Separation of BTX Fraction from Reservoir Brines by Sorption onto Hydrophobized Biomass in a Fixed-Bed-Column System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
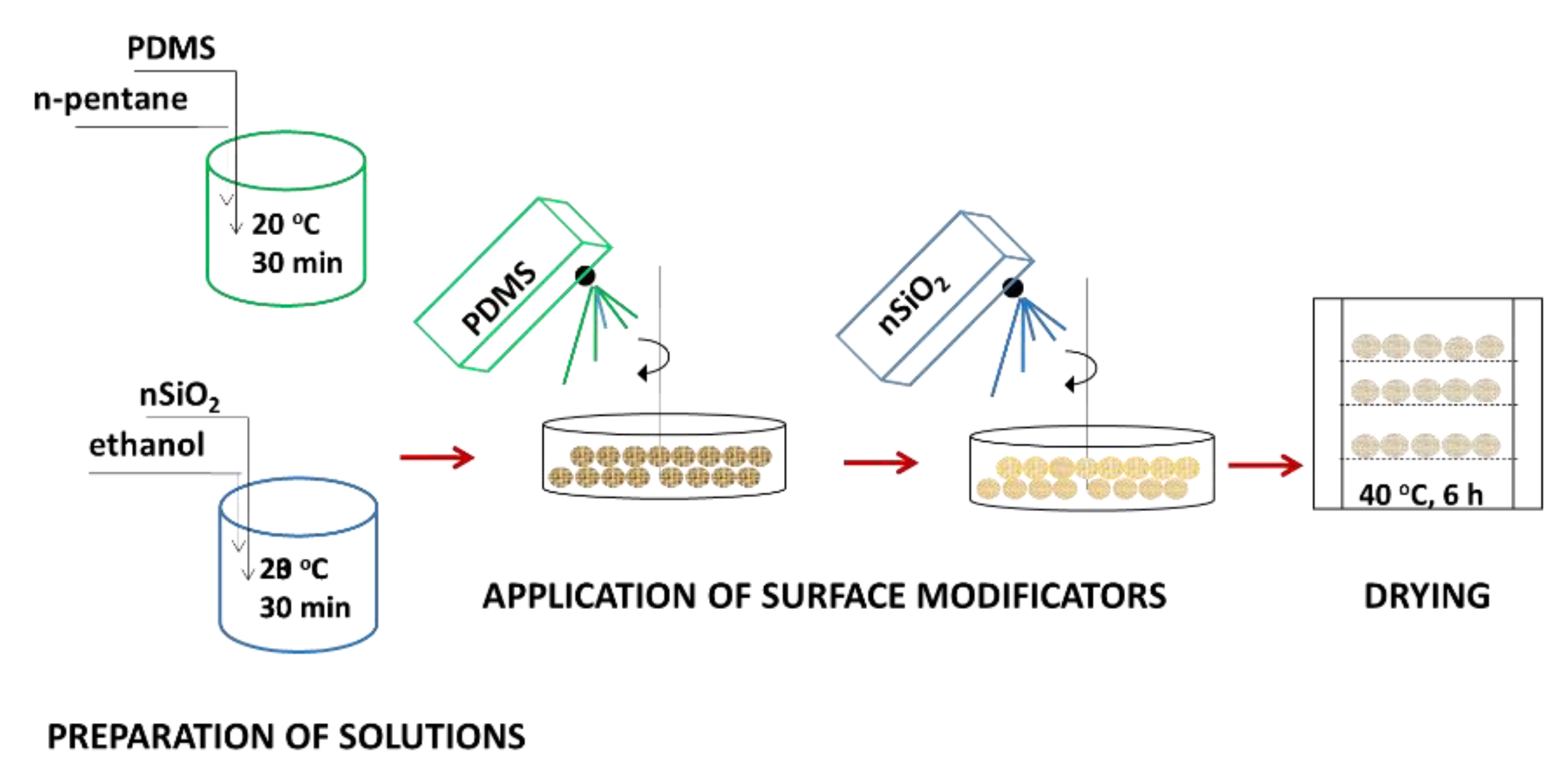
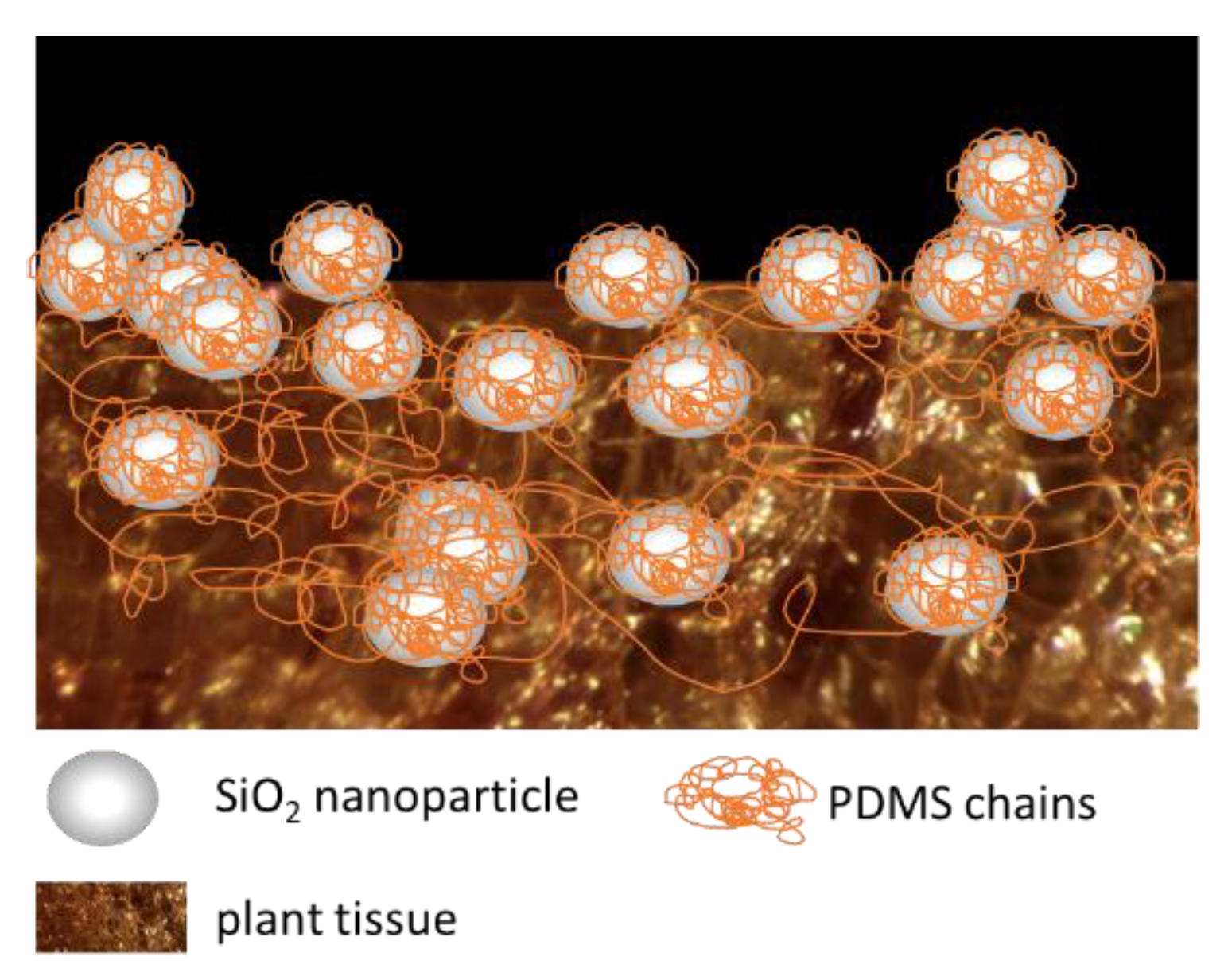
2.2.1. Preparation of Hydrophobized Plant Fibers
2.2.2. Scanning Electron Microscope Measurement
2.2.3. Filtration Process on Adsorption Beds
2.2.4. Crude Oil Characteristic
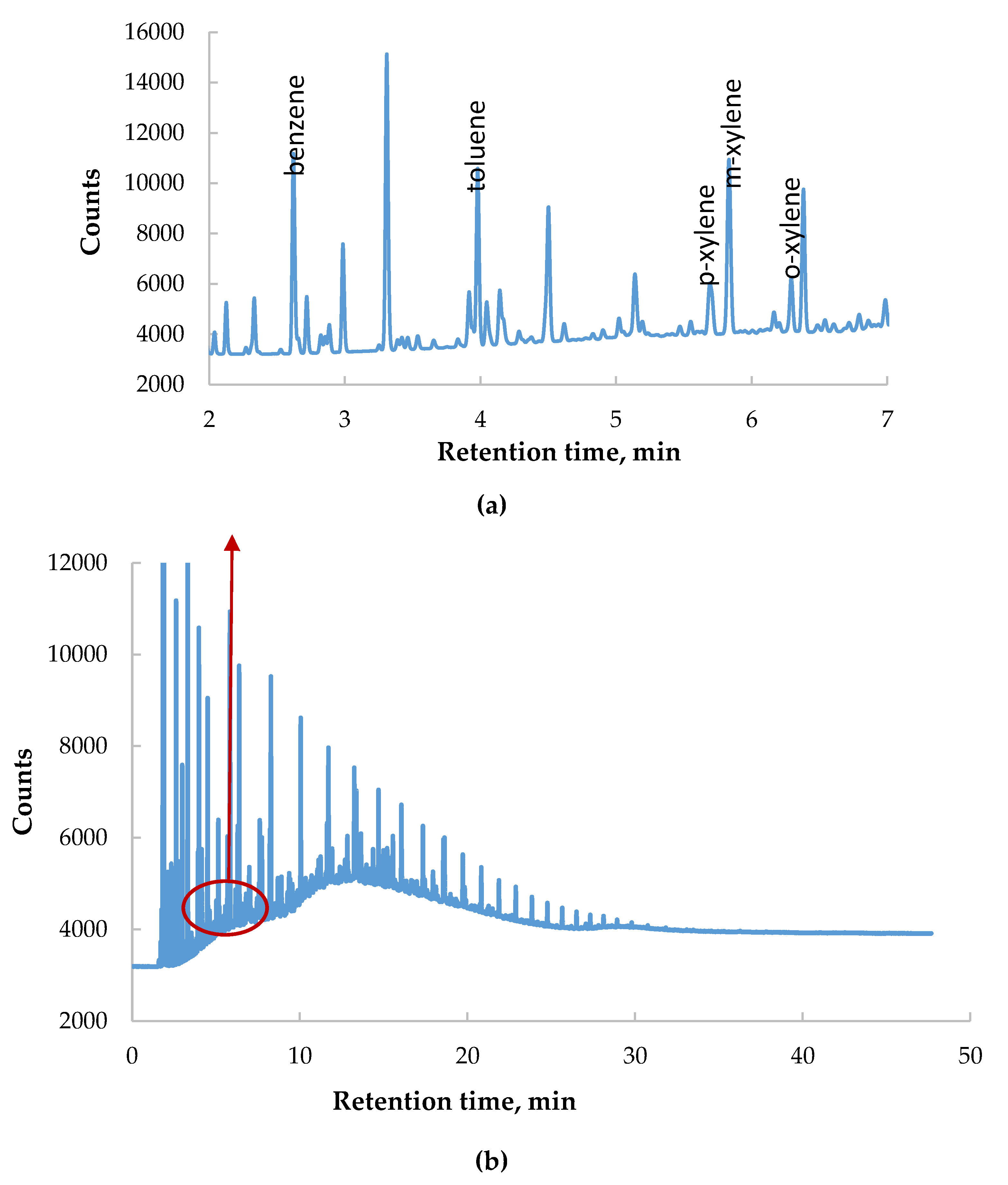
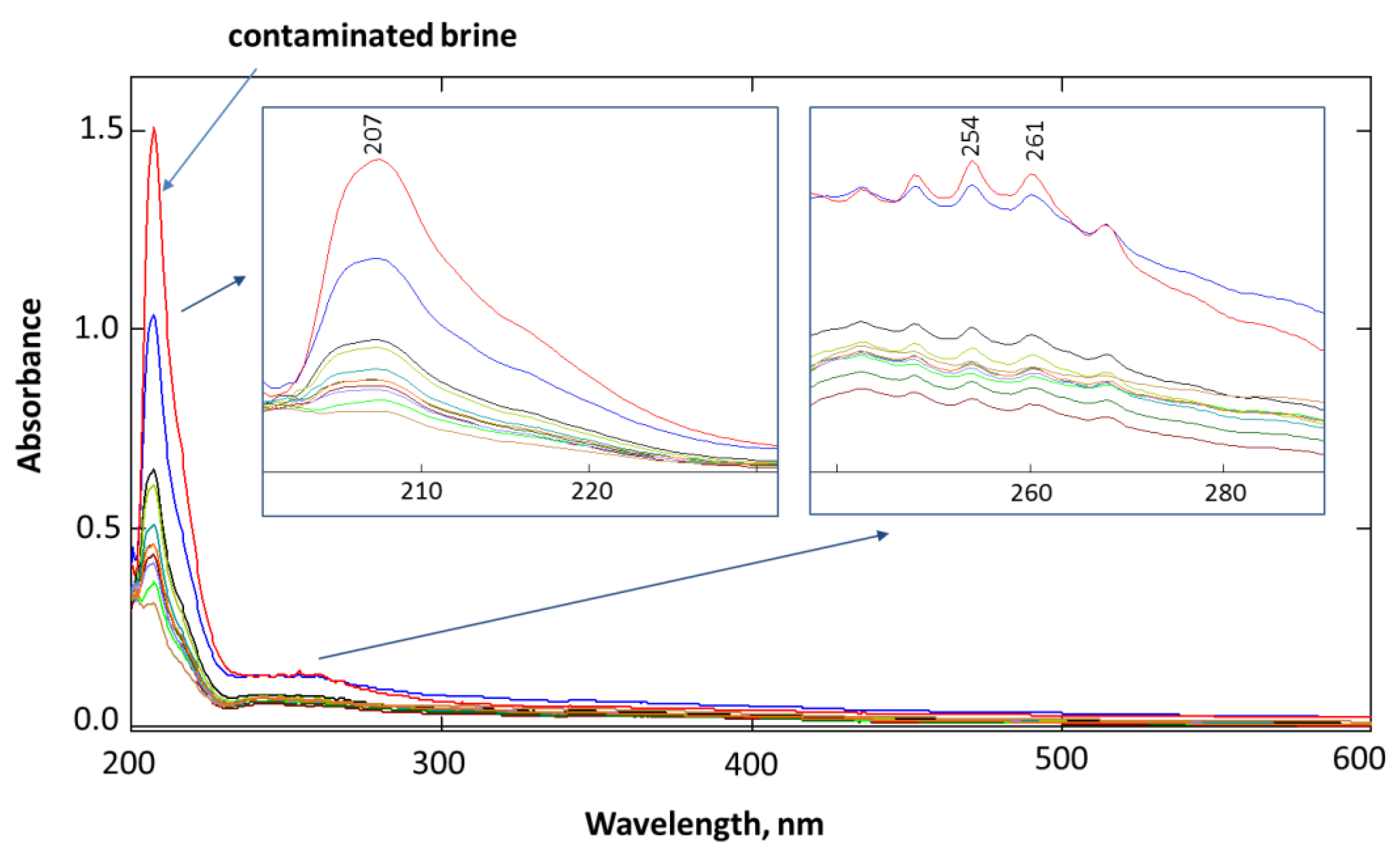
2.2.5. UV-Vis Spectroscopy Measurement
3. Results and Discussion
3.1. Crude oil Characteristic
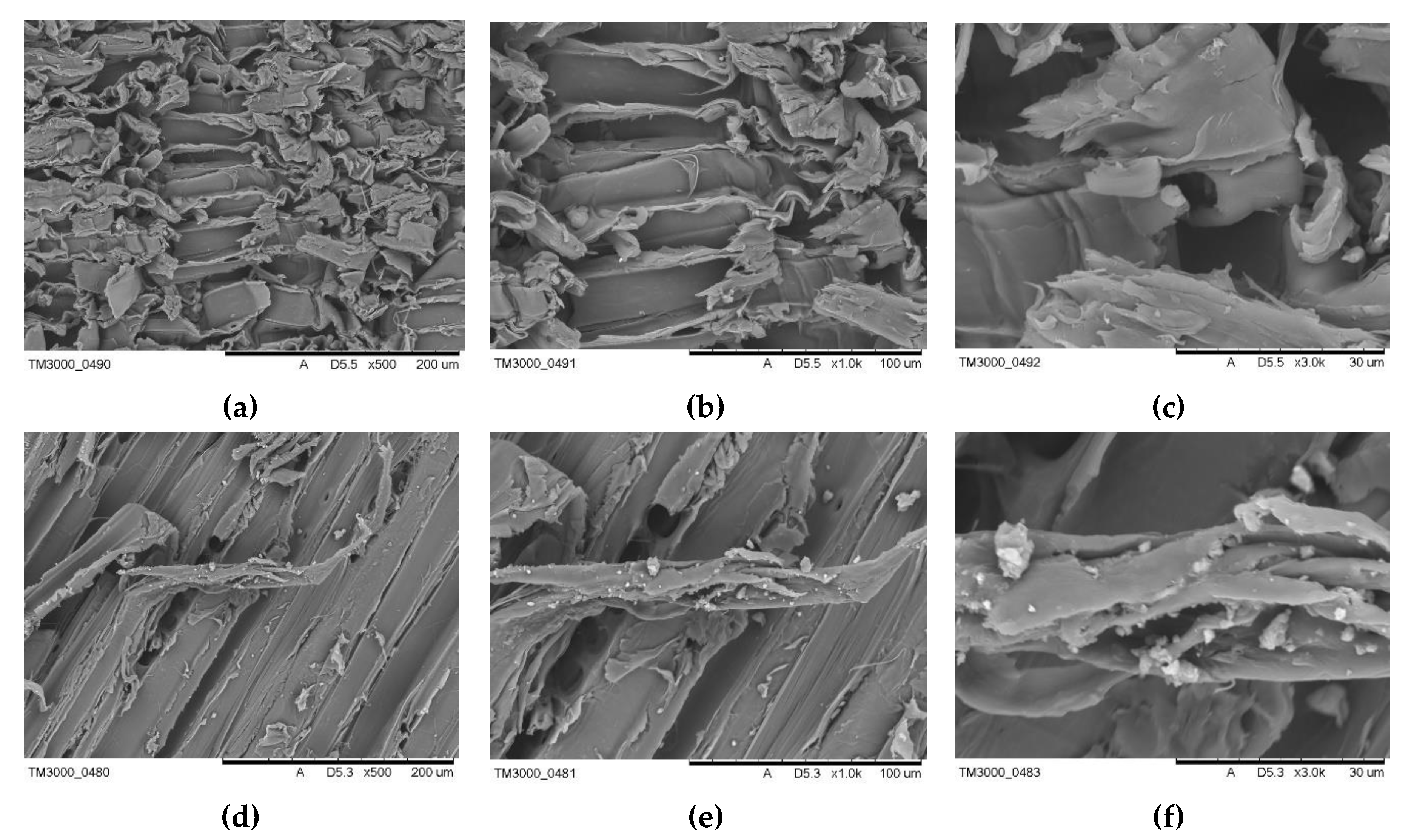
3.2. Morphology Analyses
