1. Introduction
Ozone has been recently extensively applied as a potent oxidizing agent in many fields of science, technology and everyday life including chemical synthesis, semiconductor surface treatment, water disinfection, destruction of pollutants and odor removal, purification of products, decolorization (bleaching), vermin and insects control, automotive fuel afterburning, skin treatment and dental care, just to name a few [
1,
2,
3,
4]. However, at the same time, ozone is considered a highly toxic gas—its PEL (Permissible Exposure Limit) and REL (Recommended EL) health limits are as low as 0.1 ppm (0.2 mg/m
3) while IDLH (Immediate Danger Lethal Dose) is set at 5 ppm [
5]. Lowest lethal concentrations (LCLo) range from 4.8 ppm in rats (for 4 h exposure) to 50 ppm in humans (for 30 min contact) [
6]. Ozone (discovered by F. Schoenbein in 1839) is a colorless or, at higher concentrations, pale blue gas containing three oxygen atoms in its molecule (O
3), thus it is a triatomic allotrope of O
2. It is highly unstable and readily reacts with other substances as a powerful oxidizer. In some references, it is regarded as odorless although other sources quote it as having “very pungent odor” or even “characteristic, pleasant odor” [
6] (however, such inconsistency is rather related to secondary smell sensation caused by malodorous products of olfactory tissue oxidized by O
3 than its primary olfaction). O
3 is generated naturally in the Earth stratosphere by photolysis of molecular O
2 by short-wave (λ < 200 nm) solar ultraviolet (UV) radiation, which produces excited oxygen O* atoms, undergoing binding collisions with O
2 to form O
3 molecules afterward.
O
3 is also readily produced (in so-called three-body reaction, involving O, O
2 and O
3) in electrical discharges generated in pure oxygen or in atmospheric air and such processes are commonly applied in laboratory and industrial ozone generators. Such systems commonly make use of non-thermal plasma generated in dielectric barrier discharges (DBD), corona or surface discharges as it allows to attain elevated effectiveness of O
3 generation process and to perform it on-demand and on-site, directly when and where ozone is required as it essentially cannot be effectively stockpiled [
7].
Corona discharge (also referred to as pre-discharge) arises when the dielectric strength of a discharge gap is exceeded only locally in a strongly non-uniform electric field resulting in a situation when a discharge channel cannot develop into a spark or flashover discharge. It appears as a dim filamentary discharge emanating outward from the high voltage electrode of a small curvature radius. Corona discharge term is commonly used to describe discharges developing between gas-insulated bare metal electrodes without a solid dielectric involved however, some authors use this term in conjunction or even interchangeably with DBD [
8]. Corona discharges exist in several forms depending on the electrode geometry and supply voltage type and polarity, including intermittent, glow, burst, pulse, pulseless, streamer and leader corona. Corona discharge may be generated using either DC or AC voltage although both types of pre-discharge differ in character. The term surface corona discharge (or creeping discharge) is used in a situation when the electrode configuration results in an electric field with a substantial tangential gas–solid interphase component and thus the corona discharges propagate (slide) along the interphase. A comprehensive study of topics related to corona discharge and its use in O
3 making may be found in the relevant literature [
8,
9,
10].
On the other hand, DBD (named also as silent discharge or ozonizer discharge) is also a non-thermal electrical discharge developing in a gas phase except that a layered barrier insulation system containing solid dielectric(s) is involved. In such configuration, a uniform (or slightly non-uniform) electric field is strong enough to produce a localized breakdown in the entire gas gap but not in the dielectric barrier. As such an arrangement can only pass the displacement current, DBDs are generated using AC electric supply. Volume DBD is in some way analogous to cavity discharge (internal partial discharge, PD) developing in a gaseous void inside a solid dielectric subjected to AC high voltage stress. To illustrate this analogy, a bird’s eye view photographs of the spatial distribution of DBD developing in a lens-like air gap formed in a polymeric body at various AC supply voltages are presented in
Figure 1. In such an exotic gas cavity, taken after gas hollows present in voided ferroelectric material, DBD starts developing mostly near the rim of the void and tends to shift towards the void’s center as the voltage is ramped up [
11].
