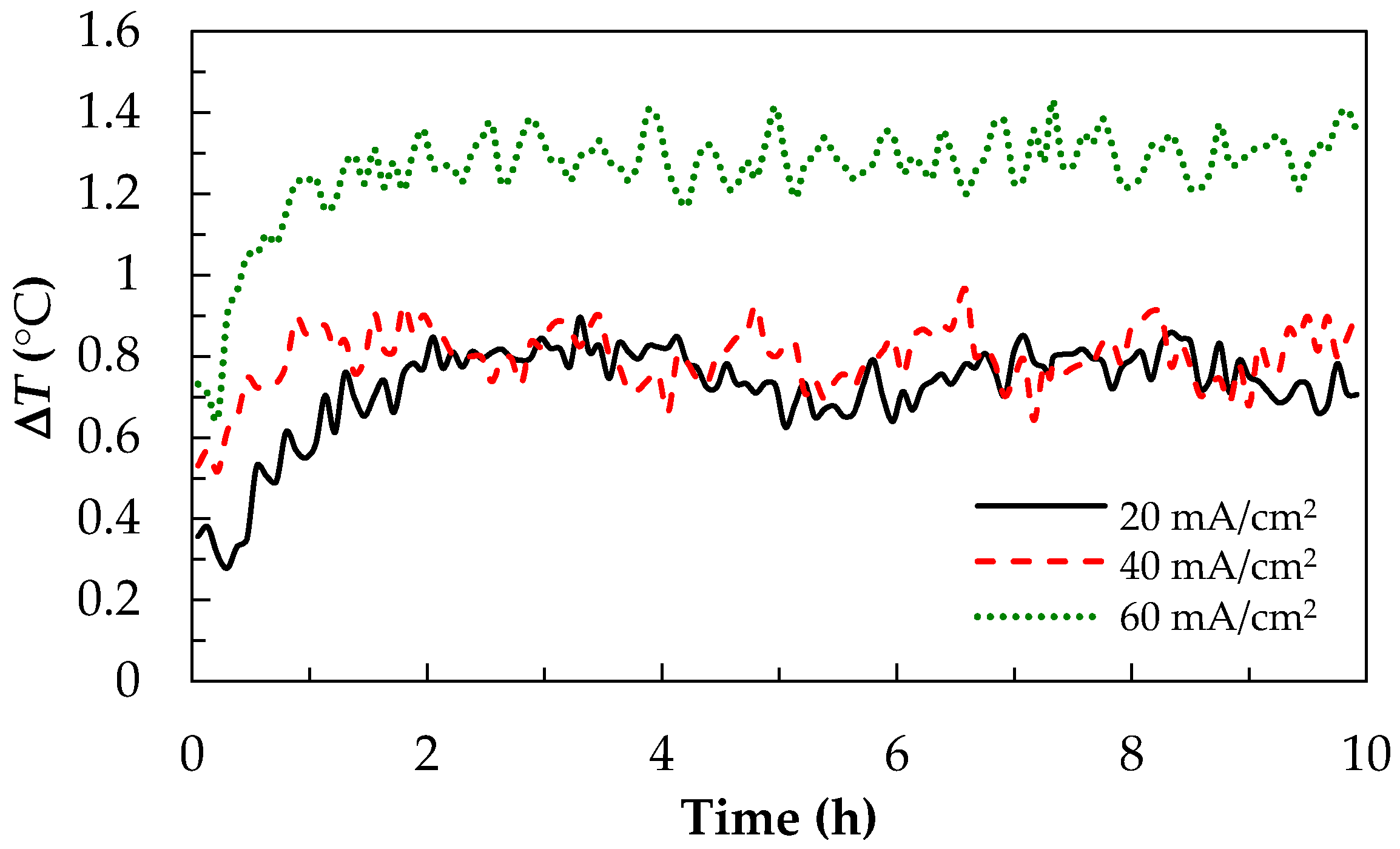
3.2. Thermal Effect of Charge-discharge Parameters for V/I RFB System
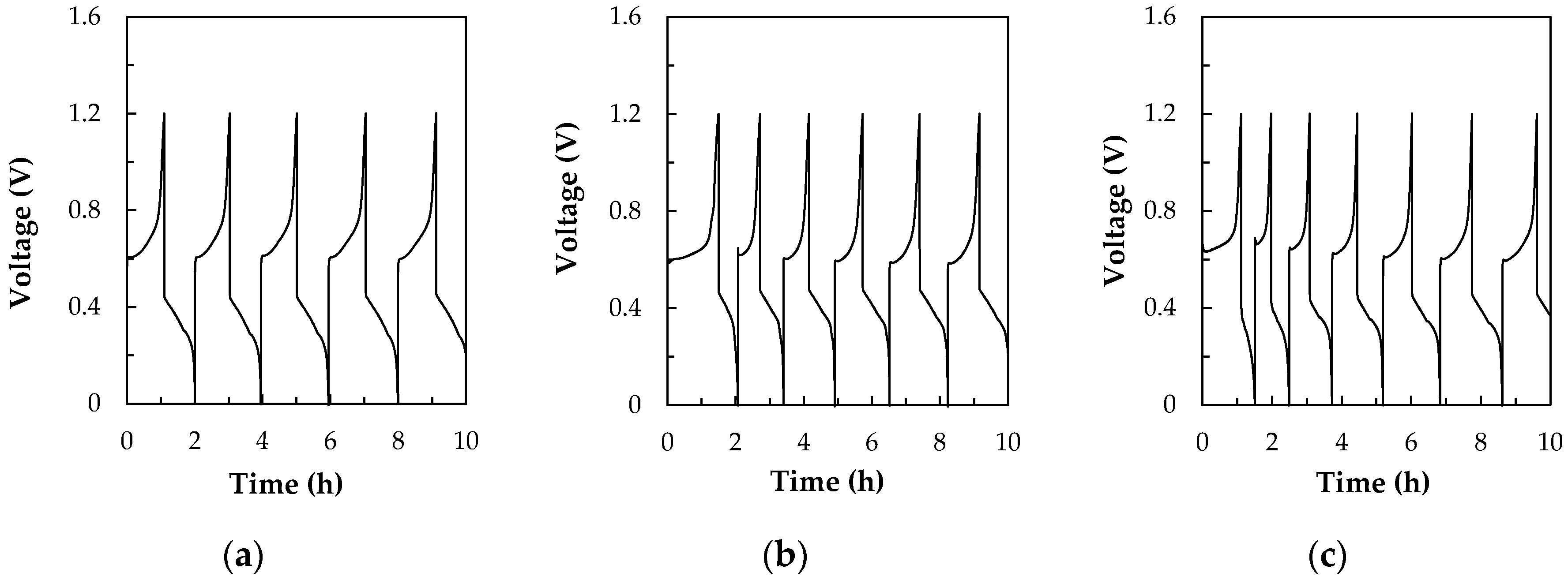
3.2.1. The Flow Rate Optimization
3.2.2. The Current Density Effect of the Charge-Discharge Test
3.2.3. The Reynold’s Number Effect
3.3. The Charge-Discharge Test of a V/I RFB by Modified Key Materials
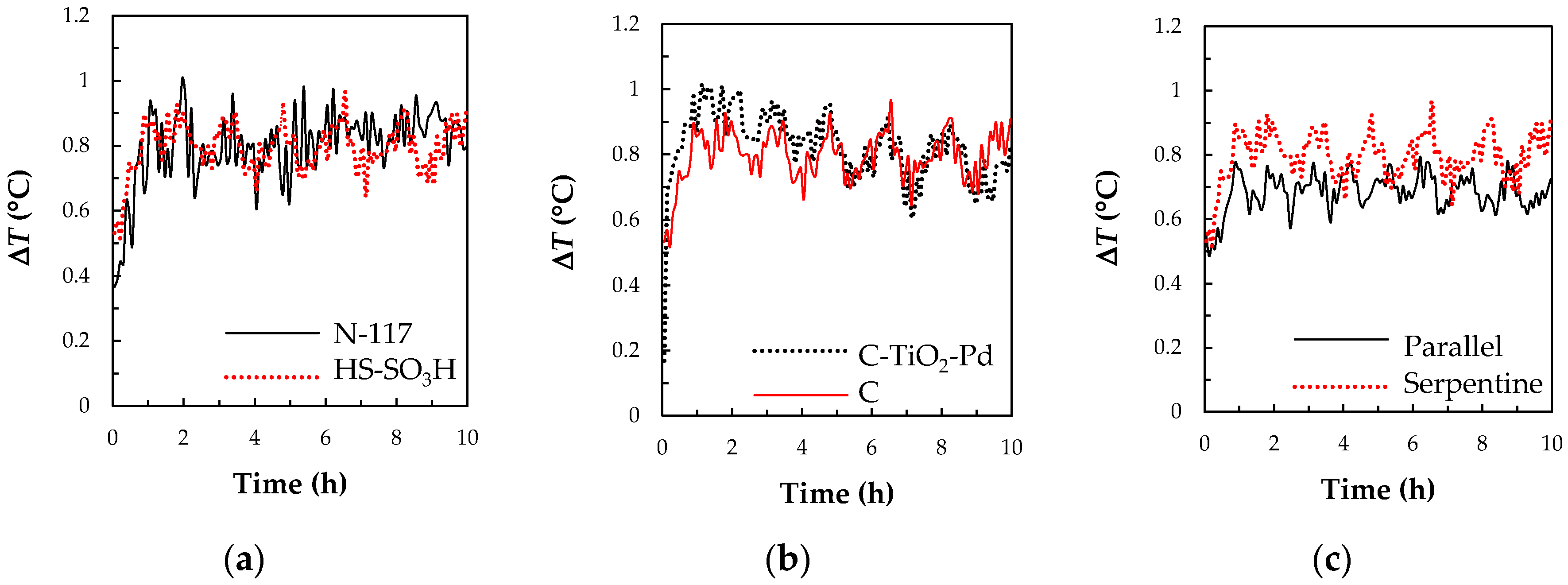
3.3.1. The Separation Membrane
3.3.2. The C-TiO2-Pd Composite Electrode
3.3.3. The Flow Channel Types
3.4. The Cell Performances of a V/I RFB System with the Key Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lucas, A.; Chondrogiannis, S. Smart grid energy storage controller for frequency regulation and peak shaving, using a vanadium redox flow battery. Int. J. Electr. Power Energy Syst. 2016, 80, 26–36. [Google Scholar] [CrossRef]
- Kazacos, M.; Cheng, M.; Skyllas-Kazacos, M. Vanadium redox cell electrolyte optimization studies. J. Appl. Electrochem. 1990, 20, 463–467. [Google Scholar] [CrossRef]
- Sha’rani, S.S.; Abouzari-Lotf, E.; Nasef, M.M.; Ahmad, A.; Ting, T.M.; Ali, R.R. Improving the redox flow battery performance of low-cost thin polyelectrolyte membranes by layer-by-layer surface assembly. J. Power Sources 2019, 413, 182–190. [Google Scholar] [CrossRef]
- Holland-Cunz, M.V.; Friedl, J.; Stimming, U. Anion effects on the redox kinetics of positive electrolyte of the all-vanadium redox flow battery. J. Electroanal. Chem. 2018, 819, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, X.; Long, R.; Si, J.; Liu, C.; Ma, S. Preparation and properties of amphoteric ion exchange membrane for all vanadium redox flow batteries. Results Phys. 2019, 14, 102373. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhang, J.; Chen, J.; Zhu, S.; Liu, X.; Wang, R. Effect of different additives with -NH2 or -NH4+ functional groups on V(V) electrolytes for a vanadium redox flow battery. J. Electroanal. Chem. 2016, 768, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, M.; Wai, N.; Veksha, A.; Giannis, A.; Lim, T.M.; Lisak, G. Sal wood sawdust derived highly mesoporous carbon as prospective electrode material for vanadium redox flow batteries. J. Electroanal. Chem. 2019, 834, 94–100. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Skyllas-Kazacos, M.; Xiong, B. Dynamic thermal-hydraulic modeling and stack flow pattern analysis for all-vanadium redox flow battery. J. Power Sources 2014, 260, 89–99. [Google Scholar] [CrossRef]
