Abstract
Energy crops are not easily converted by microorganisms because of their recalcitrance. This necessitates a pretreatment to improve their biodigestibility. The effects of different pretreatments, as well as their combination on the enzymatic digestibility of Arundo donax L. were systematically investigated to evaluate its potential for bioconversion. Dilute alkaline pretreatment (ALP) using 1.2% NaOH at 120 °C for 30 min resulted in the highest reducing sugar yield in the enzymatic hydrolysis process because of its strong delignification and morphological modification, while ferric chloride pretreatment (FP) was effective in removing hemicellulose and recovering soluble sugars in the pretreatment stage. Furthermore, an efficient two-step ferric chloride-alkaline pretreatment (FALP) was successfully developed. In the first FP step, easily degradable cellulosic components, especially hemicellulose, were dissolved and then effectively recovered as soluble sugars. Subsequently, the FP sample was further treated in the second ALP step to remove lignin to enhance the enzymatic hydrolysis of the hardly degradable cellulose. As a result, the integrated two-step process obtained the highest total sugar yield of 420.4 mg/g raw stalk in the whole pretreatment and enzymatic hydrolysis process; hence, the process is a valuable candidate for biofuel production.
1. Introduction
At present, public health, resource, energy, food safety, and environmental issues are the most important focus areas for global sustainable development [1,2,3,4,5,6,7]. Considering current energy and environmental crises, biofuel production from energy crops and lignocellulosic biomass wastes, which can produce renewable energy and balance the emission of carbon dioxide, is attracting increasing attention [1,2,3,4,6,8]. Arundo donax L., which is a prolific herbaceous plant, is widely distributed in Asia (e.g., China), Oceania, Africa, etc. While annual dry matter yields of 13~21 t/ha have been obtained from some traditional agricultural wastes, yields of Arundo donax L. have reached 30~40 t/ha. These values are similar to that of energy crops such as Pennisetum grass (0~50.2 t/ha) and miscanthus (27~38 t/ha) [9,10,11]. Given its high yield, low cost, and environmentally benign production, Arundo donax L. is considered as a potential candidate for biofuel production, and hence, it was chosen as a substrate in this study to investigate its potential for bioconversion.
The bioconversion process is still constrained due to the recalcitrance of these energy crops. Different pretreatment strategies such as pretreatments using acid, alkaline or hot water, explosion pretreatments with steam or ammonia, and biological pretreatment have been reported for reducing the recalcitrance of lignocellulosic biomass wastes, and hence, enhancing the digestibility of these biomass wastes [12,13,14,15,16,17,18]. Chemical pretreatment has been extensively investigated, from lab-scale studies to large-scale ethanol fermentation applications, especially those using low lignin biomass (e.g., agricultural wastes) as substrates [15,16,17,18]. Therefore, it could be a useful method for pretreating Arundo donax L.
Acid pretreatment, which has substantial advantages such as its low cost and high efficiency, is a commonly used technique [12,16]. It is usually performed with acid addition of 0.01~5% (w/w) at 120~210 °C for 5~120 minutes, and it cleaves the glucosidic bonds of polysaccharides and effectively removes hemicellulosic components with less impact on lignin [12,13,14,15,16,17]. The main drawbacks of acid pretreatment with inorganic acids (e.g., sulfuric acid) are the formation of inhibitory compounds and its requirement for corrosion-resistant reactors [12]. Pretreatment using Lewis acids such as FeCl3 also enhances sugar recovery from pretreated substrates by removing hemicellulose from lignocellulosic wastes [19,20]. The corrosion problem in acid pretreatment could be reduced by using Lewis acids to some extent. On the other hand, alkaline pretreatment has less corrosion problems in comparison with these processes that involve acid addition. Alkaline pretreatment, which can effectively remove lignin from lignocellulosic biomass, has significantly improved the enzymatic hydrolysis of various wastes [13,15,16].
Although these acid and alkaline pretreatments are effective for enhancing the enzymatic hydrolysis of the pretreated sample, sugar recovery from cellulosic components still needs improvement because of the different structural features of waste biomass. Consequently, two-step processes that combine acid, alkaline, and/or other pretreatments have been suggested so as to maximize sugar recovery [21,22,23,24,25]. Previous studies have reported that the sugar yield of pretreated sweet sorghum substrates with 0.5%–2.0% sulfuric acid, followed by 0.5%–2.0% sodium hydroxide was significantly higher than that observed in one-step pretreatment of the biomass [23]. The level of glucose in the enzymatic hydrolysate of samples pretreated with acid/alkaline reached as high as 84 g/L, against 28 g/L obtained in the single pretreatment process [24]. However, Guilherme et al. [25] observed significantly higher sugar yields in alkaline treatment alone than that obtained in the two-step processes combining acid/hydrothermal and alkaline pretreatment.
In this study, sugar recovery from different pretreatments and the subsequent enzymatic hydrolysis of Arundo donax L. biomass were systematically studied to analyze its potential in the bioconversion industry. The main objective of this work was to evaluate (i) the effect of dilute acid pretreatment (AP), dilute alkaline pretreatment (ALP), and ferric chloride pretreatment (FP) on the enzymatic hydrolysis of Arundo donax L. grass, (ii) the mechanisms of enzymatic hydrolysis enhancement based on the composition and microstructure observations, and (iii) the possibility of developing an integrated two-step process by combining ferric chloride and alkaline pretreatments for enhanced total sugar recovery.
2. Materials and Methods
2.1. Materials
Sun-dried Arundo donax L. samples were obtained from Fujian, China. They were then dried in a thermoelectric oven at 60 °C for more than 24 h until a constant weight was achieved. A plant miller with a 20- mesh sieve was then used to mill the solid samples. The cellulose, hemicellulose, and lignin contents of the raw stalks were 33.0%, 29.7%, and 23.0%, respectively.