Volume DBD at atmospheric pressure consists of a large number (ca. 10
6/cm
2 s) of randomly appearing but spatially-localized short-lived (ca. 100 ns) microdischarges. They take the form of thin (ca. 100 μm wide) cylindrical columns (filaments) normal to the gas-dielectric interphase and developing into much larger footings at the dielectric exterior while individual columns are separated by a non-plasma gaseous medium. The microdischarge activity results in short (a few ns), high-frequency current pulse trains registered in the discharge circuit during each AC voltage raise period. In the filament mode, observed at elevated (atmospheric-like) pressure in most gases (including air) each weakly ionized microdischarge channel is formed by a fully-developed but transient glow discharge dissipating just microjoules of energy. Volume DBD channels contain thus non-thermal non-equilibrium plasma. Consequently, thermal effects of volume DBD related to gaseous medium heating are minute (typically below 10 °C is observed in the air in mm-range discharge gaps). The spatial and temporal DBD parameters depend on the supply power density while the strength of DBD is mainly determined by discharge gap width, gas properties (composition, pressure, humidity and flow rate) and dielectric barrier properties. Similarly to surface corona discharges, stable surface DBD (SDBD) may also be initiated over a dielectric surface using a tangential electric field shaped by a system of electrodes in creepage configuration located on opposite walls of a dielectric barrier. The electrodes may be partially or completely embedded in the dielectric or a three-electrode variation of such set-up may be used, which involves additional capacitively coupled floating-potential surface electrode. A detailed and comprehensive discussion of the DBD and SDBD physics, plasma chemistry, developments and applications may be found in the relevant literature [
12,
13,
14,
15,
16].
Application of volume DBD for ozone production has been well-known for over a century as it was introduced by W. von Siemens already in 1857 in his air/oxygen-fed AC-energized ozone generator [
17]. The original design consisted of two coaxial glass pipes forming a narrow annular gas flow channel in which silent discharges were sustained using alternating electric field formed by two electrodes located also coaxially but outside the discharge channel.
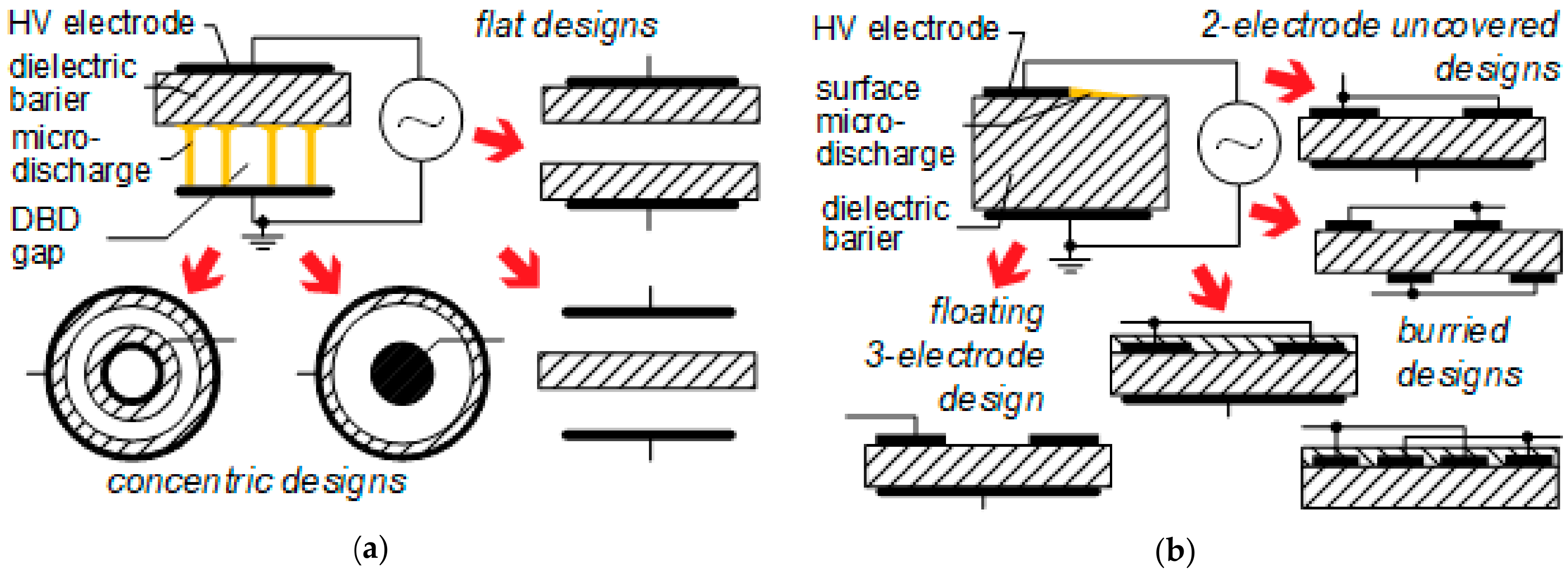
Figure 2a briefly illustrates various flat and concentric electrode–barrier–gap configurations, commonly used in volume DBD while
Figure 2b schematically outlines conventional SDBD electrode–barrier arrangements (including 2- and 3-electrode as well as uncovered and buried electrode designs).
