Abstract
Kinetic hydrate inhibitors (KHI) and anti-agglomerants (AA) rather than thermodynamic hydrate inhibitors (THI) are often used for flow assurance in pipelines. This is because they require much lower dosages than thermodynamic inhibitors. Although the hydrate-phase equilibria are not affected, KHI and AA prevent the formed hydrate crystals from growing to a bulky state causing pipeline blockage. However, these KHIs might have huge environmental impact due to leakages from the pipelines. In this study, two biodegradable AA candidates from natural sources (that is, lecithin and lanolin) are proposed and their performances are evaluated by comparing them with and without a conventional AA (Span 80, sorbitan monooleate). At 30% and 50% water cut, the addition of AA materials was found to enhance the flow characteristics substantially in pipelines and hardly affected the maximum value of the rotational torque, respectively. Considering the cost-effective and environmental advantages of the suggested AA candidates over a conventional AA such as Span 80, the materials are thought to have potential viability for practical operation of oil and gas pipelines. However, additional investigations will be done to clarify the optimum amounts and the action mechanisms of the suggested AAs.
1. Introduction
Gas hydrates are crystal compounds formed and stabilized by the accommodation of guest species with low molecular weights in cages among the lattice structures of hydrogen-bonded water molecules [1]. Recently, they have been investigated for the potential application to gas separation and recovery technologies because such compounds can hold huge amounts of gas molecules in a unit volume of the solid phase. However, gas hydrates are still regarded as hazardous materials in the oil and gas industry because they are known to cause blockages or explosion problems for oil and gas pipelines by its unexpected formation [2]. Gas hydrates can be formed in pipelines when oils, water and light hydrocarbon gases pass through the pipelines under low temperature and high pressure conditions. Once the solid crystals are formed, they attach to the wall of a pipeline and grow until the oil and gas production is stopped due to the obstruction [1,2]. To avoid such damage from blockages, thermodynamic hydrate inhibitors (THIs, such as alcohols, glycols and salts) can be used [3,4,5,6,7,8]. They shift the hydrate equilibrium so as to make the operating pipelines safe from hydrate formation. However, usage of the THIs is not cost-effective and not practical in limited spaces such as ocean plants for loading or continuous supply of large quantities of the THIs because, to be effective, the THIs must be kept at high concentrations (10–40 wt%) [1,9,10].
In this regard, kinetic hydrate inhibitors (KHIs) play an inhibition role at a lower dosage by retarding crystal formation and particle growth in ways that limit bulk [8,9,10,11]. Recently, KHIs were reported to cause anomalous hydrate dissociation inside the hydrate stability zone [12]. This provides flow assurance of the produced oil and gas, for which reason KHIs are applied in the field more often than THIs, although gas hydrate can still form in the pipelines under hydrate-forming conditions. Because KHIs are generally water-soluble polymers with a size similar to the unit structure of the gas hydrate, they have affinity with the formed gas hydrate particles (or unit crystals) [7,13]. Such affinity between KHIs and the formed hydrate particles prevents water molecules from approaching the formed hydrate (and allowing it to grow bigger) and significantly retards the blockage problem even under hydrate-forming conditions. There are also reports of a few ionic liquids that act as dual function inhibitors (playing both thermodynamic and kinetic inhibition roles) [14,15,16,17]. Basically, a dual function inhibitor is designed to improve inhibiting performance by playing two inhibition roles. This is done by changing the molecular properties determined mainly by a cation and/or an anion in an ionic liquid molecule [15,16]. Anti-agglomerants (AAs) are another type of hydrate inhibitor. When AAs are applied to pipeline flows (generally those with low water content), they hinder the agglomeration of formed hydrate crystals and support the flow of the mixture of fluids with hydrates of small particle sizes in the pipelines [18,19,20]. Because the AAs are generally not biodegradable, they can have a large environmental impact if they are released from the pipelines into the ocean. To resolve such environmental issues, a few biodegradable surfactants have been suggested as potential AAs, but they still do not have sufficient performance compared with conventional AAs [21,22,23]. Although low dosages of KHIs or AAs have recently been used to prevent hydrate formation, the materials used in the industry are toxic and accumulate in living things.