2.2. Pretreatments
In the cases of one-step pretreatments, 1.0%~4.0% (w/v) sulfuric acid, 0.6%~1.2% (w/v) NaOH and 0.8%~4.8% (w/v) FeCl3 solutions were added to bottles for AP, ALP, and FP, respectively. Dried Arundo donax L. samples were then added to these bottles on the basis of a 10% solid loading rate [6,8,17,20]. These bottles containing the mixtures of biomass samples and acid or alkaline solutions were kept in an autoclave at 121 °C for 30 min [6,8,17,20]. The pretreated mixtures were centrifuged, and the supernatants were separated and stored at −20 °C until composition analysis. The remaining solid residues were collected and washed with distilled water. After the filtrates became neutral, the washed stalk residues were then dried in a thermoelectric oven at 105 °C until the weight was found to be constant.
In the case of two-step ferric chloride-alkaline pretreatment (FALP), the dried Arundo donax L. samples were firstly added to the glass bottles. FeCl3 solutions of 1.6% or 3.2% (w/v) were then added on the basis of a 10% solid loading rate. The above Arundo donax L. mixtures were kept in an autoclave at 121 °C for 30 min for the first FP. The solid residues of the pretreated mixtures were washed and then mixed in glass bottles with 1.0% or 1.2% (w/v) NaOH on the basis of a 10% solid loading rate for the second step ALP. The above mixtures were then kept in an autoclave at 120 °C for 30 min. After the second step of pretreatment, the solid residues and supernatants were collected and stored for further analysis and/or subsequent enzymatic hydrolysis based on the same procedure used in the one-step process.
2.3. Enzymatic Hydrolysis of Untreated and Treated Samples
A 250 mL conical flask containing 50 mM sodium acetate buffer (pH 5) and 40 μL tetracycline hydrochloride was used for the enzymatic hydrolysis of the untreated and treated samples. The stalk samples were added on the basis of a 2.5% of solid loading. The enzymatic hydrolysis was carried out in a shaker at 50 °C for 72 h. The shaking speed was set at 150 rpm. The cellulase was obtained from Utter Biochemical Co., Ltd. (Hunan, China). The cellulase was added at 15 FPU/g of raw or pretreated sample. One FPU represents the amount of the cellulase that releases glucose at 1 μmol/min in the enzymatic hydrolysate at 50 °C and pH 5. The mixtures were centrifugated and the reducing sugar levels in the enzymatic hydrolysate were determined by the standard method of 3, 5-dinitrosalicylic acid (DNS) assay [14,15,26]. All enzymatic hydrolysis experiments were performed in triplicate.
2.4. Scanning Electron Microscopy (SEM) Analysis
The untreated and treated stalks were observed by scanning electron microscopy (SEM). First, a specimen holder was used to fix the obtained stalk samples with aluminum tape. The samples were placed in a JEOL JEC−1200 sputter-coater (Tokyo, Japan) for sputtering gold. All samples were then observed with a JEOL JSM−5600 LV SEM (Tokyo, Japan) under high vacuum (500× magnification). An accelerating voltage of 5.0 kV was used for the SEM observations.
2.5. Analytical Methods
After all pretreatments, the solid–liquid mixtures were centrifuged and the supernatants were used to analyze total soluble sugar content by a phenol-sulfuric acid method [27,28]. The solid residues were dried and total solid was determined on the basis of standard methods [9,28]. Solid yields were then calculated on the basis of residual total solid of stalk samples after different pretreatments [17,28,29]:
where mB (g) and mA (g) are the total solid of stalk samples before and after pretreatments, respectively.
Solid yield (%) = 100×mA/mB
The compositional contents (i.e. cellulose, hemicellulose, and lignin contents) were determined gravimetrically according to the standard method of Goering and Van-Soest [30]. The compositional contents were calculated on the basis of residual total solid. In brief, the neutral detergent fiber (NDF) level was measured gravimetrically by extracting the solid residue remaining with neutral detergent. The acid detergent fiber (ADF) level was determined gravimetrically by extracting the NDF solid residue remaining with acid detergent solution. Lignin content was then determined gravimetrically from the free ash after the ADF solid residue was extracted with 72% H2SO4 solution. Cellulose content was calculated by subtracting the pre-ash lignin level from the ADF level. The ADF level was subtracted from the NDF level to obtain the hemicellulose content. Solid biomass samples were extracted subsequently by using neutral detergent, acid detergent and 72% H2SO4 solution, and the remaining solid residue was collected to measure the ash level in a muffle furnace at 550 ℃ over 6 h. All experiments were carried out in triplicate and all results are the mean of triplicates ± S.D.
The pretreatment severity factor (log R0) of each pretreatment was obtained with the following equation [31,32]:
where Tr is the pretreatment temperature (°C), 100 is the reference temperature (°C), and t is the pretreatment time (min). The fitted value of 14.75 is the arbitrary constant ѡ.
R0=t*exp[(Tr−100)/14.75]
3. Results and Discussion
3.1. One-Step Pretreatment
3.1.1. Solid Yields and Compositional Changes
Energy crops are not easily converted by microorganisms because of their recalcitrance, which is a result of their highly ordered crystalline structure and the lignin barrier. To decrease the recalcitrance of the biomass and improve its enzymatic hydrolysis, several promising pretreatments, including AP, FP and ALP, were chosen to pretreat the Arundo donax L. samples. The selected acid and alkali concentrations in different pretreatments were as follows: (1) AP, 1.0%–4.0% H2SO4, w/v, (2) FP, 0.8%–4.8% FeCl3, w/v, and (3) ALP, 0.6%–1.2% NaOH, w/v. Results show that lignocellulosic components were significantly changed during all the pretreatments tested. As shown in Table 1, the solid yields varied from 59.4% to 50.8% in AP with acid addition of 1.0% to 4.0%. The weight loss could mainly be due to the hemicellulose removal [14,15]. After AP, the hemicellulose contents of the pretreated samples decreased from 29.7% to 12.3%~18.2%. Cellulose content increased remarkably due to hemicellulose removal. In AP of Arundo donax L. samples, lignin content did not change significantly.