A growing environmental awareness of air pollution originated from fuel combustion exhausts (of either internal combustion engines or gas burners) has resulted in research aiming at fuel-burning performance improvement relying on combustion promotion attained by means of plasma/ozone processing of the intake air [
18,
19,
20]. The latter may be realized in an automotive application using by-pass scheme DBD (exemplified in
Figure 3) however it requires effective yet energy- and space-constrained air-fed non-thermal plasma and ozone generator. Thus, experimental verification of the electrical and thermal performance of a newly-designed volume DBD flow-through ozone generator (OG) of a spiral-tubular electrode design was presented in relation to a conventional volume cylindrical DBD as well as a commercial OG making use of SDBD generated in a floating electrode arrangement (advertised by its supplier as having amazingly high 8 g/h O
3 production). Improvement of O
3 generation effectiveness through high voltage (HV) electrical supply tuning was also investigated and discussed.
2. Materials and Methods
A volume DBD OG of a tubular design, shown in
Figure 4a, consisted of a metallic inner HV rod electrode (∅18x250 mm) enclosed inside a silica (SiO
2) glass tube (∅20/∅25 mm) providing a 1 mm wide flow-through tubular gas channel, connected to gas ports, installed at end caps of the tube (as detailed in
Figure 1a upper inset). The inner rod electrode was made either of brass (EN-CW617N grade) or duralumin (EN-2017 grade); in the latter case, its surface was covered with anodic-deposited Al
2O
3 layer. The silica glass tube was fitted with a grounded (GND) electrode made of Sn-platted thin Cu foil with a conductive adhesive layer (1183, 3M). It was firmly affixed to the silica tube external wall leaving no air bubbles at the Cu-SiO
2 interphase. Foil electrodes of a cylindrical as well a spiral design (as detailed in
Figure 4a) were investigated; in each case 20 mm clearance between the foil electrode and the HV electrode metallic end caps ensured no surface sliding discharges in this area. The volume DBD OG was operated in a horizontal position with no forced cooling.
A commercial surface DBD OG (Sihon Ozone, China), used for comparative study, was claimed by its manufacturer to produce 8.0 g/h O
3 thanks to intensive surface DBDs generated in floating three-electrode arrangement. Metal-ceramic OG, shown in
Figure 4b, consisted of two 1.3 mm thick duralumin (of unknown make) electrodes spaced by 5 mm and embedded in 2.8 mm thick alumina body forming 106x54x2.8 mm OG composite plate. The OG plate was fitted at both alumina faces with two 0.2 mm thick stainless steel, unprotected slotted electrodes (as drafted in the schematic cross-section shown in
Figure 4b). Laboratory tests were conducted using two such SDBD OG plates collocated one over another, spaced vertically by 10 mm and placed in a hermetic metal vessel fitted with gas and electric supply ports.