3.3. Measurement of BTX Fraction in Model Brine During the Filtration Process on Absorption Beds
3.4. Column Study
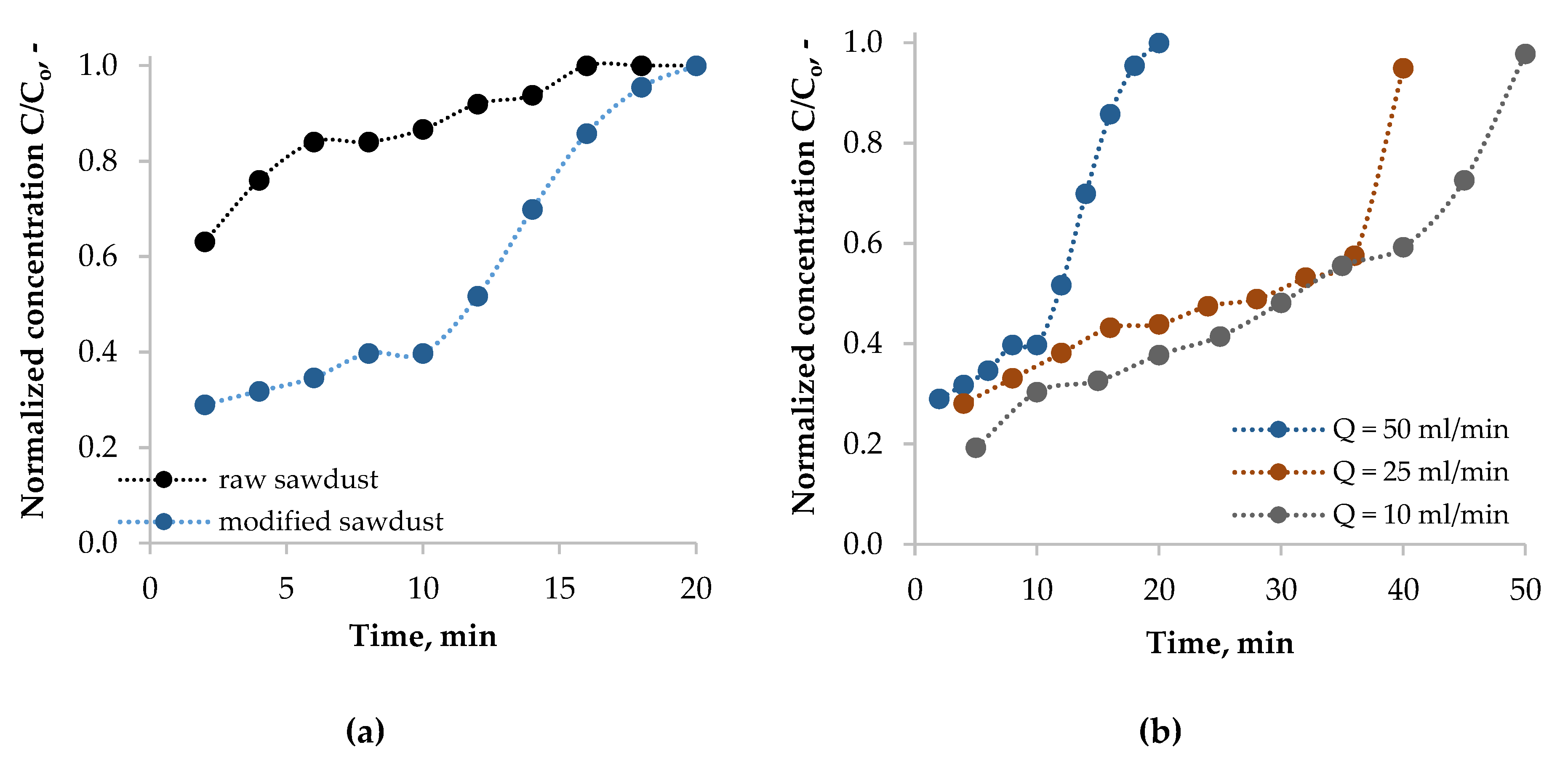
3.4.1. Effect of Feed Flow Rate and Sorbent Wettability on the BTX Breakthrough Curves
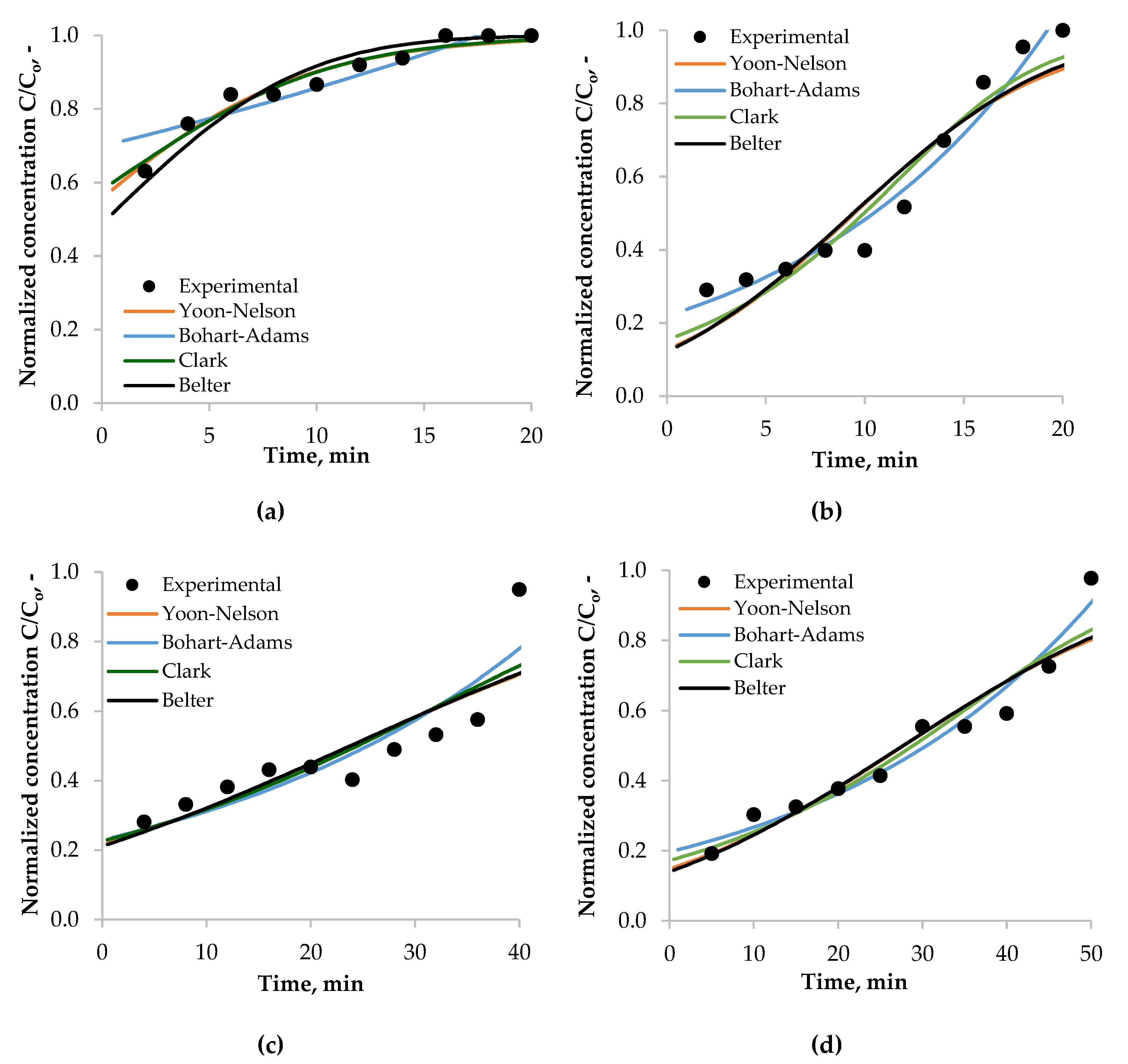
3.4.2. Modeling of Breakthrough Dynamics
3.4.3. Breakthrough Curves—Modeling and Determination of Model Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alley, B.; Beebe, A.; Rodgers, J.; Castle, J.W. Chemical and physical characterization of produced waters from conventional and unconventional fossil fuel resources. Chemosphere 