Table 1.
Effect of dilute acid pretreatment (AP), ferric chloride pretreatment (FP), dilute alkaline pretreatment (ALP) of Arundo donax L. on its chemical composition.
Similar changes in the lignocellulosic components were observed in the FP process of Arundo donax L. samples. The solid yield varied from 69.7% to 52.4%. The corresponding hemicellulose contents of the FP samples decreased from 28.3% to 10.6%~15.4%. Cellulose content of the FP sample reached as high as 62.9% with the addition of 4.8% FeCl3. These results proved that the removal of hemicellulose components was much easier than the removal of cellulose during acid pretreatments [8,17]. Different acid pretreatment processes can randomly break glycosidic bonds and dissolve hemicellulose [16,17]. In the pretreated biomass samples, cellulose content is thereby increased, which will increase the substrate accessibility to cellulases [16,17].
The solid yields in ALP were about 53.8%–80.3%. The hemicellulose contents of ALP samples decreased to 16.2%~24.4%. The corresponding cellulose contents gradually increased and reached as high as 62.7% (1.2% NaOH). Moreover, the ALP samples obtained from relatively severe conditions (1.0%~1.2% NaOH) indicated lower lignin contents compared to those observed in AP and FP (Table 1). Effective lignin removal has also been observed in previous alkaline pretreatments of various biomass wastes [6,13,16].
3.1.2. Sugar Recovery from Pretreatment Stage
Cellulosic components, mainly hemicellulose dissolved in AP and FP, will be further converted into soluble sugars [15,20]. In the case of ALP, the soluble sugar recovery from the pretreatment liquid was not very significant despite its obvious weight loss in the pretreatment stage. Soluble sugar levels gradually increased from 52.5 mg/g raw stalk (RS) to 90.8 mg/g RS. As compared to ALP, a relatively higher soluble sugar recovery was observed in the case of AP (Table 1). As the H2SO4 concentrations increased from 1.0% to 4.0%, the levels of soluble sugars in the aqueous phase of the pretreated mixtures reached 114.3 mg/g RS (Table 1).
Among all of the pretreatments tested, the FP process showed the best balance between dissolving lignocellulosic components and recovering fermentable sugars from the pretreatment stage. As shown in Table 1, the solid yield of FP was similar to that of AP (52.4% vs. 50.8%), indicating a similar level of dissolved components. However, soluble sugar levels in the case of FP were much higher than that observed in AP (197.8 mg/g RS vs. 114.3 mg/g RS). These observations showed that soluble sugars in AP could be more easily converted into some by-products such as HMF and furfural by H2SO4 as compared to FP [17,20,33].
3.1.3. SEM Observation
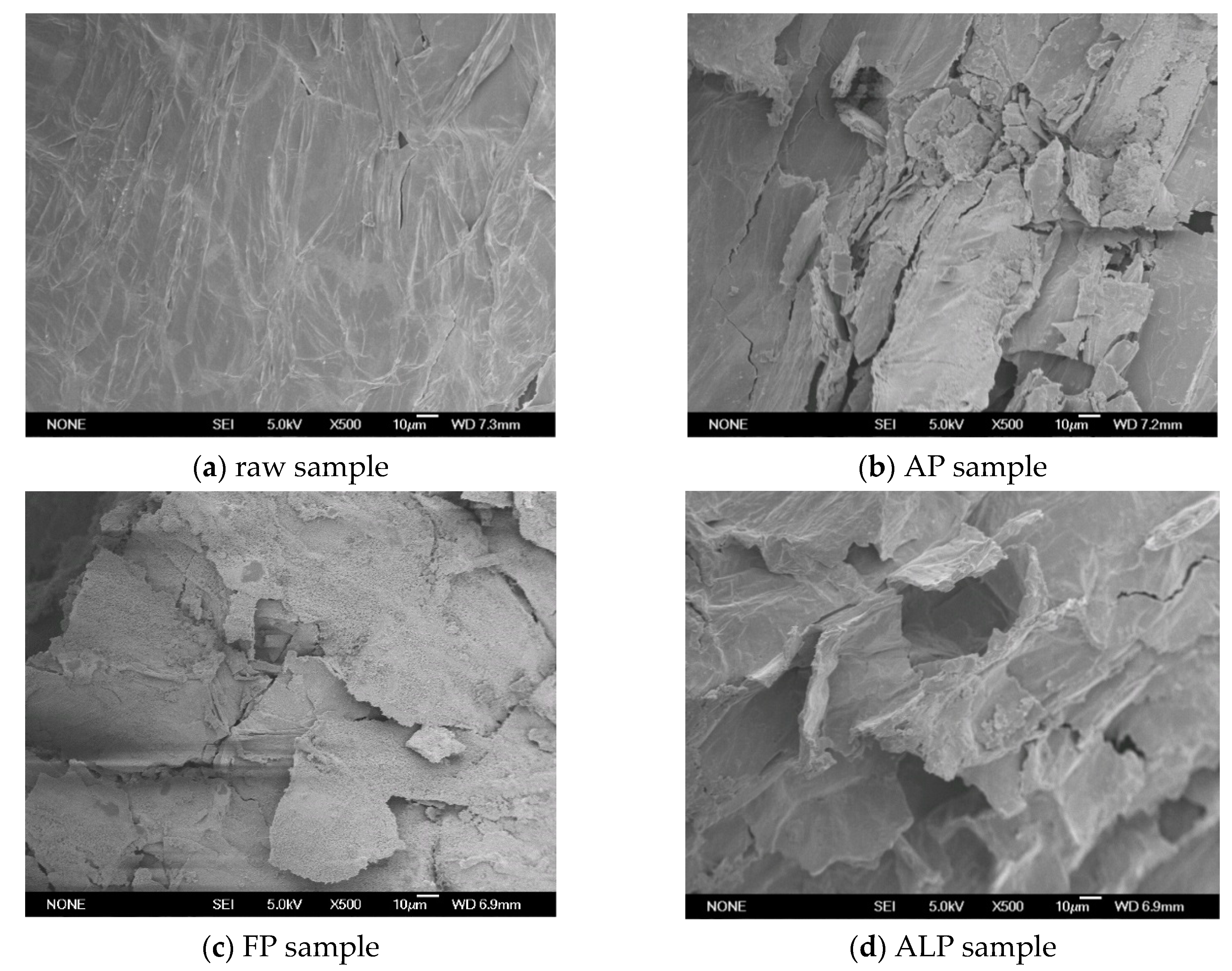
A scanning electron microscope made in Japan was used to observe cell wall structure changes during the pretreatments and the results are shown in Figure 1. The raw Arundo donax L. stalk sample had a rigid and smooth surface structure. The recalcitrant structure was obviously destroyed by different pretreatments. As shown in Figure 1b,c, distinct differences were observed in the AP and FP samples as compared to the raw stalk sample. The rectangular cell wall boundaries of the FP sample were broken to some extent. Some cracks and powder-like surface structures were observed in the FP sample. AP produced significant morphological changes. The cell wall surfaces of the AP sample exhibited cracks and small particle-sized debris. Some porous structures appeared in the AP sample. These changes in microstructure were closely related to the removal and/or rearrangement of lignocellulosic components. Both AP and FP significantly removed hemicellulose components and destroyed the original dense structure of the biomass, which could produce a positive effect on the subsequent enzymatic hydrolysis.
