Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production
Abstract
:1. Introduction
2. Fundamental of Nanomaterials
3. Biomass
3.1. Lignocellulose for Conversion of Cellulose to Biofuel
3.2. Nanotechnology for Bioenergy Production from Microalgal Biomass
4. Impacts of Nanomaterial for Enhancement of Biofuels Production
4.1. Nanomaterial Incorporation as Nanocatalyst in Microalgae Processing
4.2. Nano-Additives Blended Biodiesel in Diesel Engines
5. Bioelectrochemical System (BES)
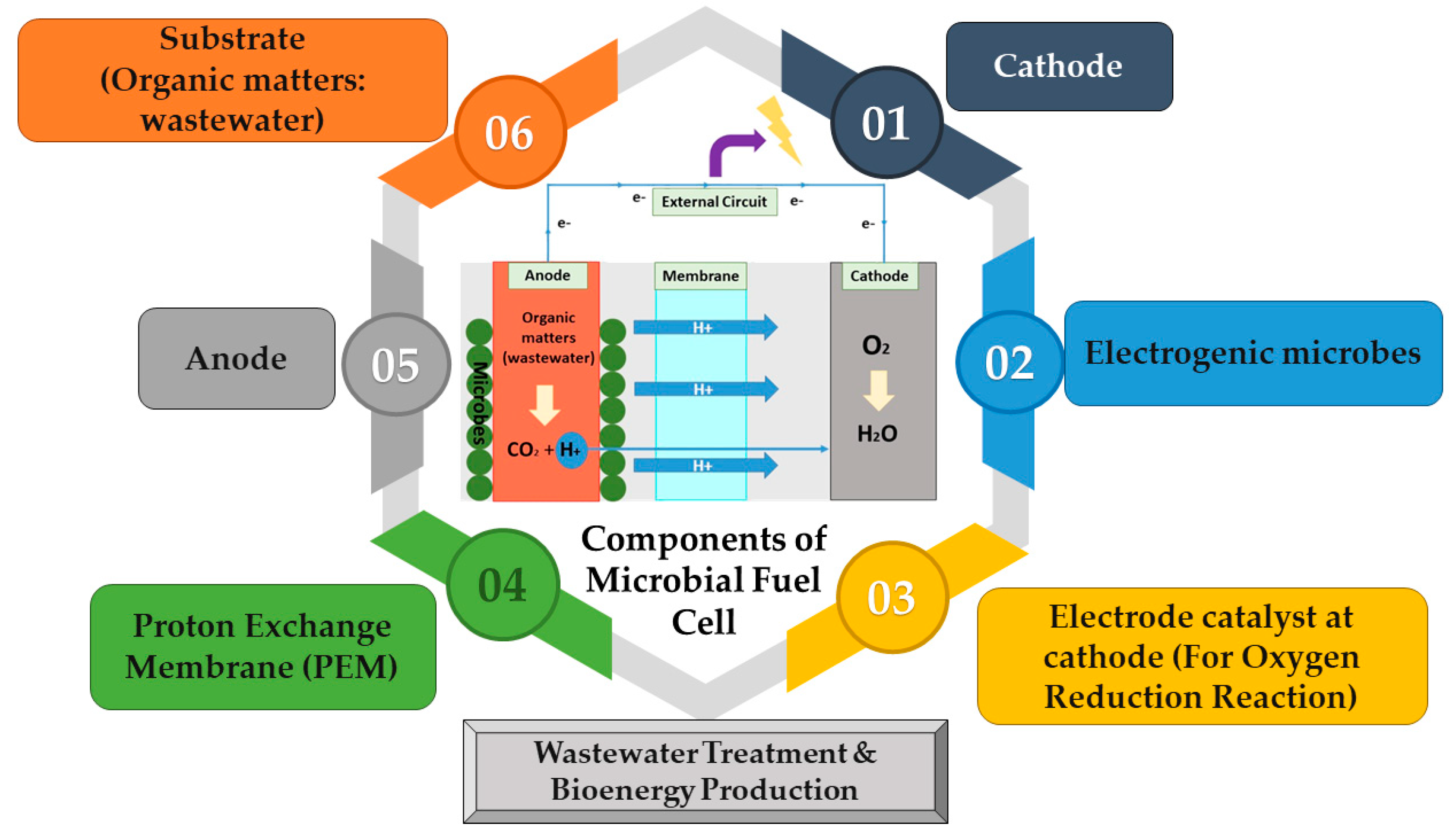
5.1. What Is MFC
- Good electrical conductivity;
- Good thermal stability;
- Low resistance;
- Good biocompatibility with the system;
- Strong stability and anti-corrosion toward the chemical used in MFC;
- Large surface area;
- Good mechanical strength;
- Low cost.