- Trovò, A.; Saccardo, A.; Giomo, M.; Guarnieri, M. Thermal modeling of industrial-scale vanadium redox flow batteries in high-current operations. J. Power Sources 2019, 424, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Xiong, B.; Yang, Y.; Tang, J.; Li, Y.; Wei, Z.; Su, Y.; Zhang, Q. An enhanced equivalent circuit model of vanadium redox flow battery energy storage systems considering thermal effects. IEEE Access 2019, 7, 162297–162308. [Google Scholar] [CrossRef]
- Trovò, A.; Picano, F.; Guarnieri, M. Comparison of energy losses in a 9 kW vanadium redox flow battery. J. Power Sources 2019, 440, 227144. [Google Scholar] [CrossRef]
- Guarnieri, M.; Trovò, A.; Picano, F. Enhancing the efficiency of kW-class vanadium redox flow batteries by flow factor modulation: An experimental method. Appl. Energy 2020, 262, 114532. [Google Scholar] [CrossRef]
- Pugach, M.; Parsegov, S.; Gryazina, E.; Bischi, A. Output feedback control of electrolyte flow rate for vanadium redox flow batteries. J. Power Sources 2020, 455, 227916. [Google Scholar] [CrossRef]
- Trovò, A.; Guarnieri, M. Standby thermal management system for a kW-class vanadium redox flow battery. Energy Convers. Manag. 2020, 226, 113510. [Google Scholar] [CrossRef]
- Al-Fetlawi, H.; Shah, A.A.; Walsh, F.C. Non-isothermal modelling of the all-vanadium redox flow battery. Electrochim. Acta 2009, 55, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhang, H.; Xing, F.; Ma, X.; Li, X.; Ning, G. A three-dimensional model for thermal analysis in a vanadium flow battery. J. Appl. Energy 2014, 113, 1675–1685. [Google Scholar] [CrossRef]
- Wei, Z.B.; Zhao, J.Y.; Xiong, B.Y. Dynamic electro-thermal modeling of all-vanadium redox flow battery with forced cooling strategies. Appl. Energy 2014, 135, 1–10. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, J.; Tseng, K.J.; Skyllas-Kazacos, M.; Lim, T.M.; Zhang, Y. Thermal hydraulic behavior and efficiency analysis of an all-vanadium redox flow battery. J. Power Sources 2013, 242, 314–324. [Google Scholar] [CrossRef]
- Hudak, N.S. Practical thermodynamic quantities for aqueous vanadium-and iron-based flow batteries. J. Power Sources 2014, 269, 962–974. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, J.; Jordan, J. Standard Potentials in Aqueous Solution, 1st ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Driggers, R.G.; Friedman, M.H.; Nichols, J.M. Introduction to Infrared and Electro-Optical Systems, 2nd ed.; Artech House: Norwood, MA, USA, 2012. [Google Scholar]