Both tested OGs were supplied with unprocessed (not dehumidified) atmospheric laboratory air at 23–25 °C and 42%–45% RH (exemplifying commonplace laboratory conditions). The airflow rate was controlled using either a mass flow controller (F-201-CV, Bronkhorst) or a flow meter (SD6000, IFM Electronik), depending on the operational airflow rate. In low flow rate experiments (1.0 Nl/min) the entire gas stream was passed through the O3 analyzer while at moderate flow rates (10–100 Nl/min) the air-O3 output stream was sampled at 1.0 Nl/min (as required by the O3 analyzer).
O
3 concentration was monitored using a photometric analyzer at 254 nm UV radiation (BMT964, BMT Messtechnik), it was thus insensitive to N
2, H
2O or NO
x. O
3 volumetric concentration was expressed in ppm
v and all data (including flow rates expressed in Nl/min) was corrected for normal 1 atm and 0 °C conditions (expressing O
3 concentration in volumetric (ppm
v)=0.0001 (%v/v) is unambiguous and it does not require information on the carrier gas density as in case of (%wt/wt) [
21]. The temperature of the OG was monitored using a metal sheath K-type thermocouple attached directly to one of its electrodes while thermal imaging camera (i3, Flir) was also used to monitor the silica glass tube temperature in case of the spiral-tubular OG assay.
Commercial surface DBD OG was electrically supplied using either its build-in HV power supply (~230 V mains-operated) or a laboratory variable sinusoidal HV supply system consisted of Agilent 33521a signal generator and Trek 20/20C HV amplifier. Only the latter laboratory HV supply set-up was used in case of the volume DBD OG of the spiral-tubular design. HV electric supply parameters were recorded using a digital oscilloscope (MSO2014, Tektronix) fitted with 1:1000 HV probe (HP 1137A) for HV (peak value) measurement and 1:10 probe (P1110, Tektronix) interfaced to 98.0 nF low loss capacitor connected in series with DBD OG for charge measurement.
All experimental data points presented if the figures were supplemented with dashed lines drawn to guide the eyes only, thus indicating no intended numerical data fit.
4. Discussion
The stability of gaseous O
3 is strongly temperature-, gas velocity- and humidity-dependent as its half-life time is reduced from approximately 1500 min (at 24 °C, 0% RH and no gas movement) to approximately 800 min at 40 °C, to approximately 700 min at 45% RH and to approximately 210 min at 100 m
3/h gas streaming [
22]. Thus, if the relative humidity of the supply air is not varied significantly, correct thermal management of the OG operated at low gas velocity is mandatory when its optimal performance is targeted. As it was experimentally shown, tweaking the HV supply enabled effective thermal control of the volume DBD OG and its performance optimization. However, its extent was limited by self-cooling of the volume OG as no forced cooling was intended to be put into operation in the tested design.
The spiral design of the outer GND electrode enhanced O
3 throughput. First of all, spiral electrode geometry resulted in boosting O
3 production by keeping the process temperature at a moderate level at increased power supply conditions due to decreased volume in which discharge-generated heat transfer was commenced. However, it has also created recurring discharge-reach and discharge-free zones. Even narrowed discharge regions provided effective O
2 dissociation into excited O* atoms due to the fast nature (ns time scale) of electron and ion collision ionization processes. When conveyed to the discharge-free zones, O* atoms were effectively recombining with O
2 to form O
3 and this process was not interfered with by the discharges. A high density of discharges and thus generation of excessively energetic and concentrated excited species is known to lead to unfavorable recombination of O* into diatomic O
2 (resulting in no O
3 production) as well as to the reaction of O
3 with O* and electron-triggered dissociation of O
3 leading to the destruction of already formed O
3. According to Kitayama and Kuzumoto, the dissociation rate of O
3 is 6–8 times higher than the dissociation rate of O
2 molecule in the discharge channel [
23]. Moreover, as the spiral design of the electrode system resulted in a subsided volume in which DBD non-thermal plasma was ignited, it may be accounted for diminished generation of atomic nitrogen N* (originating from electron impact dissociation of N
2). Thus, less NO and NO
2 nitrous oxides would be formed and therefore so-called autocatalytic cycle for O
3 destruction [
24] would be less sizable (however such stipulation has not been verified experimentally in the presented research).