2011, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.; Lee, K.; DeBlois, E.M. Produced Water: Overview of Composition, Fates, and Effects. In Produced Water Environmental Science Research; Ray, J.P., Engelhardt, F.R., Eds.; Springer: Boston, MA, USA, 1992; Volume 46, pp. 3–54. [Google Scholar]
- Tibbetts, P.J.C.; Buchanan, I.T.; Gawel, L.J.; Large, R. A Comprehensive Determination of Produced Water Composition. In Produced Water Environmental Science Research; Ray, J.P., Engelhardt, F.R., Eds.; Springer: Boston, MA, USA, 1992; Volume 46, pp. 97–112. [Google Scholar]
- Stewart, M.; Arnold, K. Produced Water Treatment Field Manual, 1st ed.; Gulf Professional Publishing: New York, NY, USA, 2011; pp. 1–134. [Google Scholar]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-Of-The-Art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Khan, N.A.; Engle, M.; Dungan, B.; Holguin, F.O.; Xu, P.; Carroll, K.C. Volatile-Organic molecular characterization of shale-Oil produced water from the Permian Basin. Chemosphere 2016, 148, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, G.T. Comparison of purge and trap GC/MS and spectrophotometry for monitoring petroleum hydrocarbon degradation in oilfield produced waters. Microchem. J. 2005, 81, 12–18. [Google Scholar] [CrossRef]