- Meola, C. Infrared Thermography Recent Advances and Future Trends; Bentham Science Publishers: Sharjah, UAE, 2012. [Google Scholar]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F.G. Infrared thermography for temperature measurement and non-destructive testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, M.; Möllmann, K.P. Infrared Thermal Imaging: Fundamentals, Research and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Jadin, M.S.; Taib, S. Recent progress in diagnosing the reliability of electrical equipment by using infrared thermography. Infrared Phys. Technol. 2012, 55, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Huang, F.Z. A novel intelligent fault diagnosis method for electrical equipment using infrared thermography. Infrared Phys. Technol. 2015, 73, 29–35. [Google Scholar] [CrossRef]
- Huda, A.S.N.; Taib, S. Application of infrared thermography for predictive/preventive maintenance of thermal defect in electrical equipment. Appl. Therm. Eng. 2013, 61, 220–227. [Google Scholar] [CrossRef]
- Kateb, B.; Yamamoto, V.; Yu, C.; Grundfest, W.; Gruen, J.P. Infrared thermal imaging: A review of the literature and case report. NeuroImage 2009, 47, T154–T162. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, S.L.; Chen, M.L.; Tsai, T.J.; Lin, Y.S. Improving electrochemical activity in a semi-V-I redox flow battery by using a C-TiO2-Pd composite electrode. J. Nanomater. 2019, 2019, 7460856. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.L.; Yu, H.F.; Lin, Y.S. Modification of Nafion® membrane via a sol-gel route for vanadium redox flow energy storage battery applications. J. Chem. 2017, 2017, 4590952. [Google Scholar] [CrossRef]
- Wang, W.; Kim, S.; Chen, B.; Nie, Z.; Zhang, J.; Xia, G.-G.; Li, L.; Yang, Z. A new redox flow battery using Fe/V redox couples in chloride supporting electrolyte. Energy Environ. Sci. 2011, 4, 4068–4073. [Google Scholar] [CrossRef]
- Sankarasubramanian, S.; Zhang, Y.; Ramani, V. Methanesulfonic acid-based electrode-decoupled vanadium-cerium redox flow battery exhibits significantly improved capacity and cycle life. Sustain. Energy Fuels 2019, 3, 2417–2425. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M. Novel vanadium chloride/polyhalide redox flow battery. J. Power Sources 2003, 124, 299–302. [Google Scholar] [CrossRef]