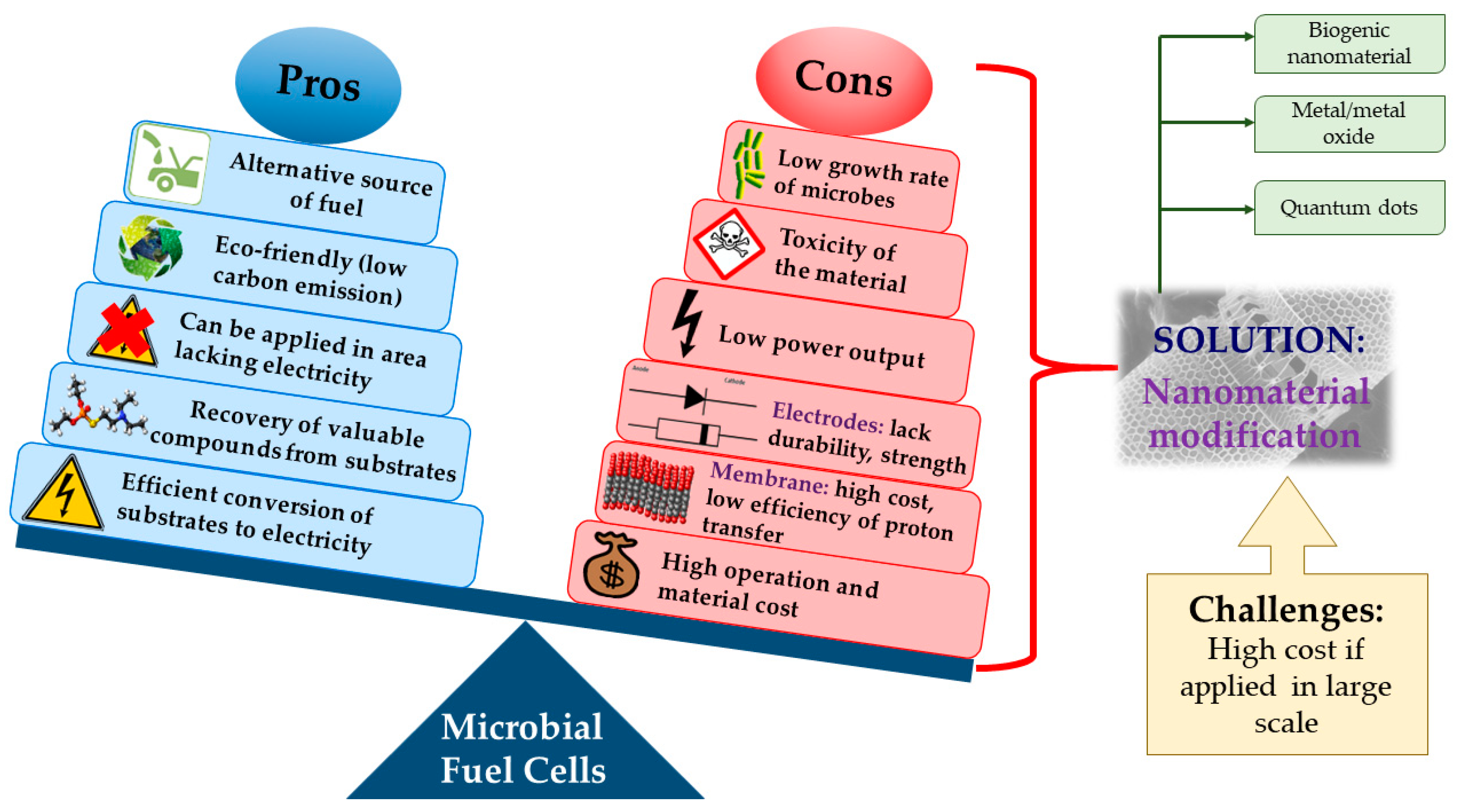
5.2. Modification of MFC Components with Nanomaterials
6. Future Works
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SS | Simultaneous saccharification |
| BES | Bioelectrochemical system |
| SSCF | Simultaneous saccharification and co-fermentation |
| CB | Consolidated bioprocessing |
| Si-NPs | Silica-based NPs |
| MS-NPs | Mesoporous silica NPs |
| EET | Extracellular electron transport |
| H+ | Proton |
| Ni-NPs | Nickel-based NPs |
| MFC | Microbial fuel cells |
| NPs | Nanoparticles |
| MNPs | Magnetic nanoparticles |
| ORR | Oxygen reduction reaction |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| SWCNTs | Single-walled carbon nanotubes |
| ROS | Reactive oxygen species |
| LCA | Life cycle analysis |
References
- Senjyu, T.; Howlader, A.M. Chapter 3—Operational aspects of distribution systems with massive DER penetrations. In Integration of Distributed Energy Resources in Power Systems; Funabashi, T., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 51–76. [Google Scholar]
- Lijó, L.; González-García, S.; Lovarelli, D.; Moreira, M.T.; Feijoo, G.; Bacenetti, J. Life Cycle Assessment of Renewable Energy Production from Biomass. In Life Cycle Assessment of Energy Systems and Sustainable Energy Technologies: The Italian Experience; Basosi, R., Cellura, M., Longo, S., Parisi, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–98. [Google Scholar]
- Strzalka, R.; Schneider, D.; Eicker, U. Current status of bioenergy technologies in Germany. Renew. Sustain. Energy Rev. 2017, 72, 801–820. [Google Scholar] [CrossRef]
- Li, Y.; Khanal, S.K. Bioenergy: Principles and Applications; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hallenbeck, P.C.; Grogger, M.; Mraz, M.; Veverka, D. Solar biofuels production with microalgae. Appl. Energy 2016, 179, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Rai, M.; dos Santos Júlio, C.; Soler Matheus, F.; Franco Marcelino Paulo, R.; Brumano Larissa, P.; Ingle Avinash, P.; Gaikwad, S.; Gade, A.; da Silva Silvio, S. Strategic role of nanotechnology for production of bioethanol and biodiesel. Nanotechnol. Rev. 2016, 5, 231–250. [Google Scholar] [CrossRef]
- Patumsawad, S. 2nd Generation biofuels: Technical challenge and R and D opportunity in Thailand. J. Sustain. Energy Environ. Spec. Issue 2011, 47–50. [Google Scholar]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Contreras, J.E.; Rodriguez, E.A.; Taha-Tijerina, J. Nanotechnology applications for electrical transformers—A review. Electr. Power Syst. Res. 2017, 143, 573–584. [Google Scholar] [CrossRef]
- Nizami, A.-S.; Rehan, M. Towards nanotechnology-based biofuel industry. Biofuel Res. J. 2018, 5, 798–799. [Google Scholar] [CrossRef] [Green Version]
- Palaniappan, K. An overview of applications of nanotechnology in biofuel production. World Appl. Sci. J. 2017, 35, 1305–1311. [Google Scholar]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of Metal and Metal Oxide Nanoparticles by Algae and their Toxic Effects. Nanoscale Res. Lett. 2016, 11, 363. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Lohse, S.E.; Murphy, C.J. Applications of Colloidal Inorganic Nanoparticles: From Medicine to Energy. J. Am. Chem. Soc. 2012, 134, 15607–15620. [Google Scholar] [CrossRef]
- Lamberti, M.; Zappavigna, S.; Sannolo, N.; Porto, S.; Caraglia, M. Advantages and risks of nanotechnologies in cancer patients and occupationally exposed workers. Expert Opin. Drug Deliv. 2014, 11, 1087–1101. [Google Scholar] [CrossRef]
- Pantic, I. Magnetic nanoparticles in cancer diagnosis and treatment: Novel approaches. Rev. Adv. Mater. Sci. 2010, 26, 67–73. [Google Scholar]