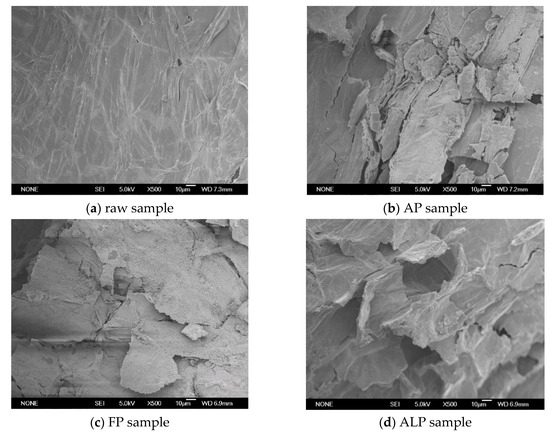
Figure 1.
SEM images of raw stalk and pretreated stalk samples (500×). (a) raw sample; (b) AP sample; (c) FP sample; (d) ALP sample.
As compared to AP and FP, ALP resulted in more significant structural changes. As shown in Figure 1d, the cell surface of the ALP sample was remarkably changed. Cell boundaries of the raw biomass sample become extremely distorted and blurry after ALP. Moreover, some fiber bundles and particle-sized debris were observed on the surface of the ALP sample. The strong structural changes could be attributed to lignin/hemicellulose removal and cellulose swelling during ALP. All these changes make the ALP sample of Arundo donax L. more accessible to enzymes in the subsequent enzymatic hydrolysis.
3.1.4. Enzymatic Hydrolysis of Stalk Samples
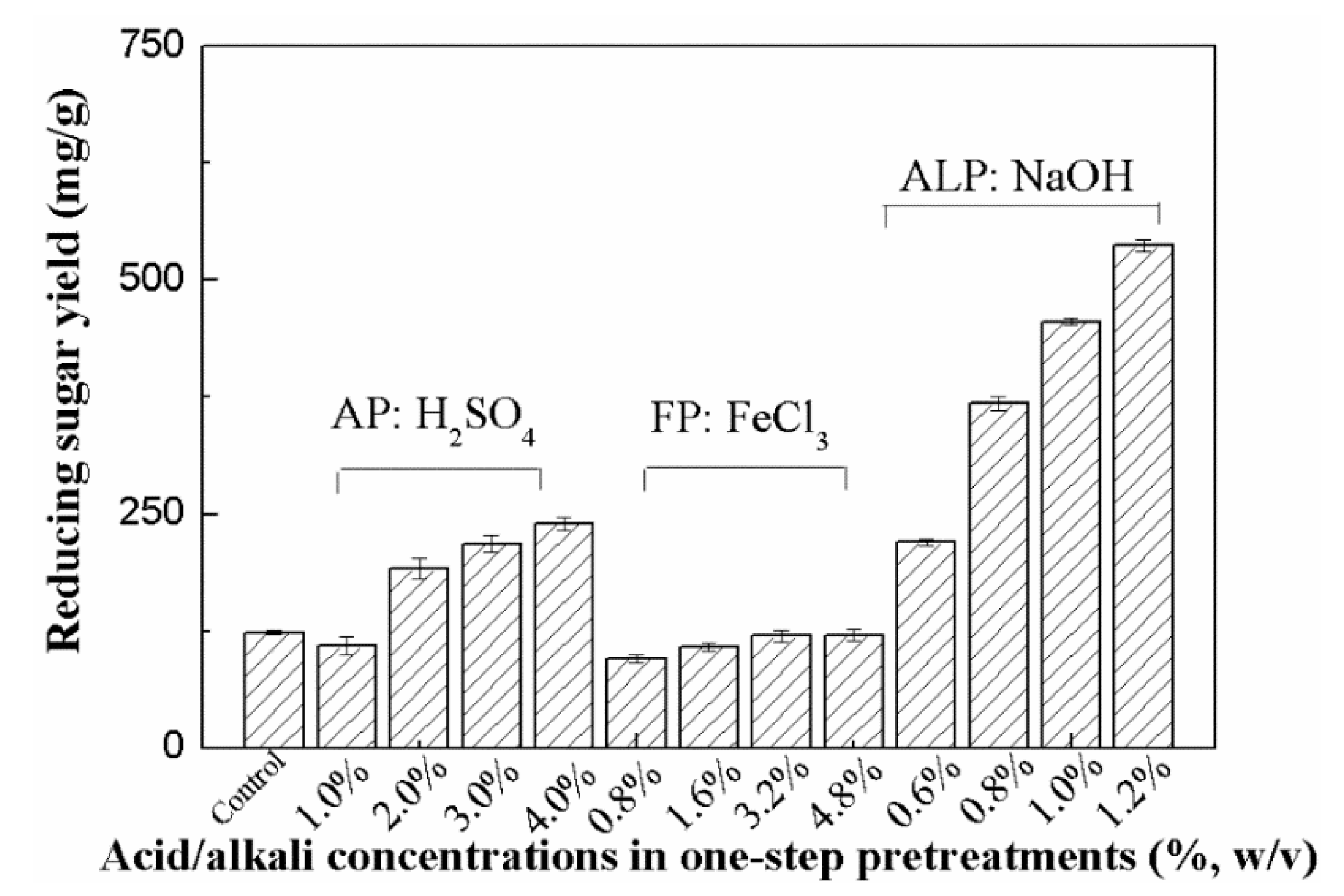
The solid samples separated from different pretreatments were collected for the subsequent enzymatic hydrolysis. The results shown in Figure 2 indicate that the reducing sugar yield of the untreated Arundo donax L. sample in its enzymatic hydrolysate was very low after 72 h (123.6 mg/g RS) because of its recalcitrance. In the case of AP with 2% H2SO4 solution, the yield clearly increased (191.2 mg/g pretreated stalk (PS)). With the increase in acid concentrations in AP (3%−4% H2SO4), the levels of reducing sugars reached 218.1~240.0 mg/g PS, increasing by 76%~94% as compared to the levels obtained in the untreated Arundo donax L. sample.