- Huang, S.L.; Chen, M.L.; Lin, Y.S. Chitosan-silica anion exchange membrane for the vanadium redox flow energy storage battery applications. React. Funct. Polym. 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, M.C.; Wei, H.J. Amino-silica modified Nafion membrane for vanadium redox flow battery. J. Power Sources 2015, 282, 562–571. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Burton, S.D.; Huang, C.; Li, L.; Yang, Z.; Graff, G.L.; Liu, J.; Hu, J.; Skyllas-Kazacos, M. Nuclear magnetic resonance studies on vanadium(IV) electrolyte solutions for vanadium redox flow battery. J. Power Sources 2010, 195, 7709–7717. [Google Scholar] [CrossRef]
- Manohar, A.K.; Kim, K.M.; Plichta, E.; Hendrickson, M.; Rawlings, S.; Narayanan, S.R. A high efficiency iron-chloride redox flow battery for large-scale energy storage. J. Electrochem. Soc. 2016, 163, A5118–A5125. [Google Scholar] [CrossRef]
- Lu, W.; Yang, H.; Prakash, J. Determination of the reversible and irreversible heats of LiNi0.8Co0.2O2/mesocarbon microbead Li-ion cell reactions using isothermal microcalorimetery. Electrochim. Acta 2006, 51, 1322–1329. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176–A192. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 1st English ed.; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Hill, J.O.; Worsley, I.G.; Hepler, L.G. Thermochemistry and oxidation potentials of vanadium, niobium, and tantalum. Chem. Rev. 1971, 71, 127–137. [Google Scholar]
- Bratsch, S.G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Heintz, A.; Illenberger, C. Thermodynamics of vanadium redox flow batteries-electrochemical and calorimetric investigations. Berichte der Bunsengesellschaft für phyikalische Chemie 1998, 102, 1401–1409. [Google Scholar] [CrossRef]
- Ling, C.Y.; Cao, H.; Chng, M.L.; Han, M.; Birgersson, E. Pulsating electrolyte flow in a full vanadium redox battery. J. Power Sources 2015, 294, 305–311. [Google Scholar] [CrossRef]
- Wang, Z.; Sankarasubramanian, S.; Ramani, V. Reactant-transport engineering approach to high-power direct borohydride fuel cells. Cell Rep. Phys. Sci. 2020, 1, 100084. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Shell momentum balances and velocity distributions in laminar flow. In Transport Phenomena, revised 2nd ed.; Anderson, W., Kulek, P., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2007; p. 46. [Google Scholar]