- Antunes, F.A.F.; Gaikwad, S.; Ingle, A.P.; Pandit, R.; dos Santos, J.C.; Rai, M.; da Silva, S.S. Bioenergy and Biofuels: Nanotechnological Solutions for Sustainable Production. In Nanotechnology for Bioenergy and Biofuel Production; Rai, M., da Silva, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P. Saving the environment by nanotechnology and waste raw materials: Use of chitin nanofibrils by EU research projects. J. Appl. Cosmetol. 2013, 31, 89–96. [Google Scholar]
- Rahim, A.H.A.; Khoo, K.S.; Yunus, N.M.; Hamzah, W.S.W. Ether-functionalized ionic liquids as solvent for Gigantochloa scortechini dissolution. AIP Conf. Proc. 2019, 2157, 020025. [Google Scholar] [CrossRef]
- Peña, L.; Hohn, K.; Li, J.; Sun, X.; Wang, D. Synthesis of propyl-sulfonic acid-functionalized nanoparticles as catalysts for cellobiose hydrolysis. J. Biomater. Nanobiotechnol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Lai, D.M.; Deng, L.; Guo, Q.X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Erdem, S.; Erdem, B.; Öksüzoğlu, R.M. Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis. Open Chem. 2018, 16, 923–929. [Google Scholar] [CrossRef]
- Su, T.-C.; Fang, Z.; Zhang, F.; Luo, J.; Li, X.-K. Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave. Sci. Rep. 2015, 5, 17538. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, A.; Zarafeta, D.; Galanopoulou, A.P.; Stamatis, H. Enhanced Catalytic Performance of Trichoderma reesei Cellulase Immobilized on Magnetic Hierarchical Porous Carbon Nanoparticles. Protein J. 2019, 38, 640–648. [Google Scholar] [CrossRef]
- Chang, R.H.-Y.; Jang, J.; Wu, K.C.W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem. 2011, 13, 2844–2850. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hosaka, Y.; Hara, K.; Feng, B.; Hirosaki, Y.; Fukuoka, A. Control of selectivity, activity and durability of simple supported nickel catalysts for hydrolytic hydrogenation of cellulose. Green Chem. 2014, 16, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.; Rawat, R.; Sharma, R.; Oberoi, H.S.; Srivastava, M.; Singh, J. Effect of nickel–cobaltite nanoparticles on production and thermostability of cellulases from newly isolated thermotolerant Aspergillus fumigatus NS (Class: Eurotiomycetes). Appl. Biochem. Biotechnol. 2014, 174, 1092–1103. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Mubarak, N.; Wong, J.; Tan, K.; Sahu, J.; Abdullah, E.; Jayakumar, N.; Ganesan, P. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Ooi, C.W.; Ong, H.C.; Ling, T.C.; Show, P.L. Extraction of natural astaxanthin from Haematococcus pluvialis using liquid biphasic flotation system. Bioresour. Technol. 2019, 290, 121794. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent Advances in Biorefinery of Astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Duraiarasan, S.; Razack, S.A.; Manickam, A.; Munusamy, A.; Syed, M.B.; Ali, M.Y.; Ahmed, G.M.; Mohiuddin, M.S. Direct conversion of lipids from marine microalga C. salina to biodiesel with immobilised enzymes using magnetic nanoparticle. J. Environ. Chem. Eng. 2016, 4, 1393–1398. [Google Scholar] [CrossRef]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of bio-oil extracted from Chlorella vulgaris micro algae to biodiesel via modified superparamagnetic nano-biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Zaidi, A.; Feng, R.; Malik, A.; Khan, S.; Shi, Y.; Bhutta, A.; Shah, A. Combining microwave pretreatment with iron oxide nanoparticles enhanced biogas and hydrogen yield from green algae. Processes 2019, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Windt, W.D.; Aelterman, P.; Verstraete, W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ. Microbiol. 2005, 7, 314–325. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.; Mahoney, P.; Gibson, K. Nanofarming technology extracts biofuel oil without harming algae. News released from Office of Public Affairs.