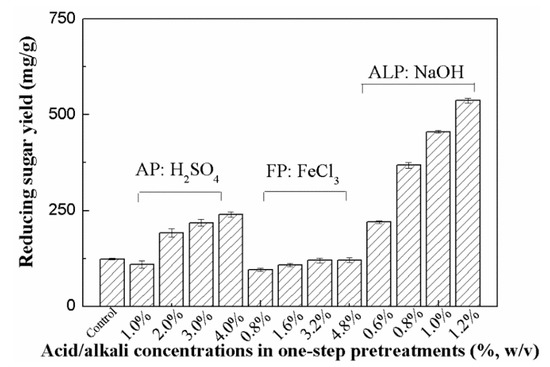
Figure 2.
Effect of AP, FP, ALP on enzymatic hydrolysis (Note: The levels of reducing sugars were determined by DNS assay).
The yields of reducing sugars from the FP samples were not high. As shown in Figure 2, the yields in the case of the Arundo donax L. samples pretreated with 0.8%~4.8% FeCl3 solution were only 95.7~120.7 mg/g PS. Among all pretreatments tested, ALP appeared to be a promising pretreatment method for improving the enzymatic digestibility of the pretreated stalk samples. The yield of reducing sugars in the case of the Arundo donax L. sample pretreated with dilute NaOH solution (0.8% NaOH) was significantly higher than those obtained in all AP and FP samples (368.2 vs. 95.7~240.0 mg/g PS). Moreover, the efficiency of the enzymatic hydrolysis resulted in a further enhancement, that is, the NaOH concentration increased to 1.2% and the highest reducing sugar yield reached as high as 537.0 mg/g PS, which was 434% of that observed in the untreated Arundo donax L. sample.
The biomass recalcitrance, including the lignin barrier, dense cellulosic structure, and a rigid cellulose–hemicellulose–lignin network structure, significantly impeded its enzymatic hydrolysis [12,13,14]. Previous studies have showed that hemicellulose removal has a positive effect on increasing the cellulose accessibility. As a result, enzymatic hydrolysis of cellulose was linearly linked to the ratio of hemicellulose removal for acid pretreatments [34]. Results in Table 1 and Figure 2 support the positive relationship between hemicellulose removal and the yields of reducing sugars in the enzymatic hydrolysis of the AP samples. However, the enhancement of enzymatic hydrolysis by AP and FP was still limited, which was consistent with some previous observations [16,35]. Reducing sugar yield in the enzymatic hydrolysis of bamboo pretreated by dilute acid was only 77.0 mg/g stalk, while glucose yields of 7%–11% were observed in another study by dilute acid pretreatment [16,35]. These results indicate that other factors such as delignification could play an important role in enhancing enzymatic hydrolysis of the Arundo donax L. samples besides hemicellulose removal. More significant delignification and much higher reducing sugar recovery were observed in ALP when compared to that obtained in AP and FP (537.0 mg/g PS vs. 120.7~240.0 mg/g PS) despite its relatively lower hemicellulose removal. Previous studies have also indicated that the enzymatic hydrolysis of different agricultural wastes and energy crops was obviously enhanced by alkaline pretreatments, which could be due to their abilities, including lignin removal, cellulose swelling, and the partial de-crystallization of cellulose [12,13,16]. Moreover, alkaline pretreatments also caused significant changes in the morphological structures in the pretreated samples (Figure 1). These results suggest that lignin removal and microstructural changes, which help decrease the unproductive binding of enzymes to lignin and improve the cellulose accessibility, appear to be some of the most important factors, which also result in higher sugar release in the enzymatic hydrolysis of the ALP sample [34].
3.2. Two-Step Pretreatment
An ideal pretreatment process aims (a) to improve enzymatic hydrolysis by dissolving lignocellulosic components and changing the microstructure; (b) to minimize the loss of sugars for an efficient sugar recovery in the pretreatment steps; and (c) to reduce the cost and energy demands. FP was effective at dissolving hemicellulose and recovering soluble sugars, but it failed to enhance the enzymatic hydrolysis. Although ALP significantly enhanced the enzymatic digestibility, the loss of soluble sugars in the pretreatment step was remarkable. There is no single strategy that could sufficiently meet all the requirements for the ideal pretreatment. Given the differences between FP and ALP in the way they deconstruct biomass and increase the hydrolysis, a two-step pretreatment using ferric chloride followed by alkaline pretreatment was proposed. In this process, easily degradable cellulosic components, especially hemicellulose, were designed to be effectively recovered from the first FP step. The FP sample was further treated by NaOH for delignification, hence improving the following enzymatic hydrolysis.
