Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. ZSM-5 Extrudates Preparation
2.3. Characterization of ZSM-5 Extrudates
2.4. Adsorption Equilibrium of CO2, CH4, and H2O
3. Results
3.1. Characterization of ZSM-5 Extrudates
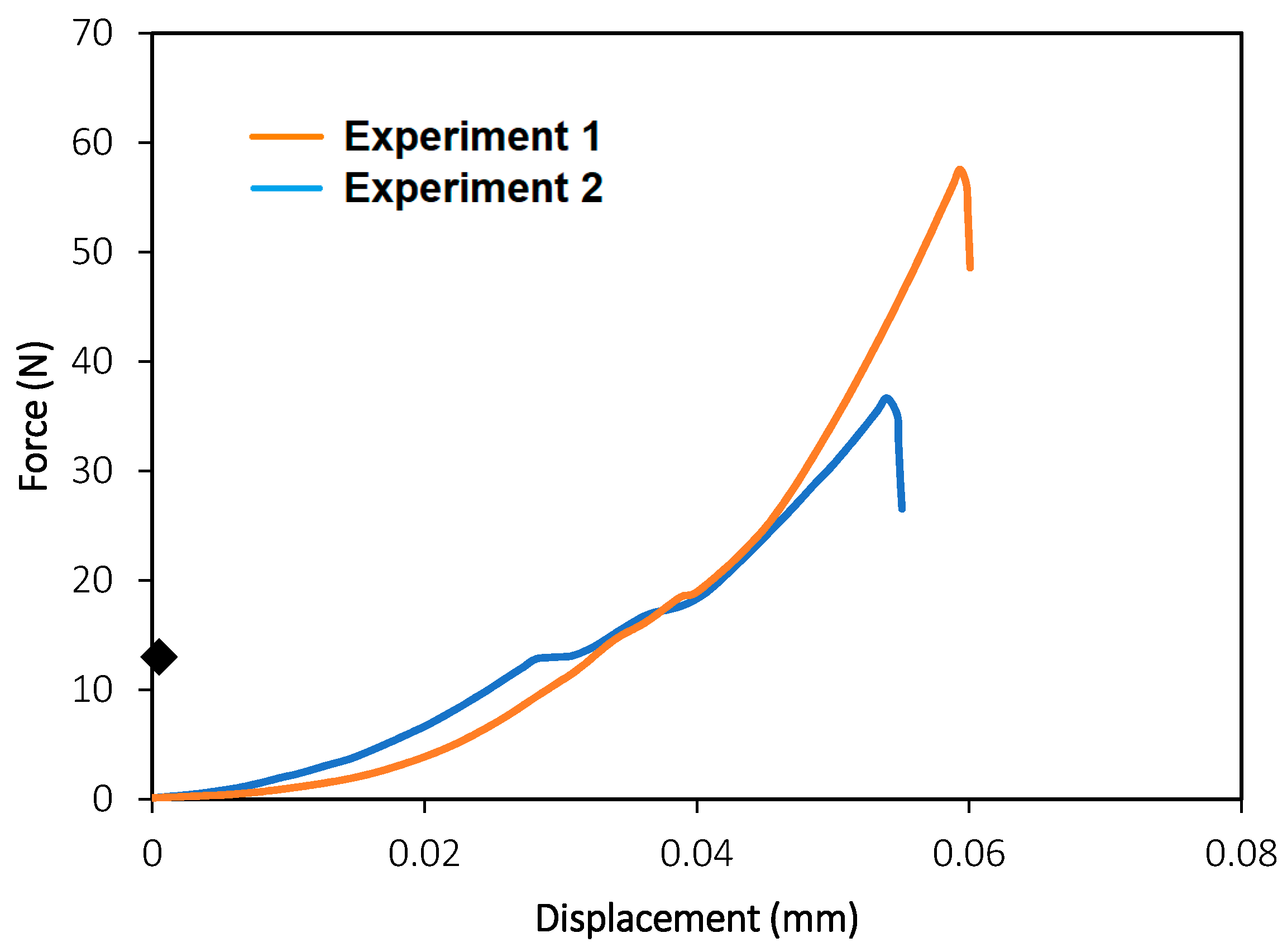
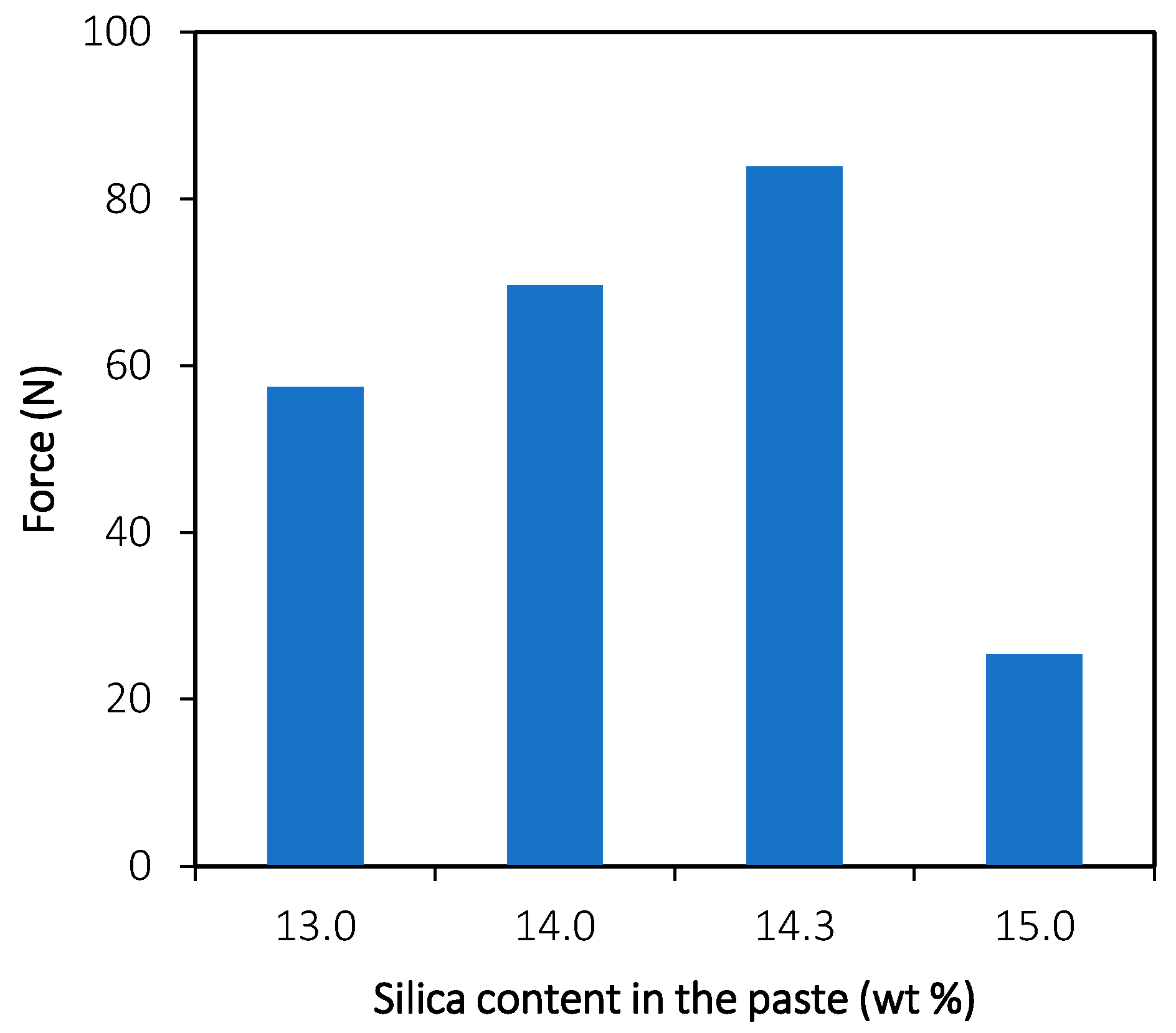
3.1.1. Compression Tests