- Allen, E. Process water treatment in Canada’s oil sands industry: I. Target pollutants and treatment objectives. J. Environ. Eng. Sci. 2008, 7, 123–138. [Google Scholar] [CrossRef]
- Fillo, J.P.; Koraido, S.M.; Evans, J.M. Sources, characteristics, and management of produced waters from natural gas production and storage operations. In Produced Water Environmental Science Research; Ray, J.P., Engelhardt, F.R., Eds.; Springer: Boston, MA, USA, 1992; Volume 46, pp. 151–161. [Google Scholar]
- ATSDR 2017 Substance Priority List. Available online: https://www.atsdr.cdc.gov/SPL/ (accessed on 20 January 2020).
- Forero, J.E.; Ortiz, O.-P.; Duque, J.-P. Design and application of flotation systems for the treatment of reinjected water in a Colombian petroleum field. Cienc. Tecnol. y Futuro 2007, 3, 147–158. [Google Scholar]
- Qiao, X.; Zhang, Z.; Yu, J.; Ye, X. Performance characteristics of a hybrid membrane pilot-Scale plant for oilfield-Produced wastewater. Desalination 2008, 225, 113–122. [Google Scholar] [CrossRef]
- Ali, S.A.; Henry, L.R.; Darlington, J.W.; Occapinti, J. New filtration process cuts contaminants from offshore produced. Oil Gas. J. 1998, 96, 73–78. [Google Scholar]
- Oil and Gas Produced Water Management and Beneficial Use in the Western United States. Available online: https://www.usbr.gov/research/dwpr/reportpdfs/report157.pdf (accessed on 20 January 2020).