In this study, we propose two biodegradable AA candidates from natural sources having very low environmental impact even in the case of leakage from pipelines. We selected lecithin and lanolin as the AA candidates (or in principal, water-in-oil emulsifiers) because they are used mainly in oil-dominated systems (oil contents of 50% and higher). We then investigated their anti-agglomeration performance under hydrate-forming temperatures and pressure conditions. In addition, their anti-agglomeration performances were compared with and without a conventional AA (in this study, Span 80, one of the most investigated surfactants). Lecithin, a mixture of neutral and polar lipids, is currently obtained from vegetable or animal sources. Lecithin has wide range of applications such as industrial, cosmetic and food products, because it can strongly emulsify so as to decrease the interfacial tension between oil and water phases. Lanolin, a fat-like grease obtained from wool, also possesses a strong emulsifying characteristic. Therefore, it can also be applied to industrial, dermatological and cosmetic fields as well. Their emulsifying functions make us consider that these two natural chemicals may be good candidates for biodegradable AAs by preventing hydrate plug formation with good anti-agglomeration performance. Experimental results obtained in this study suggest that the new biodegradable emulsifiers have potential as AAs because they have similar or better performance than that of the conventional Span 80. Further studies at various water cuts and various additive concentrations are expected to shed light on the action mechanisms of AAs from natural sources and the formation mechanisms of natural gas hydrates in oil-dominated fluid systems.
2. Materials and Methods
The synthetic ternary natural gas used in this study was manufactured and supplied by Anjeon Gas (Republic of Korea). The synthetic ternary natural gas includes methane (CH4, 92.5 mol%), ethane (C2H6, 4.6 mol%) and propane (C3H8, 2.9 mol%), which is the same synthetic ternary natural gas that used in our previous report [21]. The HPLC grade water and mineral oil (light reflective index of n20/D 1.467, density of 0.838 g/mL at 25 °C) were purchased from the Duksan Company (Republic of Korea) and Sigma-Aldrich, respectively. While the mineral oil and water can be dispersed with mixing, emulsions are prepared using surfactants, basically in the same way of our previous investigation [22]. Two biodegradable surfactant candidates from natural sources (lanolin and lecithin) and a third control group (Span 80) were used in this study. Lanolin, generally known as wool grease or wool wax, and lecithin from soybeans were respectively purchased from Sigma-Aldrich (L7387) and Junsei Chemical (Japan). Span 80 (sorbitan monooleate) was purchased from Sigma-Aldrich. All the materials used in this work were used without further treatment after purchase. Liquid samples had 24 mL of a total volume, and their water cuts (volume of water per total liquid volume, or simply water contents (vol%) in mixed oil and water liquids) were set at 30% or 50%.
Figure 1a shows an experimental diagram used to perform the kinetic inhibition tests for gas hydrate formation with the synthetic natural gas, which was similar to the one in our previous report [21,22]. First, water was dispersed or emulsified in the liquid phase by means of the mixing of a certain volume of light mineral oil and deionized water in the presence or absence of surfactants (0.5, 1.0 and 1.5 wt%) at 7500 rpm for 2.0 min with a T25 ULTRA-TURRAX homogenizer (IKA, Staufen, Germany). Then, the liquid phase with dispersed or emulsified water in oil was introduced into a Midiclave reactor (Büchiglasuster, Uster, Switzerland) in a steel pressure reactor with an internal volume of 1.0 L. This reactor was connected with a temperature controller and a circulating chiller (FP 88, Julabo, Seelbach, Germany) using an aqueous ethylene glycol solution [21,22]. A thermocouple, a pressure transducer and a torque magnetic drive were inserted into the reactor, whose accuracies were ±0.1 K, ±0.01 bar, and ±0.01 N·cm, respectively. The temperature, pressure and torque values in the reactor were measured with the above instruments and recorded by a data acquisition system every second until the end of each inhibition test. A 300 mL volume of liquid with water cut of 30% or 50% was introduced in the pressure reactor. When the aqueous phased with an AA, the concentrations of the AAs were expressed as the weight percentage in aqueous phase. After the synthetic ternary natural gas in the cylinder was pressurized using a gas booster, the reactor was purged using the pressurized gas three times to remove residual air in the reactor system. For the kinetic hydrate inhibition tests after purging, the synthetic natural gas was charged into the pressure vessel up to the desired pressures and the liquid phase was agitated using a magnetic torque drive at 1000 rpm [21]. Because the temperature of the vessel was set to decrease from 298.2 to around 275.0 K, the final pressure in the reactor was reduced from 100 bar to about 80 bar due to gas consumption or enclathration into the hydrate phase (Figure 1b). The cooling rate was approximately 0.40 K/min. In addition, the sub-cooling temperature of such a condition was calculated to be about 18.0 K by CSMGem. Every kinetic hydrate inhibition test with and without an AA material was performed three times at least.