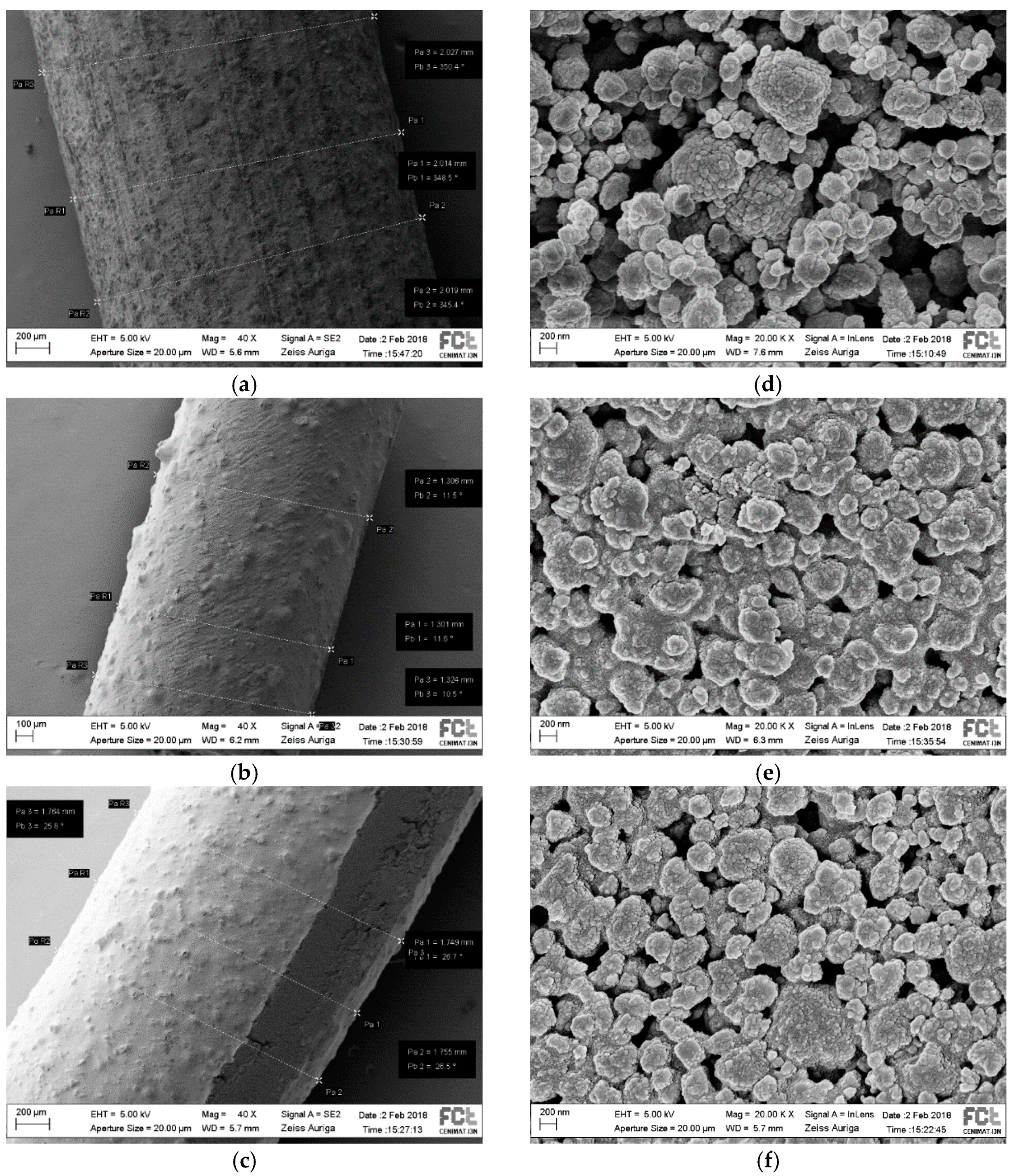
3.1.2. Scanning Electron Microscopy (SEM) Imaging