- Hamad, B.K.; Noor, A.M.; Afida, A.R.; Asri, M.N.M. High removal of 4-Chloroguaiacol by high surface area of oil palm shell-Activated carbon activated with NaOH from aqueous solution. Desalination 2010, 257, 1–7. [Google Scholar] [CrossRef]
- Kayvani, A.; Mckay, G.; Manawi, Y.; Malaibari, Z.; Hussien, M.A. Outstanding adsorption performance of high aspect ratio and super-Hydrophobic carbon nanotubes for oil removal. Chemosphere 2016, 164, 142–155. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hussien, M.; Abou-Rady, R. Equilibrium and kinetics behavior of oil spill process onto synthesized nano-Activated carbon. Am. J. Appl. Chem. 2015, 3, 22–30. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and facile fabrication of carbon aerogels from cellulose-Based waste newspaper for solving organic pollution. Carbohydr. Polym. J. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Blumenschein, C.D.; Severing, K.W.; Boyle, E. Walnut shell filtration for oil and solids. AISE Steel Technol. 2001, 78, 33–37. [Google Scholar]
- Hirs, G. Method of Filtering Oil from Liquids. US Patent No 3992291, 16 November 1976. [Google Scholar]
- Srinivasan, A.; Viraraghavan, T. Removal of oil by walnut shell media. Bioresour. Technol. 2008, 99, 8217–8220. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, C.; Sadeghi, F. Experimental study on oil removal in nutshell filters for produced-Water treatment. SPE Prod. Oper. 2017, 33, 1–9. [Google Scholar]
- Dong, T.; Wang, F.; Xu, G. Theoretical and experimental study on the oil sorption behavior of kapok assemblies. Ind. Crops Prod. 2014, 61, 325–330. [Google Scholar] [CrossRef]
- Dong, T.; Xu, G.; Wang, F. Adsorption and adhesiveness of kapok fiber to different oils. J. Hazard. Mater. 2015, 296, 101–111. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Zhu, Y.; Wang, A. Research and application of kapok fiber as an absorbing material: A mini review. J. Environ. Sci. 2017, 27, 21–32. [Google Scholar] [CrossRef]
- Khan, E.; Virojnagud, W.; Ratpukdi, T. Use of biomass sorbents for oil removal from gas station runoff. Chemosphere 2004, 57, 681–689. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.; Muñoz, G.A.; Arboleda-Faini, X.; Streitwieser, A.D. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Ribeiro, T.H.; Rubio, J.; Smith, R.W. A dried hydrophobic aquaphyte as an oil filter for oil/water emulsions. Spill Sci. Technol. Bull. 2003, 8, 483–489. [Google Scholar] [CrossRef]
- El-Sayed, M.; Ramzi, M.; Hosny, R.; Fathy, M.; Abdel Moghny, T. Breakthrough curves of oil adsorption on novel amorphous carbon thin film. Water Sci. Technol. 2016, 73, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption separation of condensate oil from produced water using ACTF prepared of oil palm leaves by batch and fixed bed techniques. Egypt. Fournal Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Cambiella, A.; Ortea, E.; Rios, G.; Benito, J.; Pazos, C.; Coca, J. Treatment of oil-In-Water emulsions: Performance of a sawdust bed filter. J. Hazard. Mater. 2006, 131, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S. Preparation and evaluation of fatty-Sawdust as a natural biopolymer for oil spill sorption. Chem. J. 2015, 5, 80–85. [Google Scholar]
- Ismail, A.S.; El-Sheshtawy, H.S.; Khalil, N.M. Bioremediation process of oil spill using fatty-Lignocellulose sawdust and its enhancement effect. Egypt. J. Pet. 2019, 28, 205–211. [Google Scholar] [CrossRef]
- Hussein, S.N.C.M.; Othman, N.H.; Dollah, A.; Rahim, A.N.C.A.; Japperi, N.S.; Ramakrishnan, N.S.M.A. Study of acid treated mixed sawdust as natural oil sorbent for oil spill. Mater. Today Proc. 2019, 19, 1382–1389. [Google Scholar] [CrossRef]
- PN-EN ISO 9377-2:2003 Water Quality – Determination of Hydrocarbon Oil Index – Part 2: Method Using Solvent Extraction and Gas Chromatography. Available online: https://infostore.saiglobal.com/en-us/Standards/PN-EN-ISO-9377-2-2003-943737_SAIG_PKN_PKN_2220471/ (accessed on 20 January 2020).