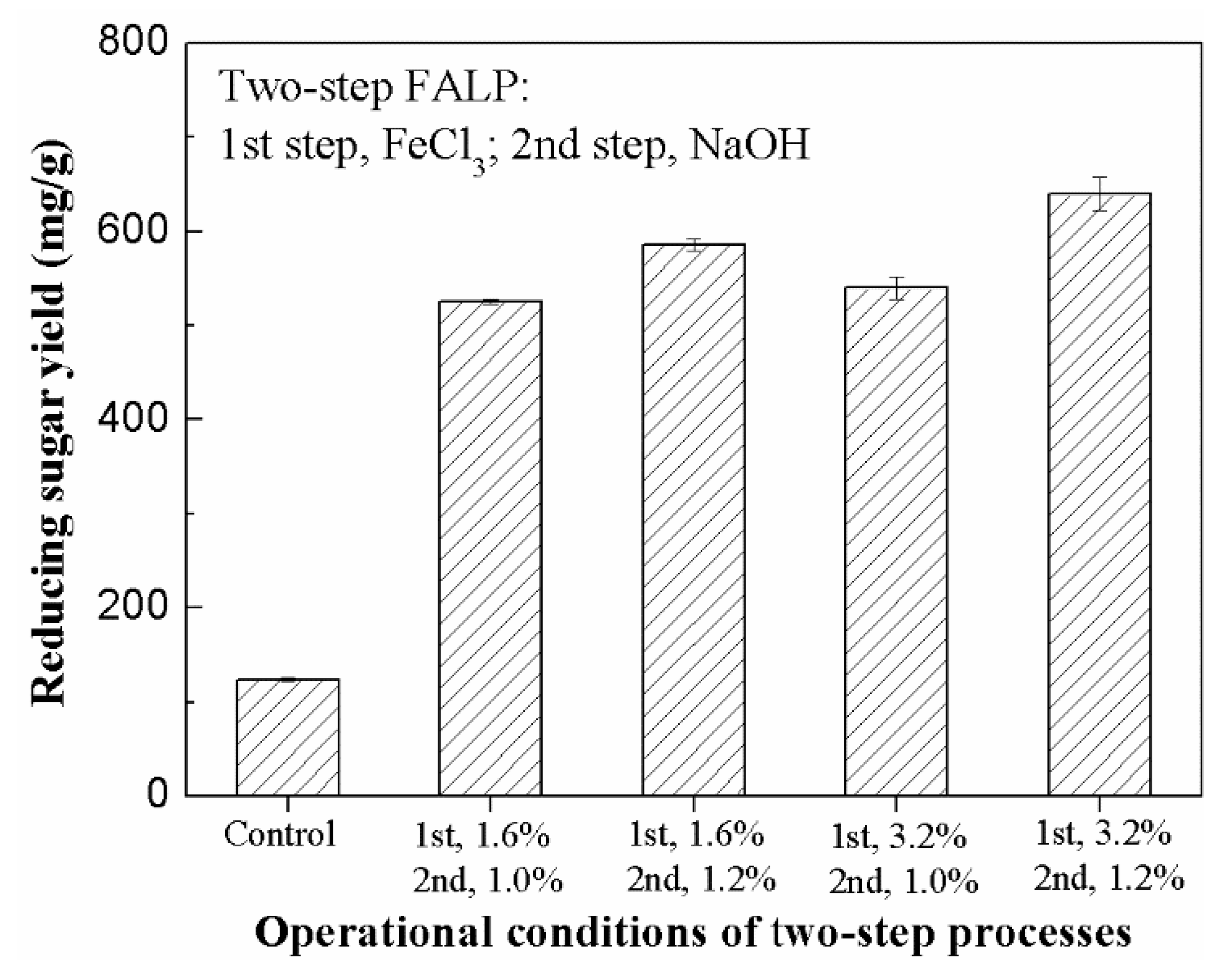
The samples were firstly treated using 1.6% or 3.2% FeCl3 at 120 °C for 30 min. The FP samples treated under these conditions were collected for the second ALP with 1.0% or 1.2% NaOH, respectively. The obtained FALP sample was used for the subsequent enzymatic hydrolysis. As shown in Figure 3, the reducing sugar level in the enzymatic hydrolysis of the FALP sample from relatively mild conditions (step 1, 1.6% FeCl3; step 2, 1.0% NaOH) reached as high as 525.3 mg/g PS, an increase of 338% compared to that obtained in the one-step FP process. In the enzymatic hydrolysis of the FALP sample from relatively severe pretreatment conditions (step 1, 3.2% FeCl3; step 2, 1.2% NaOH), the sugar yield reached the highest level of 639.7 mg/g PS, which was much higher than those obtained from both raw stalk and all of the AP, FP, and ALP samples.
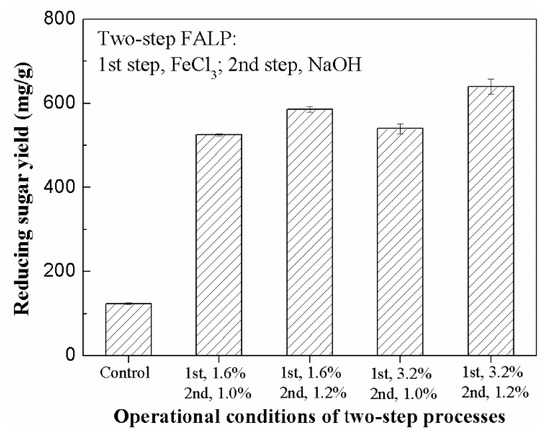
Figure 3.
Effect of two-step ferric chloride-alkaline pretreatment (FALP) on enzymatic hydrolysis (Note: The levels of reducing sugars were determined by DNS assay).