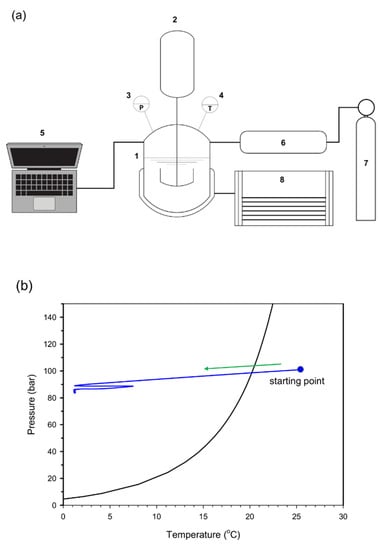
Figure 1.
(a) Schematic diagram of the experimental system. The synthetic natural gas (7) was injected into the reactor (1) through a booster (6). The experimental temperature was controlled by an external chiller (8), while the temperatures (4), the pressures (3) and the torques of a magnetic drive (2) were recorded in a data acquisition computer (5). (b) Temperature profiles of the experimental measurements starting at 298.2 K and 100 bar.
3. Results and Discussion
In this research, we proposed using two biodegradable surfactants (AAs) from natural sources with higher performance than conventional AAs. We selected lecithin and lanolin as the biodegradable surfactant candidates and compared their performance for anti-agglomeration of formed hydrates with and without the control AA (that is, the most popular conventional AA, Span 80). The experimental results were obtained at 30% and 50% water cut (volume of water per total liquid volume, or simply water contents (vol%) in mixed oil and water liquids), assuming that the produced fluid was transported through sub-ocean oil and gas pipelines in which the temperature and pressure conditions were within the hydrate-forming range [21].
Figure 2 shows the rotational torques as a function of time in the absence or presence of the AA additives at 30% water cut. When the mixtures of water and oil, with and without the AA additives, were injected into the reactor and the experimental conditions were controlled at high pressure and low temperature conditions, solid particles of the gas hydrate formed in the reactor so as to increase the resistance to the flow (that is, the rotational torque increased) of the liquid-solid hydrate mixture. For about 2 h, the torque values were obtained as a constant value of about 9.5 N·cm, which did not mean there was no hydrate formation, nor included the induction time (the time elapsed until the appearance of a detectable volume of hydrate phase). We suggest that formed hydrate particles did not affect the torque values because they were distributed in a large volume of the mixed oil and water liquids. Then, as shown in the figure, the rotational torque increased rapidly up to the maximum of about 22 N·cm after 3.0 h when no additive was used. The formed hydrate particles were then dispersed evenly in the liquid phase through agitation, and the rotational torque decreased and converged to the terminal value. However, when the AAs were added to inhibit hydrate formation in the system, a rapid increase of the rotational torque due to hydrate formation was not observed and the terminal value of the rotational torque was also lower than that with no additive. When 0.5 wt% Span 80, 0.5 wt% lecithin and 1.0 wt% lanolin were added to the system, the maximum torque values were 13.0, 11.6 and 12.2 N·cm and the terminal torque values were 10.5, 10.4 and 11.5 N·cm. The time-torque measurements with and without an AA material were performed three times at least. Although there were a few deviations in exact values (rotational torques, time) in the plots, the overall trends (the final rotational values at the end of the experiments, particularly) showed good repeatability within ±3.0%. In the case of lanolin, effective anti-agglomeration was observed at concentrations of 1.0 wt% or higher due to its high molecular weight (or no significant observations were found at lower concentrations of 0.5 wt%, for example). In particular, when the concentration of the lanolin added was increased to 1.5 wt%, the flow characteristics of the fluid improved notably (i.e., the rotational torque changed little throughout the experiment). The water conversions into the hydrate formed at the end of the experiments were found to be 27.8–29.2% with and without the AA materials. The conversions changed little because the addition of the AA species did not change the hydrate-phase equilibria, and so the consumed gas was almost the same when the experimental conditions were the same. Considering the above results, it can be said that the biodegradable AA candidates (lecithin and lanolin) showed much higher anti-agglomeration effects than with no additive, and showed improved or equivalent anti-agglomeration effects compared with the conventional AA, Span 80.