All those detrimental processes are electron energy- and temperature-amplified and decrease the overall effectiveness of O
3 formation. Thus, the geometrical introduction of discharge-free zones (supplying no energy to electrons and ions and providing moderate temperature) but still reach in the active oxygen atoms favored global O
3 synthesis effectiveness. To address the above remarks, electrostatic and conjugated heat transfer finite element method (FEM) simulations were performed using the
Comsol Multiphysics software package. The volume DBD OG design (with optimized 19/35mm spiral electrode width/pitch) was simplified by representing the outer helical electrode as 5 rings of the same total surface area separated by 16 mm distance (thus furnishing the same surface power density and similar heating and cooling conditions) while other dimensions of the model were kept analogous to the real design.
Figure 9a illustrates simulated static electric field distribution in the volume DBD OG axisymmetric cross-section constrained to one of the outer electrode segments at the maximal 10 kV supply voltage. In order to better visualize the DBD region,
Figure 9b expands the inter-electrode area in which the electric field was shown only in the region where the air breakdown strength of 4.37 MV/m was surpassed (as calculated for 1 mm discharge gap and 1 atm pressure according to Hussain and Nema [
25]). As might be expected, the DBD region was limited merely to the ring-like sub-volume of the flow-through tubular gas channel zoned by the outer foil electrode.
For the purpose of the conjugated heat transfer FEM simulation, it was stipulated that only those inter-electrode ring narrow gas volumes were the sources of the heat generated by DBD and that the entire active supply energy was converted into heat in those volumes. Simulation results obtained for such a simplified model were shown in
Figure 10a for the case of 1 Nl/min laminar airflow and 8.1 W active supply power. The simulated temperature of the outer silica surface was generally lower than that registered experimentally; 45 °C was modeled in the locations in the middle between the last two ring electrodes (at the point marked with X in
Figure 10a) while 46 °C was observed experimentally in the corresponding location in the middle between the last twist of the real helical electrode. Similarly, underestimated temperature data was obtained when the simulation was run at 100 Nl/min air flow rate yielding 29 °C (as shown in
Figure 10b) while 33 °C was registered empirically. The negative temperature bias discovered in both simulation cases might be related to the overestimated natural cooling modeled by the mean heat transfer coefficient (3.5 W/m
2 K) of the outer silica glass and copper electrode surface as well as to their overrated radiative cooling. The turbulent airflow conditions (which exist in the real tubular pneumatic system at 100 Nl/min air flow rate) have also not been taken into account in the FEM simulation.
There are literature references related to catalytic effects of Al
2O
3 in DBD ozone synthesis, however, such effects are pronounced in packed-bed-type ozonizers or if surface discharges are employed [
26,
27]. However, as it was verified experimentally a perceptible catalytic action of Al
2O
3 was also perceived in the presented volume-type DBD OG, accounting for approximately 5% increase in its performance in relation to the non-catalytic scheme using a brass internal electrode. As the optimal process temperature (manifested by the silica tube temperature) was almost identical in both cases (brass- and alumina-covered electrode) thus it seems to be justified that Al
2O
3 surface catalytic action was not temperature-activated in this low-temperature region.
On the other hand, the operation of a popular commercial SDBD OG was mostly bringing about thermal destruction of O
3. Very high active power consumption as well as the vigorous surface discharges advancing on the alumina plate exterior were responsible for the observed excessive thermal effects. Taking into account the estimated surface area covered by surface discharges (approximately 2.7 cm
2; calculated using digitally processed discharge image shown in
Figure 5a inset) it was translated to 33–42 W/cm
2 active power density (a value comparable to power density used in electrical resistive water heaters [
28]) and thus low O
3 generation efficiency of 8.4 g/kWh.