- Lee, Y.-C.; Huh, Y.S.; Farooq, W.; Han, J.-I.; Oh, Y.-K.; Park, J.-Y. Oil extraction by aminoparticle-based H2O2 activation via wet microalgae harvesting. RSC Adv. 2013, 3, 12802–12809. [Google Scholar] [CrossRef]
- Kang, N.K.; Lee, B.; Choi, G.-G.; Moon, M.; Park, M.S.; Lim, J.; Yang, J.-W. Enhancing lipid productivity of Chlorella vulgaris using oxidative stress by TiO2 nanoparticles. Korean J. Chem. Eng. 2014, 31, 861–867. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lee, K.; Oh, Y.-K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: A review. Bioresour. Technol. 2015, 184, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.J.; Das, R.K.; Brar, S.K.; Le Bihan, Y.; Buelna, G.; Verma, M.; Soccol, C.R. Application of magnesium sulfate and its nanoparticles for enhanced lipid production by mixotrophic cultivation of algae using biodiesel waste. Energy 2014, 78, 16–22. [Google Scholar] [CrossRef]
- Wang, X.; Dou, P.; Zhao, P.; Zhao, C.; Ding, Y.; Xu, P. Immobilization of lipases onto magnetic Fe3O4 nanoparticles for application in biodiesel production. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 947–950. [Google Scholar]
- Kaieda, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wang, J.-Q.; Xu, Z.-K. Immobilization of lipase from Candida rugosa on electrospun polysulfone nanofibrous membranes by adsorption. J. Mol. Catal. B Enzym. 2006, 42, 45–51. [Google Scholar] [CrossRef]
- Liu, C.-H.; Huang, C.-C.; Wang, Y.-W.; Lee, D.-J.; Chang, J.-S. Biodiesel production by enzymatic transesterification catalyzed by Burkholderia lipase immobilized on hydrophobic magnetic particles. Appl. Energy 2012, 100, 41–46. [Google Scholar] [CrossRef]
- Ng, I.-S.; Tang, M.S.; Show, P.L.; Chiou, Z.-M.; Tsai, J.-C.; Chang, Y.-K. Enhancement of C-phycocyanin purity using negative chromatography with chitosan-modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 132, 615–628. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.-L.; Ng, I.-S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Sakai, S.; Liu, Y.; Yamaguchi, T.; Watanabe, R.; Kawabe, M.; Kawakami, K. Production of butyl-biodiesel using lipase physically-adsorbed onto electrospun polyacrylonitrile fibers. Bioresour. Technol. 2010, 101, 7344–7349. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sajith, V.; Sobhan, C.; Peterson, G. Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel. Adv. Mech. Eng. 2010, 2, 581407. [Google Scholar] [CrossRef]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Impact of hydrogen generated by splitting water with nano-silicon and nano-aluminum on diesel engine performance. Int. J. Hydrog. Energy 2014, 39, 8098–8105. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Elango, A.; Prathima, A. Performance and Emission Study on Zinc Oxide Nano Particles Addition with Pomolion Stearin Wax Biodiesel of CI Engine. J. Sci. Ind. Res. 2014, 73, 187–190. [Google Scholar]
- Singh, N.; Bharj, R. Effect of CNT-emulsified fuel on performance emission and combustion characteristics of four stroke diesel engine. Int. J. Curr. Eng. Technol. 2015, 5, 477–485. [Google Scholar]
- Aalam, S.; Saravanan, C.; PremAnand, B. Reduction of emissions from CRDI diesel engine using metal oxide nanoparticles blended diesel fuel. Int. J. Appl. Eng. Res. 2015, 10, 3865–3869. [Google Scholar]