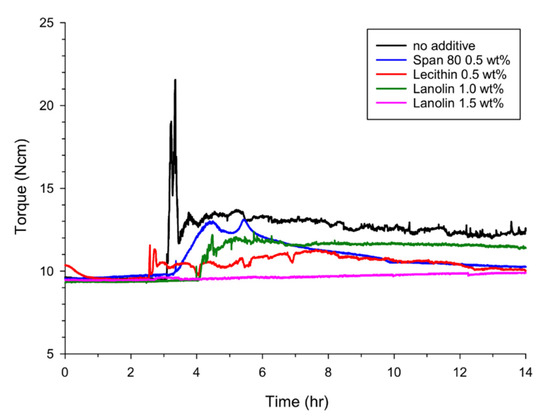
Figure 2.
Rotational torques depending on time in the absence or presence of the anti-agglomerant (AA) additives at 30% water cut. 0.5 wt% Span 80, 0.5 wt% lecithin, 1.0 wt% lanolin and 1.5 wt% lanolin were added as the AAs.
Although AAs are normally used when the fluid in a system has a low water content, the rotational torque depending on the time at 50% water cut (with and without AAs) was also measured to verify the anti-agglomeration effect of the AAs under conditions favoring rapid hydrate formation (Figure 3). As expected, hydrate formation progressed faster with increased water content, which led to rapid changes of the rotational torque with and without the AAs. The starting points of the rotational torque changes were found to be at about 2.0 h, which was faster than 3.0 h at a 30% water cut. Under four experimental conditions, no additive, 0.5 wt% Span 80, 0.5 wt% lecithin and 1.0 wt% lanolin added to the system, the maximum torque was 27.4, 28.0, 30.0 and 23.9 N·cm, respectively. This indicated that no significant anti-agglomeration effect was obtained from the addition of the AAs. However, the terminal values of rotational torque after 16 h were found to be 19.5, 19.5 and 17.0 N·cm from adding Span 80, lecithin and lanolin, respectively, which showed some improvement from 23.7 N·cm with no additive. In addition, when the concentration of lanolin added was increased to 1.5 wt%, the maximum and terminal torque values obtained were 24.0 and 13.9 N·cm. The water conversions into the hydrate formed at the end of the experiments were found to be 28.2–29.4% regardless of the AA materials. In this case (50% water cut), higher water conversions were observed because the amount of water was increased while the amount of gas (experimental pressure) remained constant. All of these results meant that the flow characteristics of the fluid were enhanced in spite of the rapid hydrate formation.