3.3. Mass Balance for One-Step/Two-Step Processes
The mass balance of the whole process was calculated to evaluate the total soluble sugar recovery. As shown in Table 2, both the soluble sugar level in the pretreatment liquid of AP and the reducing sugar yield in the enzymatic hydrolysis of the FP sample were not very high. As a result, total soluble sugar yields in both AP and FP were unattractive (236.2~255.9 mg/g RS). In the case of ALP, although the yield of sugar in the pretreatment stage was also low, the total sugar recovery reached as high as 379.7 mg/g RS as a result of the significant enhancement in enzymatic hydrolysis efficiency.

Table 2.
Mass balance of the whole process.
Further enhancement occurred in the case of FALP. The soluble sugar yields from the FP step, the ALP step and the enzymatic hydrolysis of the FALP sample were 189.3 mg/g RS, 24.9 mg/g RS and 639.7 mg/g PS, respectively. The yield of the corresponding total soluble sugar reached the highest level of 420.4 mg/g RS, which was 111% and 164% more than those achieved in the one-step ALP and FP, respectively.
The obtained sugar yield from the FALP sample was comparable to that reported in previous studies [14,15,34,36]. Reducing sugar yields of 146.9~700.0 mg/g PS were reported in the case of acid or alkaline pretreatments of various lignocellulosic wastes such as wild rice grass, Napier grass, Miscanthus, bamboo, pine foliage, and Eucalyptus [14,15,34,36]. In this study, the reducing sugar yield and total soluble sugar yield obtained so far with Arundo donax L., under mild operational conditions (120 °C for 30 min, pretreatment severity factor (log R0) of 2.1), were 639.7 mg/g PS and 420.4 mg/g RS respectively. This provides a promising alternative for efficient biofuel conversion from the Arundo donax L. feedstock.
Moreover, it is well known that the bioethanol process is limited because of its high cost. A detailed economic analysis of five typical pretreatments (acid, alkali, hot water, ammonia recycle percolation, and ammonia fiber explosion) indicated that all processes were capital intensive [37]. There is little differentiation between the projected economic performances of these typical processes, including acid/alkaline pretreatments [37]. In the past years, extensive studies have been conducted to reduce the high cost by optimizing enzyme production, pretreatment, enzymatic hydrolysis, and the following fermentation [38,39,40,41,42,43,44,45]. Recent economic analysis showed that the minimum ethanol selling price of bioethanol process decreased from 4.58 USD/gallon to 1.91~2.46 USD/gallon [38,39]. Notably, high cellulase cost, which was about 0.50 USD/gallon, accounted for 20%~30% of total costs [40]. What is surprising in the two-step FALP is that a significant ratio of fermentable sugars was recovered from the pretreatment stage as compared to that recovered in one-step ALP; hence, a substantial reduction in cellulase consumption was produced (it decreased by about 40%).
4. Conclusions
An efficient two-step FALP process was successfully developed for stepwise deconstruction and recovery of cellulosic components by combining ferric chloride pretreatment with dilute alkaline pretreatment. In the first step with ferric chloride pretreatment, easily degradable cellulosic components, especially hemicellulose, were well dissolved and effectively recovered. The FP sample from the first step was further delignified in the second ALP step to improve the enzymatic hydrolysis of the hardly degradable cellulose in the FALP sample. As a result, the two-step FALP process showed the highest total sugar yield of 420.4 mg/g RS for the whole pretreatment-enzymatic hydrolysis process of Arundo donax L.
Author Contributions
Conceptualization, X.C., S.T., Y.C., C.X., and Y.L. (Yonghong Liao); methodology, S.T., P.Y. and Y.L. (Ying Luo); software, Y.W.; validation, S.T. and Y.W.; formal analysis, P.Y. and Y.L. (Ying Luo); investigation, S.T., Y.C., C.X., Y.W., L.L., P.Y., Y.L. (Ying Luo), Y.G., Y.L. (Yonghong Liao), Q.Y. and X.C.; resources, C.X., Q.Y. and X.C.; data curation, S.T.; writing—original draft preparation, S.T., Y.C. and X.C.; writing—review and editing, X.C., and Q.Y.; supervision, X.C.; project administration, X.C.; funding acquisition, X.C. The authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21306009), Fundamental Research Funds for the Central Universities (No. 2017JBM073), the Beijing Training Program of Innovation and Entrepreneurship for Undergraduates (No. 202010004103), the Open Research Fund Program of Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry (No. CP−2019-YB6).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dziekonska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric Acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; González, A.; Ballesteros, M. Study of the application of alkaline extrusion to the pretreatment of eucalyptus biomass as first step in a bioethanol production process. Energies 2018, 11, 2961. [Google Scholar] [CrossRef]
- You, Z.; Zhang, S.; Kim, H.; Chiang, P.C.; Sun, Y.; Guo, Z.; Xu, H. Effects of corn stover pretreated with NaOH and CaO on anaerobic co-digestion of swine manure and corn stover. Appl. Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, S.S.; Rogers, K.M.; Xia, Y.A.; Zhang, B.; Suo, R.; Zhao, Y. A case of milk traceability in small-scale districts-Inner Mongolia of China by nutritional and geographical parameters. Food Chem. 2020, 316, 126332. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour. Technol. 2012, 104, 373–379. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Chen, G.; Chen, A.L.; Yang, S.M.; Ye, Z.H. Recent developments in application of stable isotope analysis on agro-product authenticity and traceability. Food Chem. 2014, 145, 300–305. [Google Scholar] [CrossRef]
- Camesasca, L.; Ramı’rez, M.B.; Guigou, M.; Ferrari, M.D.; Lareo, C. Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenergy 2015, 74, 193–201. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; D’Agosta, G.M.; Sanzone, E.; Mantineo, M. First results on evaluation of Arundo donax L. clones collected in Southern Italy. Ind. Crop Prod. 2006, 23, 212–222. [Google Scholar] [CrossRef]