- Aalam, C.S.; Saravanan, C.; Premanand, B. Influence of Iron (II, III) oxide nanoparticles fuel additive on exhaust emissions and combustion characteristics of CRDI system assisted diesel engine. Int. J. Adv. Eng. Res. Sci. 2015, 2, 23–28. [Google Scholar]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Nanofuels: Combustion, engine performance and emissions. Fuel 2014, 120, 91–97. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Q.; Kobayashi, H.; Xiao, S. Biofuel Production from Bioelectrochemical Systems. In Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2018; pp. 435–461. [Google Scholar]
- Gul, M.M.; Ahmad, K.S. Bioelectrochemical systems: Sustainable bio-energy powerhouses. Biosens. Bioelectron. 2019, 142, 111576. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1911, 84, 260–276. [Google Scholar]
- Kalathil, S.; Pant, D. Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems. RSC Adv. 2016, 6, 30582–30597. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; Sun, B.; Xu, H. Anode decoration with biogenic Pd nanoparticles improved power generation in microbial fuel cells. Electrochim. Acta 2015, 182, 815–820. [Google Scholar] [CrossRef]
- Ray, S.G.; Ghangrekar, M. Enhancing organic matter removal, biopolymer recovery and electricity generation from distillery wastewater by combining fungal fermentation and microbial fuel cell. Bioresour. Technol. 2015, 176, 8–14. [Google Scholar]
- Kalathil, S.; Lee, J.; Cho, M.H. Granular activated carbon based microbial fuel cell for simultaneous decolorization of real dye wastewater and electricity generation. New Biotechnol. 2011, 29, 32–37. [Google Scholar] [CrossRef]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Steinberg, L.; Regan, J. Electricity production and microbial biofilm characterization in cellulose-fed microbial fuel cells. Water Sci. Technol. 2008, 58, 617–622. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.; De Los Ríos, A.P.; Salar-García, M.; Ortiz-Martínez, V.; Lozano-Blanco, L.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Majid, S.; Ahmad, K.S. Analysis of dopant concentration effect on optical and morphological properties of PVD coated Cu-doped Ni3S2 thin films. Optik 2019, 187, 152–163. [Google Scholar] [CrossRef]
- Majid, S.; Ahmad, K.S. Optical and morphological properties of environmentally benign Cu-Tin sulphide thin films grown by physical vapor deposition technique. Mater. Res. Express 2018, 6, 036406. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hussain, Z.; Majid, S. Synthesis, characterization and PVD assisted thin film fabrication of the nano-structured bimetallic Ni3S2/MnS2 composite. Surf. Interfaces 2018, 12, 190–195. [Google Scholar]
- Erbay, C.; Pu, X.; Choi, W.; Choi, M.-J.; Ryu, Y.; Hou, H.; Lin, F.; de Figueiredo, P.; Yu, C.; Han, A. Control of geometrical properties of carbon nanotube electrodes towards high-performance microbial fuel cells. J. Power Sources 2015, 280, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Aryal, N.; Ammam, F.; Patil, S.A.; Pant, D. An overview of cathode materials for microbial electrosynthesis of chemicals from carbon dioxide. Green Chem. 2017, 19, 5748–5760. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Hu, Y.; Li, S.; Xu, Q. Bio-cathode materials evaluation in microbial fuel cells: A comparison of graphite felt, carbon paper and stainless steel mesh materials. Int. J. Hydrog. Energy 2012, 37, 16935–16942. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Q.; Jiao, Y.; Wang, K.; Lee, D.-J.; Ren, N. Efficient electricity generation from sewage sludge usingbiocathode microbial fuel cell. Water Res. 2012, 46, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Salar-García, M.; Ortiz-Martínez, V. Nanotechnology for Wastewater Treatment and Bioenergy Generation in Microbial Fuel Cells. In Advanced Research in Nanosciences for Water Technology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 341–362. [Google Scholar]