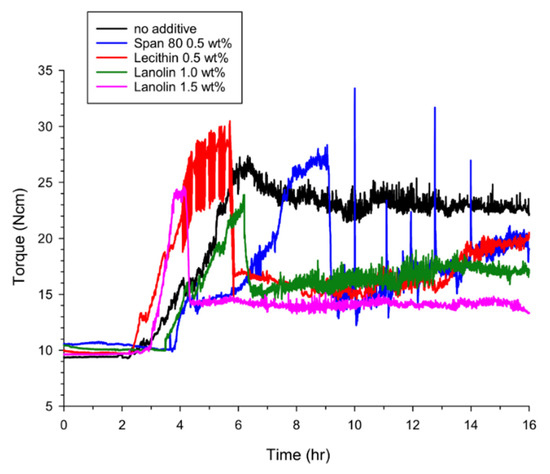
Figure 3.
Rotational torques depending on time in the absence or presence of the AA additives at 50% water cut. 0.5 wt% Span 80, 0.5 wt% lecithin, 1.0 wt% lanolin and 1.5 wt% lanolin were added as the AAs.
Next, the same kinetic experiments at 30% and 50% water cuts were also measured to identify the anti-agglomeration effects of the AAs depending on the concentrations added. Figure 4 shows the mechanical torque changes at the two water cuts for Span 80 while hydrate formation progressed in the fluid. When the concentration of Span 80 added was doubled from 0.5 wt% to 1.0 wt%, the terminal torque at 30% water cut and the maximum torques at both water cuts changed little for the same water cut. However, the terminal torque at 50% water cut was found to decrease when the concentration of Span 80 added was doubled. From the experiments, we suggest that the greater effect for anti-agglomeration of formed hydrates was not expected, even when the added concentration of Span 80 was increased under our experimental conditions. In addition, Span 80 also showed its anti-agglomeration effect at a lower concentration in the 30% water cut, which is why AAs are generally applied to industrial fields with lower water content. At 50% water cut or higher, hydrate-formation rates dominated the anti-agglomeration effects by an AA so that the flow assurance could not be secured by using an AA. In particular, drastic increases or surges (higher than the initial maximum torque value) of the rotational torques were observed repeatedly for Span 80 at 50% water cut, as illustrated in Figure 3 and Figure 4. Such surges were thought to be attributable to the repeated behaviors of the formation/agglomeration of hydrate particles near the stirring axis and then the breaking of the agglomerated state. In this regard, Span 80 is thought to present the potential hazard of crystal agglomeration, leading to complete blockages in oil and gas pipelines when it is applied to situations with higher water cuts.
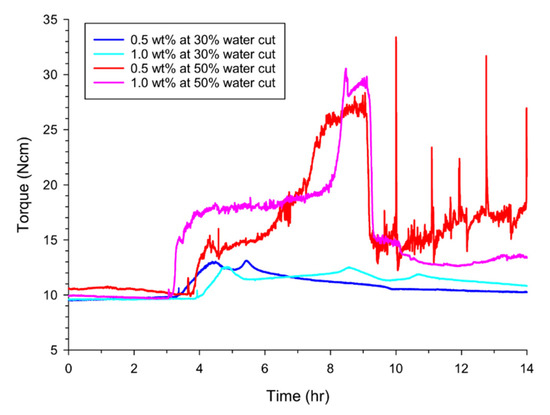
Figure 4.
Anti-agglomeration effects of Span 80 depending on the concentrations added. Hydrate formation kinetics (or rotational torques) were measured at two water cuts of 30% and 50% with two concentrations of 0.5 and 1.0 wt%.