- Utvik, T.I.R. Chemical characterization of produced water from four offshore oil production platforms in the North Sea. Chemosphere 1999, 39, 2593–2606. [Google Scholar] [CrossRef]
- Sirivedhin, T.; Dallbauman, L. Organic matrix in produced water from the Osage-Skiatook Petroleum Environmental Research site, Osage county, Oklahoma. Chemosphere 2004, 57, 463–469. [Google Scholar] [CrossRef]
- Sidirasa, D.; Batziasa, F.; Schroeder, E.; Ranjanc, R.; Tsapatsis, M. Dye adsorption on autohydrolyzed pine sawdust in batch and fixed-Bed systems. Chem. Eng. J. 2011, 171, 3883–3896. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T. Surface modification of pine bark with quaternary ammonium groups and its use for vanadium removal. Chem. Eng. J. 2020, 385, 123967. [Google Scholar] [CrossRef]
- Knapik, E. Application of Nanocomposites for the Removal of Hydrocarbons from Produced (Reservoir) Water in Surface Facilities in Oil Fields. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, 2019. [Google Scholar]
- Thomas, O.; Brogat, M. Organic Constituents. In UV-Visible Spectrophotometry of Water and Wastewater; Thomas, O., Burgess, C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 73–90. [Google Scholar]
- Negrea, P.; Sidea, F.; Negrea, A.; Lupa, L.; Ciopec, M.; Muntean, C. Studies regarding the Benzene, Toluene and o-Xylene Removal from Waste Water. Chem. Bull. POLITEHNICA Univ. (Timişoara) 2008, 53, 1–2. [Google Scholar]
- Zoccolillo, L.; Allessandrelli, M.; Felli, M. Simultaneous determination of benzene and total aromatic fraction of gasoline by HPLC-DAD. Chromatographia 2001, 54, 659–663. [Google Scholar] [CrossRef]
- Peng, S.; He, X.; Pan, X. Spectroscopic study on transformations of dissolved organic matter in coal-to-liquids wastewater under integrated chemical oxidation and biological treatment process. J. Environ. Sci. 2018, 70, 206–216. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, S.; Wu, J.; Liu, B.; Cheng, Ch.; Akbar, M.; Chai, Y.; Memon, A. Fluorescence and photophysical properties of xylene isomers in water: With experimental and theoretical approaches. R. Soc. Open Sci. 2018, 5, 171719. [Google Scholar]
- Chu, K.H. Improved fixed bed models for metal biosorption. Chem. Eng. J. 2004, 97, 233–239. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Moura, C.P.; Vidal, C.B.; Barros, A.L.; Costa, L.S.; Vasconcellos, L.C.G. Adsorption of BTX (benzene, toluene, o-xylene, and p-xylene) from aqueous solutions by modified periodic mesoporous organosilica. J. Colloid Interface Sci. 2011, 363, 626–634. [Google Scholar] [CrossRef]
- Zeinali, F.; Ghoreyshi, A.A.; Najafpour, G. Removal of toluene and dichloromethane from aqueous phase by granular activated carbon (GAC). Chem. Eng. Commun. 2012, 199, 203–220. [Google Scholar] [CrossRef]
| Parameter | Method | Value |
|---|---|---|
| Density, g/L | ASTM D287 | 0.827 |
| Viscosity at 293 K, mm2/s | ASTM D445 | 3.05 |
| Acid number, mg KOH/g | ASTM D974 | 0.02 |
| Sulfur content, % wt | ASTM D6560 | 0.09 |
| Initial boiling point, K | ASTM D86 | 331 |
| Aromatic component, g/L | ||
| Benzene | 0.219 | |
| Toluene | 0.191 | |
| p-xylene | 0.087 | |
| m-xylene | 0.257 | |
| o-xylene | 0.075 | |
| Total BTX | 0.829 | |
| System | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Model parameters | Raw sawdust Q = 10 mL/min | Modified sawdust Q = 10 mL/min | Modified sawdust Q = 25 mL/min | Modified sawdust Q = 50 mL/min |
| Yoon–Nelson | ||||
| kYN, 1/min | 0.199 | 0.063 | 0.054 | 0.203 |
| , min | −1.141 | 27.779 | 23.874 | 9.473 |
| chi2 | 0.007 | 0.076 | 0.146 | 0.172 |
| RSME | 0.024 | 0.07 | 0.096 | 0.086 |
| Bohart–Adams | ||||
| kBA, L/(g*min) | 0.056 | 0.084 | 0.084 | 0.217 |
| No, g/L | 3.367 | 2.037 | 4.613 | 3.683 |
| chi2 | 0.021 | 0.039 | 0.106 | 0.042 |
| RSME | 0.041 | 0.046 | 0.08 | 0.051 |
| Clark | ||||
| kC, - | 2.747 | 69.767 | 35.782 | 91.899 |
| rC, 1/min | 0.225 | 0.096 | 0.086 | 0.306 |
| chi2 | 0.492 | 1.537 | 0.13 | 0.178 |
| RSME | 0.187 | 0.374 | 0.091 | 0.079 |
| Belter | ||||
| , min | 0.223 | 27.703 | 23.786 | 9.415 |
| σ, - | 31.646 | 0.926 | 1.247 | 0.859 |
| chi2 | 0.015 | 0.074 | 0.146 | 0.167 |
| RSME | 0.035 | 0.069 | 0.095 | 0.084 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapik, E.; Chruszcz-Lipska, K.; Stopa, J.; Marszałek, M.; Makara, A. Separation of BTX Fraction from Reservoir Brines by Sorption onto Hydrophobized Biomass in a Fixed-Bed-Column System. Energies 2020, 13, 1064. https://doi.org/10.3390/en13051064
Knapik E, Chruszcz-Lipska K, Stopa J, Marszałek M, Makara A. Separation of BTX Fraction from Reservoir Brines by Sorption onto Hydrophobized Biomass in a Fixed-Bed-Column System. Energies. 2020; 13(5):1064. https://doi.org/10.3390/en13051064
Chicago/Turabian StyleKnapik, Ewa, Katarzyna Chruszcz-Lipska, Jerzy Stopa, Marta Marszałek, and Agnieszka Makara. 2020. "Separation of BTX Fraction from Reservoir Brines by Sorption onto Hydrophobized Biomass in a Fixed-Bed-Column System" Energies 13, no. 5: 1064. https://doi.org/10.3390/en13051064
APA StyleKnapik, E., Chruszcz-Lipska, K., Stopa, J., Marszałek, M., & Makara, A. (2020). Separation of BTX Fraction from Reservoir Brines by Sorption onto Hydrophobized Biomass in a Fixed-Bed-Column System. Energies, 13(5), 1064. https://doi.org/10.3390/en13051064