- Kaur, R.; Marwah, A.; Chhabra, V.A.; Kim, K.-H.; Tripathi, S. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2019, 119, 109551. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Choi, S. A solid phase bacteria-powered biobattery for low-power, low-cost, internet of Disposable Things. J. Power Sources 2019, 429, 105–110. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Energy harvesting from sediment microbial fuel cell to supply uninterruptible regulated power for small devices. Int. J. Energy Res. 2019, 43, 2821–2831. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, S.; Guo, J. Anode modification with formic acid: A simple and effective method to improve the power generation of microbial fuel cells. Appl. Surf. Sci. 2014, 320, 281–286. [Google Scholar] [CrossRef]
- Shantaram, A.; Beyenal, H.; Veluchamy RR, A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 39, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Zhang, C.; Zhou, X.; Xiao, Z.; Awata, T.; Katayama, A. Phenol-degrading anode biofilm with high coulombic efficiency in graphite electrodes microbial fuel cell. J. Biosci. Bioeng. 2017, 123, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Valipour, A.; Ayyaru, S.; Ahn, Y. Application of graphene-based nanomaterials as novel cathode catalysts for improving power generation in single chamber microbial fuel cells. J. Power Sources 2016, 327, 548–556. [Google Scholar] [CrossRef]
- Zou, L.; Qiao, Y.; Zhong, C.; Li, C.M. Enabling fast electron transfer through both bacterial outer-membrane redox centers and endogenous electron mediators by polyaniline hybridized large-mesoporous carbon anode for high-performance microbial fuel cells. Electrochim. Acta 2017, 229, 31–38. [Google Scholar] [CrossRef]
- Zou, L.; Lu, Z.; Huang, Y.; Long, Z.E.; Qiao, Y. Nanoporous Mo2C functionalized 3D carbon architecture anode for boosting flavins mediated interfacial bioelectrocatalysis in microbial fuel cells. J. Power Sources 2017, 359, 549–555. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Chen, Q.; Zhou, W.; Liu, Y. Advances in graphene/graphene composite based microbial fuel/electrolysis cells. Electroanalysis 2017, 29, 652–661. [Google Scholar] [CrossRef]
- Lead, J.R.; Valsami-Jones, E. Nanoscience and the Environment; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7. [Google Scholar]
- Peng, X.; Chu, X.; Wang, S.; Shan, K.; Song, D.; Zhou, Y. Bio-power performance enhancement in microbial fuel cell using Ni–ferrite decorated anode. RSC Adv. 2017, 7, 16027–16032. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Liang, Y.; Guo, K.; Chen, W.; Shen, D.; Huang, L.; Zhou, Y.; Wang, M.; Long, Y. TiO2 nanotube arrays modified titanium: A stable, scalable, and cost-effective bioanode for microbial fuel cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.; Lv, P.; Kong, X.; Sun, Y.; Wang, Z.; Yuan, Z.; Liu, H.; Yang, J. Core-shell Au-Pd nanoparticles as cathode catalysts for microbial fuel cell applications. Sci. Rep. 2016, 6, 35252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Palma, L.; Bavasso, I.; Sarasini, F.; Tirillò, J.; Puglia, D.; Dominici, F.; Torre, L. Synthesis, characterization and performance evaluation of Fe3O4/PES nano composite membranes for microbial fuel cell. Eur. Polym. J. 2018, 99, 222–229. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, P.; Yang, X.; Jiang, Y.; Bian, Y.; Chen, C.; Zhang, X.; Huang, X. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens. Bioelectron. 2016, 81, 32–38. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Zou, L.; Li, C.M.; Qiao, Y. Pectin assisted one-pot synthesis of three dimensional porous NiO/graphene composite for enhanced bioelectrocatalysis in microbial fuel cells. J. Power Sources 2018, 378, 119–124. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Pourali, S.; Ezoji, H.; ElMekawy, A.; Pant, D. Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Prog. Nat. Sci. Mater. Int. 2017, 27, 647–651. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Ren, Y.; Wang, X. In-situ modified carbon cloth with polyaniline/graphene as anode to enhance performance of microbial fuel cell. Int. J. Hydrog. Energy 2016, 41, 11369–11379. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, Y.; Shi, Z.; Tang, W.; Li, C.M. Hierarchically porous N-doped carbon nanotubes/reduced graphene oxide composite for promoting flavin-based interfacial electron transfer in microbial fuel cells. ACS Appl. Mater. Interfaces 2018, 10, 11671–11677. [Google Scholar] [CrossRef]
- Ren, H.; Tian, H.; Gardner, C.L.; Ren, T.-L.; Chae, J. A miniaturized microbial fuel cell with three-dimensional graphene macroporous scaffold anode demonstrating a record power density of over 10,000 W m−3. Nanoscale 2016, 8, 3539–3547. [Google Scholar] [CrossRef]
- Aslan, S.; Ó Conghaile, P.; Leech, D.; Gorton, L.; Timur, S.; Anik, U. Development of a Bioanode for Microbial Fuel Cells Based on the Combination of a MWCNT-Au-Pt Hybrid Nanomaterial, an Osmium Redox Polymer and Gluconobacter oxydans DSM 2343 Cells. ChemistrySelect 2017, 2, 12034–12040. [Google Scholar] [CrossRef]
- Malorni, L.; Guida, V.; Sirignano, M.; Genovese, G.; Petrarca, C.; Pedata, P. Exposure to sub-10nm particles emitted from a biodiesel-fueled diesel engine: In vitro toxicity and inflammatory potential. Toxicol. Lett. 2017, 270, 51–61. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Disadvantages |
|---|---|
|
|
| Magnetic Nanoparticles (MNPs) | Biomass Strain | Operating Condition | Yield (%) | References |
|---|---|---|---|---|
| Sulfonate-supported silica MNPs, FE3O4-SBA-SO3H | Amorphous cellulose | 1.0 g, 15 mL H2O at 150 °C for 3 h | 50 | [26] |
| Sulfonate-supported silica MNPs, FE3O4-SBA-SO3H | Cellulose | 1.0 g, 15 mL H2O at 150 °C for 3 h | 26 | [26] |
| Sulfonate-supported silica MNPs, FE3O4-SBA-SO3H | Starch | 1.0 g, 15 mL H2O at 150 °C for 3 h | 95 | [26] |
| Sulfonate-supported silica MNPs, FE3O4-SBA-SO3H | Corn cob | 1.5 g, 15 mL H2O at 150 °C for 3 h | 45 | [26] |
| Perfluoroalkylsulfonic MNPs, PFS-MNPs | Wheat straw | 2.5% (w/w) biomass, 160 °C for 24 h | 66.3 ± 0.9 | [25] |
| Alklysulfonic MNPs, AS-MNPs | Wheat straw | 2.5% (w/w) biomass, 160 °C for 24 h | 61.0 ± 1.2 | [25] |
| Carbonaceous acid MNPs, C-SO3H-Fe3O4-MNPs | Sugarcane bagasse | 0.027 g, 15 mL H2O, 160–200 °C (0.5–2.2 MPa) for 3 min | 58.3 | [28] |
| Carbonaceous acid MNPs, C-SO3H-Fe3O4-MNPs | Jatropha hulls | 0.027 g, 15 mL H2O, 160–200 °C (0.5–2.2 MPa) for 3 min | 35.6 | [28] |
| Carbonaceous acid MNPs, C-SO3H-Fe3O4-MNPs | Plukenetia hulls | 0.027 g, 15 mL H2O, 160–200 °C (0.5–2.2 MPa) for 3 min | 35.8 | [28] |
| Nanomaterial | Modified Part of MFC | Advantages | Description | Reference |
|---|---|---|---|---|
| Fabrication of bio-palladium nanoparticles using pure strain Shewanella oneidensis on carbon cloth | Anode |
| The maximum power output and coulombic efficiency were improved by 14% and 31% as compared to unmodified anode. | [69] |
| Spinel type Ni-ferrite (NiFe2O4) modified composite anode | Anode |
| As compared to control, the maximum power density achieved was increased by 26% and the internal resistance was lowered by 39%. | [98] |