Meanwhile, the mentioned disadvantages of Span 80 were improved in the case of lecithin (Figure 5a). Even though the maximum and the terminal torques showed little difference as the concentration was increased from 0.5 to 1.0 wt% at 30% water cut, the increase in the concentration greatly enhanced the situation by reducing both torque values (the maximum from 30.5 to 20.0 N·cm, and the terminal from 20.0 to 11.6 N·cm). This indicated a significant improvement of the flow characteristics. In addition, the other candidate (lanolin) also showed a major reduction of the maximum and terminal torques when the concentration added was increased from 1.0 to 1.5 wt% (Figure 5b). In particular, the torque values were maintained for at least 16 h before hydrate formation. Although the maximum torque was increased (from 24.0 N·cm at 1.0 wt% to 24.5 N·cm at 1.5 wt%), due to the increased water content and faster hydrate-forming rates at the 50% water cut, the terminal value was found to drop from 17.5 to 14.0 N·cm when the lanolin concentration was increased from 1.0 to 1.5 wt%. The observed differences between Span 80 and the two biodegradable AA candidates were associated with the action mechanism of AAs. As proposed by Majid et al. [24], the dispersion of water molecules in the oil phase may have been limited whether Span 80 was present or absent, which led to inclusion of dissolved (unreacted) water molecules during the hydrate formation. When the material transfers progressed further into the dissolved water molecules in the oil phase, rapid hydrate aggregation or transition to the bulky state readily occurred (Figure 6a). In contrast, the proposed biodegradable AA candidates had better performance in emulsifying or dispersing water molecules into the oil phase, and this was enhanced even more depending on the concentration added. Therefore, crystal aggregation was interrupted, or crystal growth occurred without dissolved water molecules, in the hydrate phase. This made slow-growing needle-like hydrate crystals, as shown in Figure 6b.
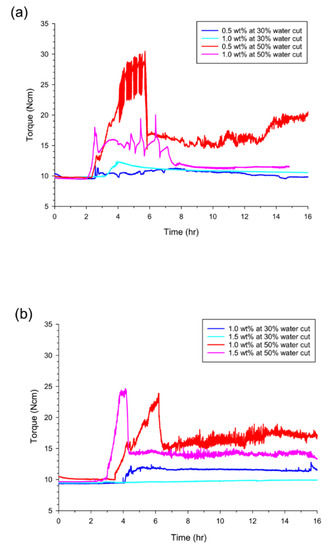
Figure 5.
Anti-agglomeration effects of (a) lecithin and (b) lanolin depending on the concentrations added. Hydrate formation kinetics (or rotational torques) were measured at two water cuts of 30% and 50% with two concentrations of 0.5 and 1.0 wt% for lecithin, and 1.0 and 1.5 wt% for lanolin, respectively.
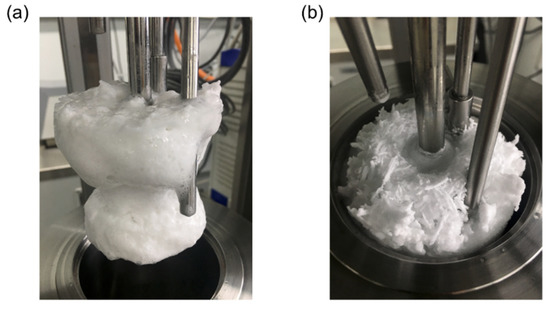
Figure 6.
Pictures of hydrate formations in the reactor after finishing the experimental tests with (a) Span 80 and (b) the biodegradable AA candidates. The morphology of the hydrate growth with the lecithin or lanolin was observed to be different from that with Span 80.
As discussed above, the two biodegradable AA candidates from natural sources (that is, lecithin and lanolin) had corresponding or better anti-agglomeration performance than the conventional AA material, Span 80. In particular, lanolin was found to enhance the anti-agglomeration performance dramatically under our experimental conditions, and sustained the flow characteristics with 1.5 wt% addition without small changes before hydrate formation, even under the hydrate-forming temperature and pressure conditions. The two candidates also cost less than Span 80 because both materials are currently produced and utilized on huge scales. Moreover, they are environmentally friendly with high biodegradability because both are made from natural sources. Considering together the experimental results and the properties mentioned, the proposed AA candidates have great potential as AA additives in oil and gas pipelines. However, this study also provided us with the necessity for additional investigations to answer the following: (1) Can the concentrations of the candidate materials added be optimized by identifying changes in the concentration at various water cuts? (2) Can the performances of the AA candidates be maintained regardless of changes in the operational temperature and pressure conditions, and in the composition of the gas phase? (3) Can any synergetic effect be expected when the candidates are used as a mixture with other additives such as KHI? [16,21,25]. Although further investigations are still necessary to answer or clarify the above questions, the viability of the new AA candidates from natural sources for practical application is thought to be demonstrated sufficiently by the experimental evidence from this research.