- Tsai, M.H.; Lee, W.C.; Kuan, W.C.; Sirisansaneeyakul, S.; Savarajara, A. Evaluation of different pretreatments of Napier grass for enzymatic saccharification and ethanol production. Energy Sci. Eng. 2018, 6, 683–692. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Effectiveness of dilute oxalic acid pretreatment of Miscanthus×giganteus biomass for ethanol production. Biomass Bioenergy 2013, 59, 540–548. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Sabanci, K.; Buyukkileci, A.Q. Comparison of liquid hot water, very dilute acid and alkali treatments for enhancing enzymatic digestibility of hazelnut tree pruning residues. Bioresour. Technol. 2018, 261, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef]
- Pandey, A.K.; Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 2015, 192, 115–125. [Google Scholar] [CrossRef]
- Li, K.N.; Wan, J.M.; Wang, X.; Wang, J.F.; Zhang, J.H. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Products 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Liu, C.Z.; Cheng, X.Y. Improved hydrogen production via thermophilic fermentation of corn stover by microwave-assisted acid pretreatment. Int. J. Hydrog. Energy 2010, 35, 8945–8952. [Google Scholar] [CrossRef]
- Menegol, D.; Schol, A.L.; Dillon, A.J.; Camassola, M. Influence of different chemical pretreatments of elephant grass (Pennisetum purpureum, Schum.) used as a substrate for cellulase and xylanase production in submerged cultivation. Bioprocess Biosyst. Eng. 2016, 39, 1455–1464. [Google Scholar] [CrossRef]
- Kang, K.E.; Park, D.H.; Jeong, G.T. Effects of inorganic salts on pretreatment of Miscanthus straw. Bioresour. Technol. 2013, 132, 160–165. [Google Scholar] [CrossRef]
- Zhang, H.D.; Lyu, G.J.; Zhang, A.P.; Li, X.; Xie, J. Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour. Technol. 2018, 265, 93–101. [Google Scholar] [CrossRef]
- Dziekonska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-stage pretreatment to improve saccharification of oat straw and Jerusalem artichoke biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Y.H.; Yu, G.; Lee, W.H.; Jin, Y.S.; Morgenroth, E. Two-stage acidic–alkaline hydrothermal pretreatment of lignocellulose for the high recovery of cellulose and hemicellulose sugars. Appl. Biochem. Biotechnol. 2013, 169, 1069–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cai, D.; Zhang, C.; Li, S.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z. Comparison of two-stage acid-alkali and alkali-acid pretreatments on enzymatic saccharification ability of the sweet sorghum fiber and their physicochemical characterizations. Bioresour. Technol. 2016, 221, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.M.; Seo, J.W.; Kim, C.H. Sequential acid-/alkali-pretreatment of empty palm fruit bunch fiber. Bioresour. Technol. 2012, 109, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.A.; Dantas, P.V.F.; Santos, E.S.; Fernandes, F.A.N.; Macedo, G.R. Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz. J. Chem. Eng. 2015, 32, 23–33. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- SEPAC. The Methods for Water and Wastewater Monitoring and Analysis, 4th ed.; State Environmental Protection Administration of China & China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Goering, H.K.; Van-Soest, P.J. Agricultural Handbook No. 379. Forage Fiber Analyses, Apparatus, Reagents, Procedures and Some Applications; U.S. Department of Agriculture: Washington, DC, USA, 1970.
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity-relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Meng, X.Z.; Wells, T., Jr.; Sun, Q.N.; Huang, F.; Ragauska, A. Insights into the effect of dilute acid, hot water or alkaline pretreatment on the cellulose accessible surface area and the overall porosity of Populus. Green Chem. 2015, 17, 4239–4246. [Google Scholar] [CrossRef]
- Xin, D.; Yang, Z.; Liu, F.; Xu, X.; Zhang, J. Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: Structure properties and enzymatic hydrolysis. Bioresour. Technol. 2015, 175, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Si, S.L.; Chen, Y.; Fan, C.F.; Hu, H.Z.; Li, Y.; Huang, J.F.; Liao, H.F.; Hao, B.; Li, Q.; Peng, L.C.; et al. Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses-rich Miscanthus species under various alkali and acid pretreatments. Bioresour. Technol. 2015, 183, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Eggeman, T.; Elander, R.T. Process and economic analysis of pretreatment technologies. Bioresour. Technol. 2005, 96, 2019–2025. [Google Scholar] [CrossRef]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-economic and environmental analysis of ethanol production from agroindustrial residues in Colombia. Energy Fuels 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Denis, B.; Mark, M.W.; Robert, B. More than ethanol: A techno-economic analysis of a corn stover-ethanol biorefinery integrated with a hydrothermal liquefaction process to convert lignin into biochemicals. Biofuels Bioprod. Biorefin. 2018, 12, 497–509. [Google Scholar] [CrossRef]
- Du, J. Novozymes accelerates cellulosic ethanol commercialized. China WTO Trib. 2010, 10, 81. (In Chinese) [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A review of recent advances in high gravity ethanol fermentation. Renew. Energy 2018, 133, 1366–1379. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Q.; Liu, H.J.; Li, S.Z.; Jiang, Z.Q. Characterization of actinidin from Chinese kiwifruit cultivars and its applications in meat tenderization and production of angiotensin I-converting enzyme (ACE) inhibitory peptides. LWT-Food Sci. Technol. 2017, 78, 1–7. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, B.; Yan, Q.J.; Jiang, Z.Q. Comparative analysis on the distribution of protease activities among fruits and vegetable resources. Food Chem. 2016, 213, 708–713. [Google Scholar] [CrossRef]
- Nosrati-Ghods, N.; Harrison, S.T.L.; Isafiade, A.J.; Tai, S.L. Ethanol from biomass hydrolysates by efficient fermentation of glucose and xylose—A Review. Chembioeng. Rev. 2018, 5, 294–311. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Yang, H.Y.; Yan, Q.J.; Yang, S.Q.; Jiang, Z.Q.; Li, S.Z. Biochemical properties and application of a novel beta-1,3-1,4-glucanase from Paenibacillus barengoltzii. Food Chem. 2017, 234, 68–75. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).