| TiO2 nanotubes (TN) on the surface of titanium anode | Anode |
| The maximum current density achieved was 12.7 Am−2, which was up to 190-fold as compared with bare titanium anode electrode. | [99] |
| Bimetallic core-shell gold-palladium nanoparticles as cathode catalyst | Cathode |
| High stability (can stable over 150 days), high durability and the power output produced was 15.98 Wm−3, twice the power obtained with hollow structures-based platinum (Pt) cathodes (7.1 Wm−3). | [100] |
| Fe3O4 nanoparticles/polyethersulfone (PES) nanocomposite membrane | Proton exchange membrane |
| High thermal stability and mechanical properties as well as the maximum power output produced was 9.59 mWm−2, higher than commercial membrane. | [101] |
| Graphene oxide (rGO)/manganese oxide (MnO2) composite on carbon felt surface | Anode |
| The internal resistance was lowered, and maximum power density achieved was 2065 mWm−2, 154% higher as compared with carbon felt anode. | [102] |
| Nickel oxide (NiO)/graphene nanocomposite with the addition of pectin into NiO | Anode |
| The maximum power density achieved was 3.632 mWm−2, higher than NiO anode and Pt/C anode. | [103] |
| Fabrication of two graphite based composite electrodes using graphene paste modified with TiO2 (GP-TiO2) or hybrid graphene (GP-HG) | Cathode |
| The power density was increased above 80 mWm−2 for GP-TiO2 and above 220 mWm−2 for GP-HG as compared to the control, graphite paste bare electrode (30 mWm−2). | [104] |
| Polyaniline/graphene modified carbon cloth | Anode |
| The maximum power density achieved was 884 ± 96 mWm−2, 1.9 times higher than the unmodified carbon cloth anode (454 ± 47 mWm−2). The voltage achieved was 573 ± 37 mV, higher than CC anode, 454 ± 34 mV. | [105] |
| Fabrication of polyaniline hybridized disorderly large mesoporous carbon nanocomposite with aid of nanoparticles, CaCO3 | Anode |
| The maximum power density obtained was 1280 mWm−2, 1.5-fold and 10-fold higher than LMC anode (878 mWm−2) plain carbon cloth anode (127 mWm−2). | [94] |
| Nitrogen-doped carbon nanotube/reduced graphene oxide (rGO) composite with polyaniline as nitrogen source | Anode |
| The power density achieved was 1137 mWm−2, 8.9 times higher than carbon cloth anode. | [106] |
| 3D graphene macroporous scaffold anode | Anode |
| The MFC system was able to accommodate a high population of microbes and the power density achieved was 5.61 Wm−2/11220 Wm−3, 3-fold higher than planar 2D control counterparts. The highest power achieved was 3320 Wm−3. | [107] |
| Graphene material (RGOHI-AcOH) and graphene nanoparticles composite (RGO/Ni) as cathode catalyst | Cathode |
| The power generated was 1683 mWm−2 and had good stability (can operate for 30 days and around 27 cycles) as compared to non-metal cathode MFCs. | [93] |
| Integration of carbon nanotube-gold-platinum nanomaterial with osmium redox polymer and Gluconabacter oxydans DSM 2343 in carbon felt electrode | Anode |
| The maximum power density and current density achieved were 32.1 mWm−2 and 1032 mAm−2, respectively, showed the improvement of the system. | [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.-H. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies 2020, 13, 892. https://doi.org/10.3390/en13040892
Khoo KS, Chia WY, Tang DYY, Show PL, Chew KW, Chen W-H. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies. 2020; 13(4):892. https://doi.org/10.3390/en13040892
Chicago/Turabian StyleKhoo, Kuan Shiong, Wen Yi Chia, Doris Ying Ying Tang, Pau Loke Show, Kit Wayne Chew, and Wei-Hsin Chen. 2020. "Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production" Energies 13, no. 4: 892. https://doi.org/10.3390/en13040892