4. Conclusions
In this report, the use of two biodegradable AA candidates from natural sources were proposed. As indirect flow characteristics of produced pipeline fluids, the rotational torques were measured with agitation during hydrate formation with and without three AAs (Span 80, lecithin and lanolin). The increase or decrease of the rotational torque indicated changes in the flow resistance (also an increase or decrease) while transporting mixed water-oil-gas fluids through pipelines. At 30% water cut, both the maximum and the terminal torques were found to decrease when the AAs were added to the system. In addition, both of the AA candidates proposed in this work were found to be comparable with the conventional AA (Span 80) in terms of their retardation effect on hydrate formation. In particular, when 1.5 wt% lanolin was added to the system at 30% water cut, the flow characteristics changed little, as compared with flows without hydrate formation. However, all the AAs were found to have little effect due to faster hydrate-forming rates when the water content was increased to 50% water cut. It is worthwhile to note that the terminal torques with the AAs after 16 h from the starting point were found to decrease significantly even at 50% water cut. This is because the AAs evenly dispersed the hydrate particles formed in the fluid, and prevented their aggregation. Considering the cost efficiency due to their abundance from large-scale production and excellent biodegradability due to the natural sources of the two suggested materials, they are thought to have experimentally demonstrated viability for practical applications in oil and gas pipelines. However, additional investigations are still required to explain such factors as action mechanisms, optimum concentrations of additives and synergetic effects with other additives before these can be used as commercial AAs.
Author Contributions
S.-P.K. organized and performed the measurements, and drafted the article. D.L. helped the investigation and the additional measurements. J.-W.L. put forward the research direction, and completed the original manuscripts. S.-P.K. and J.-W.L. were involved in revising the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Korea Ministry of Environment (MOE) as a Waste to Energy Recycling Human Resource Development Project, and also supported by the Industrial Strategic Technology Development Program (10045068) of the Korea Evaluation Institute of Industrial Technology funded by the Ministry of Trade, Industry and Energy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hammerschmidt, E.G. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Lederhos, J.P.; Long, J.P.; Sum, A.; Christiansen, R.L.; Sloan, E.D. Effective Kinetic Inhibitors for Natural Gas Hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, H. Phase Equilibria of Methane and Carbon Dioxide Hydrates in the Aqueous MgCl2 Solutions. Fluid Phase Equilibria 1998, 147, 229–238. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H. Hydrate Phase Equilibria of the Ternary CH4 + NaCl + Water, CO2 + NaCl + Water and CH4 + CO2 + Water Mixtures in Silica Gel Pores. J. Phys. Chem. B 2003, 107, 889–894. [Google Scholar] [CrossRef]
- Masoudi, R.; Tohidi, B.; Danesh, A.; Todd, A.C.; Anderson, R.; Burgass, R.W.; Yang, J. Measurement and Prediction of Gas Hydrate and Hydrated Salt Equilibria in Aqueous Ethylene Glycol and Electrolyte Solutions. Chem. Eng. Sci. 2005, 60, 4213–4224. [Google Scholar] [CrossRef]