- Vargün, E.; Usanmaz, A. Degradation of poly(2-hydroxyethyl methacrylate) obtained by radiation in aqueous solution. J. Macromol. Sci. Part A 2016, 47, 882–891. [Google Scholar] [CrossRef]
- Chen, T.S.; Huang, S.L.; Ye, Z.Y.; Lin, Y.S.; Li, C.P. A novel Fe (II/0) redox couple with key components for the V/Fe redox flow battery. J. Electroanal. Chem. 2019, 850, 113396. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, G.; Li, A.; Wei, X.; Li, F.; Zhang, J.; Chen, J.; Wang, R. Novel sulfonated poly(ether ether ketone)/triphenylamine hybrid membrane for vanadium redox flow battery applications. RSC Adv. 2019, 9, 3838–3846. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Tay, Z.Y.; Li, S.F.Y. Highly selective sulfonated poly(ether ether ketone)/titanium oxide composite membranes for vanadium redox flow batteries. J. Membr. Sci. 2017, 539, 197–205. [Google Scholar] [CrossRef]


| 9RFB | CE (%) | VE (%) | EE (%) | Discharge Capacity (mAh) | ΔTmax (°C) |
|---|---|---|---|---|---|
| all-V RFB | 79 | 79 | 62 | 416 | 1.31 |
| V/I RFB | 93 | 61 | 57 | 495 | 0.90 |
| Formula | State | (kJ/mol) | (kJ/mol) | (J/mol.K) |
|---|---|---|---|---|
| aq | −226.0 | −218.0 | −130.0 | |
| aq | −259.0 | −251.3 | −230.0 | |
| aq | −486.6 | −446.4 | −133.9 | |
| aq | −649.8 | −587.0 | −42.3 | |
| aq | −55.2 | −51.6 | 111.3 | |
| s | 0 | 0 | 116.1 | |
| aq | −285.8 | −237.2 | 69.9 | |
| aq | 0 | 0 | 0 | |
| All-V RFB | −155.6 | −129.9 | −121.7 | |
| V/I RFB | −67.4 | −45.0 | −75.0 |
| Source | Year | ΔG of Cell Reaction (kJ/mol) | E of Cell (V) | ΔS of Cell Reaction (J/mol.K) | ∂E/∂T of Cell (mV/K) | Remarks |
|---|---|---|---|---|---|---|
| All-V RFB | ||||||
| Pourbaix book [40] | 1966 | −121 | 1.26 | N/A | N/A | Standard values |
| Hill et al. review [41] | 1971 | −120 | 1.25 | −120 | −1.2 | Standard values |
| Bard et al. book [20] | 1985 | −130 | 1.34 | −120 | −1.2 | Standard values |
| Bratsch tables [42] | 1989 | −121 | 1.26 | −230 | −2.4 | Standard values |
| Heintz and Illenberger [43] | 1998 | −119.8 | 1.242 | −156 | −1.62 | Experimental results based on calorimetry and ∂E/∂T measurement |