- Yang, J.; Tohidi, B. Characterization of Inhibition Mechanisms of Kinetic Hydrate Inhibitors Using Ultrasonic Test Technique. Chem. Eng. Sci. 2011, 66, 278–283. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lim, J.I.; Kim, J.H.; Lee, J.D. Kinetic Studies on Methane Hydrate Formation in the Presence of Kinetic Inhibitor via in situ Raman Spectroscopy. Energy Fuels 2012, 26, 7045–7050. [Google Scholar] [CrossRef]
- Maekawa, T. Equilibrium Conditions for Carbon Dioxide Hydrates in the Presence of Aqueous Solutions of Alcohols, Glycols, and Glycerol. J. Chem. Eng. Data 2010, 55, 1280–1284. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas Hydrate Phase Equilibrium in the Presence of Ethylene Glycol or Methanol Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Kim, J.; Chang, D.; Seo, Y.; Lee, H.; Kang, S.-P. Thermodynamic and Kinetic Hydrate Inhibition Performance of Aqueous Ehtylene Glycol Solutions for Natural Gas. Chem. Eng. Sci. 2013, 99, 184–190. [Google Scholar] [CrossRef]
- Aminnaji, M.; Anderson, R.; Tohidi, B. Anomalous KHI-Induced Dissociation of Gas Hydrates inside the Hydrate Stability Zone: Experimental Observations & Potential Mechanisms. J. Petrol. Sci. Eng. 2019, 178, 1044–1050. [Google Scholar]
- Ohno, H.; Moudrakovski, I.; Gordienko, R.; Ripmeester, J.; Walker, V.K. Structures of Hydrocarbon Hydrates during Formation with and without Inhibitors. J. Phys. Chem. A 2012, 116, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Adidharma, H. Dual Function Inhibitors for Methane Hydrate. Chem. Eng. Sci. 2009, 64, 1522–1527. [Google Scholar] [CrossRef]
- Villano, L.D.; Kelland, M.A. An Investigation into the Kinetic Hydrate Inhibitor Properties of Two Imidazolium-based Ionic Liquids on Structure II Gas Hydrate. Chem. Eng. Sci. 2010, 65, 5366–5372. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kang, J.W.; Kang, S.-P. Tuning Ionic Liquids for Hydrate Inhibition. Chem. Commun. 2011, 47, 6341–6343. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ro, H.; Lee, J.-W.; Kang, H.; Lee, H. Morpholine-Induced Thermodynamic and Kinetic Inhibitions on Gas Hydrate Formation. Energy Fuels 2013, 27, 6581–6586. [Google Scholar] [CrossRef]
- Huo, Z.; Freer, E.; Lamar, M.; Sannigrahi, B.; Knauss, D.M.; Sloan, E.D. Hydrate Plug Prevention by Anti-agglomeration. Chem. Eng. Sci. 2001, 56, 4979–4991. [Google Scholar] [CrossRef]
- Dann, K.; Rosenfeld, L. Surfactant Effect on Hydrate Crystallization at the Oil-Water Interface. Langmuir 2018, 34, 6085–6094. [Google Scholar] [CrossRef]
- Hou, G.; Liang, D.; Li, X. Experimental Study on Hydrate Anti-agglomeration in the Presence of Rhamnolipid. RSC Adv. 2018, 8, 39511–39519. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.-S.; Kang, S.-P.; Kim, J.-N. Synergetic Performance of the Mixture of Poly(N-vinylcaprolactam) and a Pyrrolidinium-Based Ionic Liquid for Kinetic Hydrate Inhibition in the Presence of the Mineral Oil Phase. Energy Fuels 2018, 32, 4932–4941. [Google Scholar] [CrossRef]
- Lee, W.; Choi, Y.; Kim, Y.; Lim, J.-S.; Kang, S.-P. Rheological Investigation of Methane Hydrate Formation with Biodegradable Emulsifiers as Anti-agglomerants. J. Petrol. Sci. Eng. 2019, 183, 106454. [Google Scholar] [CrossRef]
- Altamash, T.; Qureshi, M.F.; Aparicio, S.; Aminnaji, M.; Tohidi, B.; Atilhan, M. Gas hydrates Inhibition via Combined Biomolecules and Synergistic Materials at Wide Process Conditions. J. Nat. Gas Sci. Eng. 2017, 46, 873–883. [Google Scholar] [CrossRef]
- Majid, A.A.A.; Wu, D.T.; Koh, C.A. A Perspective on Rheological Studies of Gas Hydrate Slurry Properties. Engineering 2018, 4, 321–329. [Google Scholar] [CrossRef]
- Kang, S.-P.; Kim, E.S.; Shin, J.-Y.; Kim, H.-T.; Kang, J.W.; Chae, J.-H.; Kim, K.-S. Unusual Synergy Effect on Methane Hydrate Inhibition When Ionic Liquid Meets Polymer. RSC Adv. 2013, 3, 19920–19923. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).