| Nicholas S. Hudak [19] | 2013 | −138 | 1.32 | −83.3 | −1.22 | Formal potential (22 °C) and dE/dT (22–80 °C) measured at 13.3% SOC |
| This work * | 2020 | −126 | 1.31 | −113 | −1.15 | |
| V/I RFB | ||||||
| Bard et al. book [29] | 1985 | −45 | 0.47 | −75 | N/A | Standard values |
| This work * | 2020 | −49 | 0.51 | −75.3 | −0.78 |
| Flow Field | Volumetric Flow Rate (mL/min) | Flow Velocity (m/s) | Re | Current Density (20 mA/cm2) | Current Density (40 mA/cm2) | Current Density (60 mA/cm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE (%) | VE (%) | EE (%) | ΔTmax | CE (%) | VE (%) | EE (%) | ΔTmax | CE (%) | VE (%) | EE (%) | ΔTmax | ||||
| Type A | 30 | 0.25 | 250 | - | - | - | - | 85 | 51 | 43 | 1.378 | - | - | - | - |
| 60 | 0.50 | 500 | 93 | 60 | 56 | 0.832 | 93 | 61 | 57 | 0.936 | 91 | 17 | 16 | 1.387 | |
| 90 | 0.75 | 750 | - | - | - | - | 91 | 55 | 50 | 0.967 | - | - | - | - | |
| Type B | 30 | 0.02 | 20 | - | - | - | - | - | - | - | - | - | - | - | - |
| 60 | 0.04 | 40 | - | - | - | - | 94 | 34 | 32 | 0.779 | - | - | - | - | |
| 90 | 0.06 | 60 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Samples | Water Uptake (wt%) | IEC (mmol/g) | Dynamic Permeability (×106 mL/s) |
|---|---|---|---|
| N-117 | 21 | 0.99 | 7.00 |
| HS-SO3H | 38 | 1.93 | 3.87 |
| Key materials | Types | CE (%) | VE (%) | EE (%) |
|---|---|---|---|---|
| Separation membrane | N-117 | 93 | 60 | 56 |
| HS-SO3H | 94 | 60 | 57 | |
| Negative electrode | Carbon paper (C) | 94 | 60 | 57 |
| C-TiO2-Pd | 93 | 81 | 75 | |
| Type of flow field | serpentine | 96 | 86 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-L.; Li, C.-P.; Chang, C.-C.; Tseng, C.-C.; Wang, M.-W.; Chen, M.-L. Real-Time Monitoring of the Thermal Effect for the Redox Flow Battery by an Infrared Thermal Imaging Technology. Energies 2020, 13, 6717. https://doi.org/10.3390/en13246717
Huang S-L, Li C-P, Chang C-C, Tseng C-C, Wang M-W, Chen M-L. Real-Time Monitoring of the Thermal Effect for the Redox Flow Battery by an Infrared Thermal Imaging Technology. Energies. 2020; 13(24):6717. https://doi.org/10.3390/en13246717
Chicago/Turabian StyleHuang, Shu-Ling, Chi-Ping Li, Chia-Chin Chang, Chen-Chen Tseng, Ming-Wei Wang, and Mei-Ling Chen. 2020. "Real-Time Monitoring of the Thermal Effect for the Redox Flow Battery by an Infrared Thermal Imaging Technology" Energies 13, no. 24: 6717. https://doi.org/10.3390/en13246717