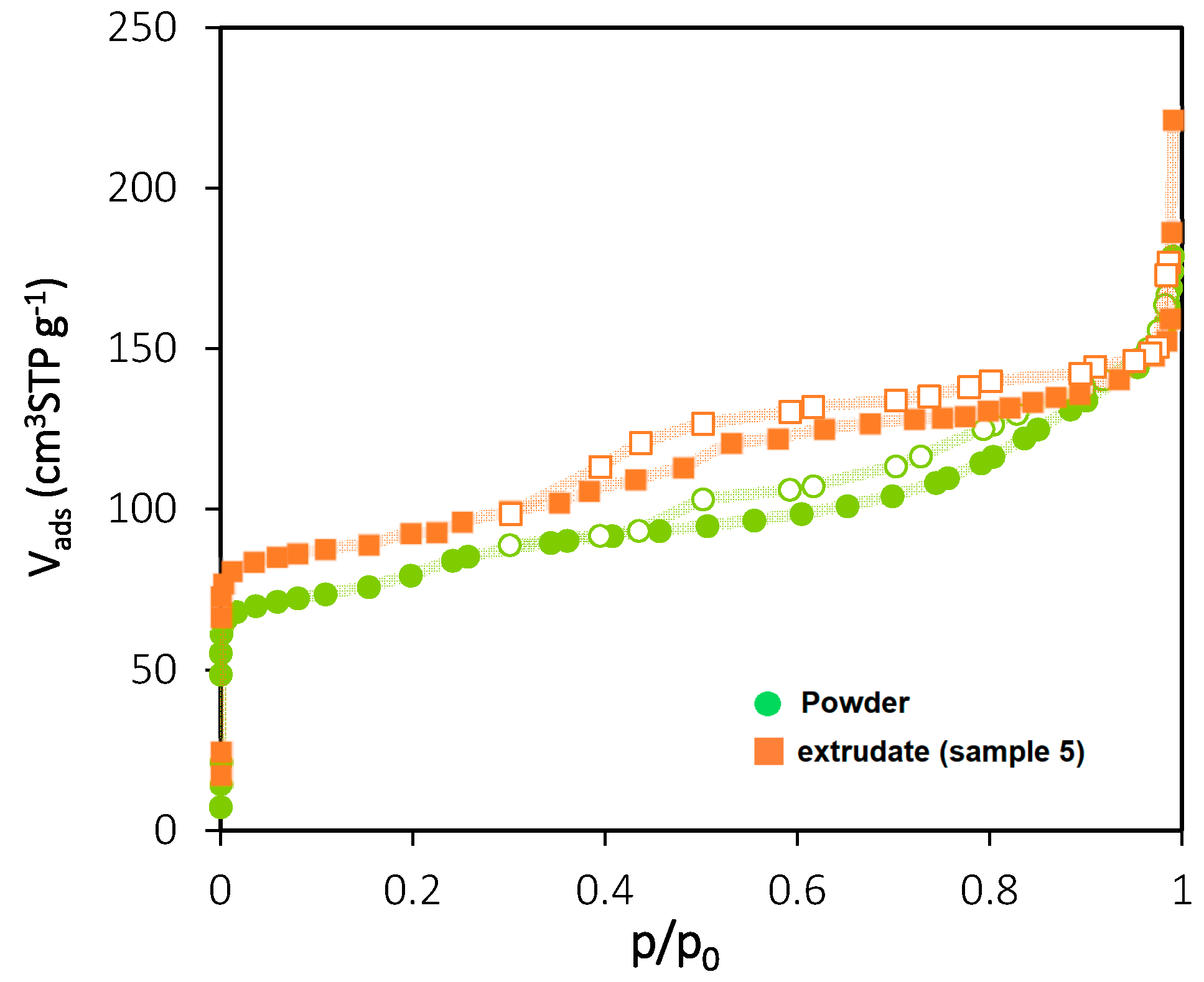
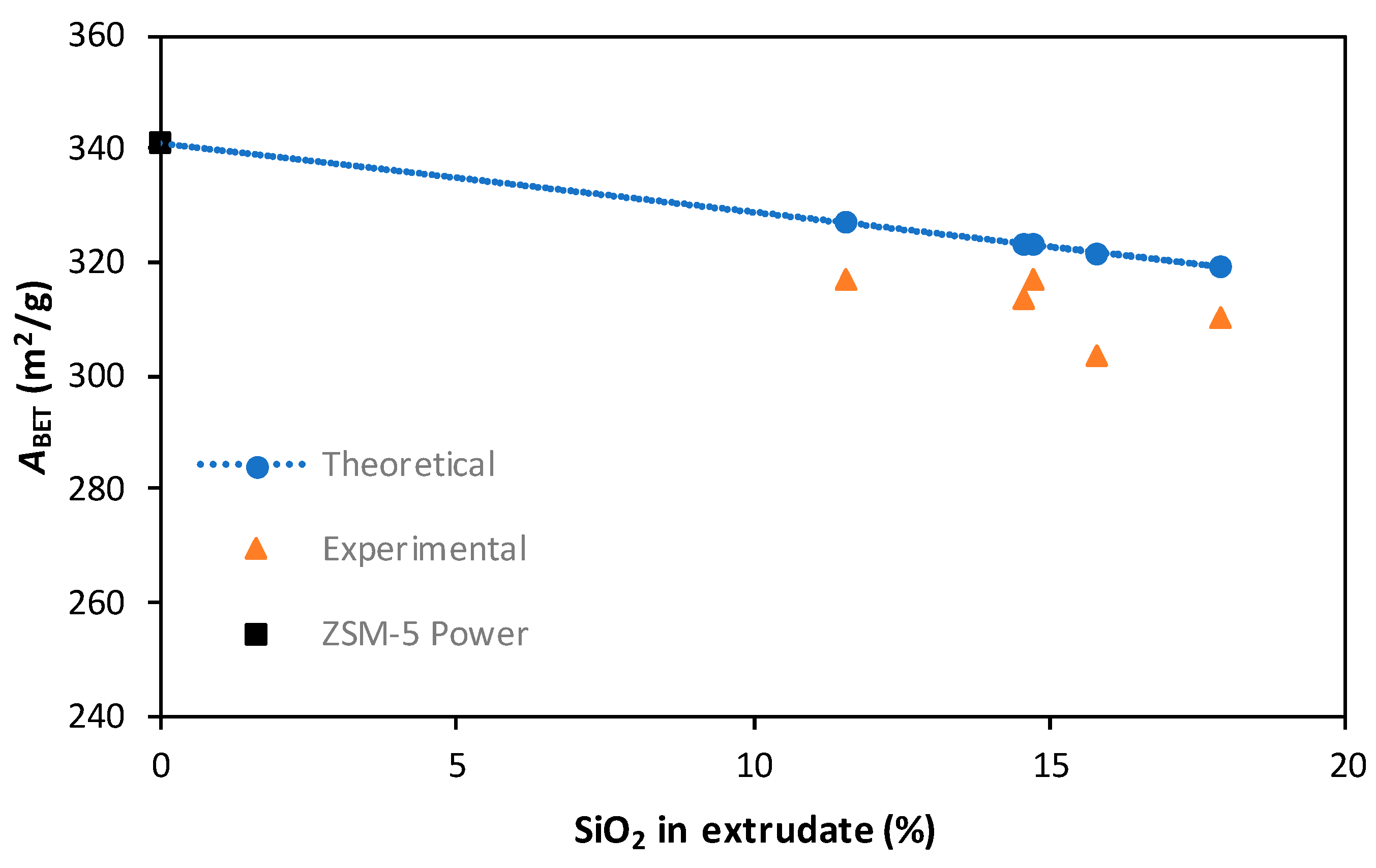
3.1.3. Nitrogen Adsorption at 77 K
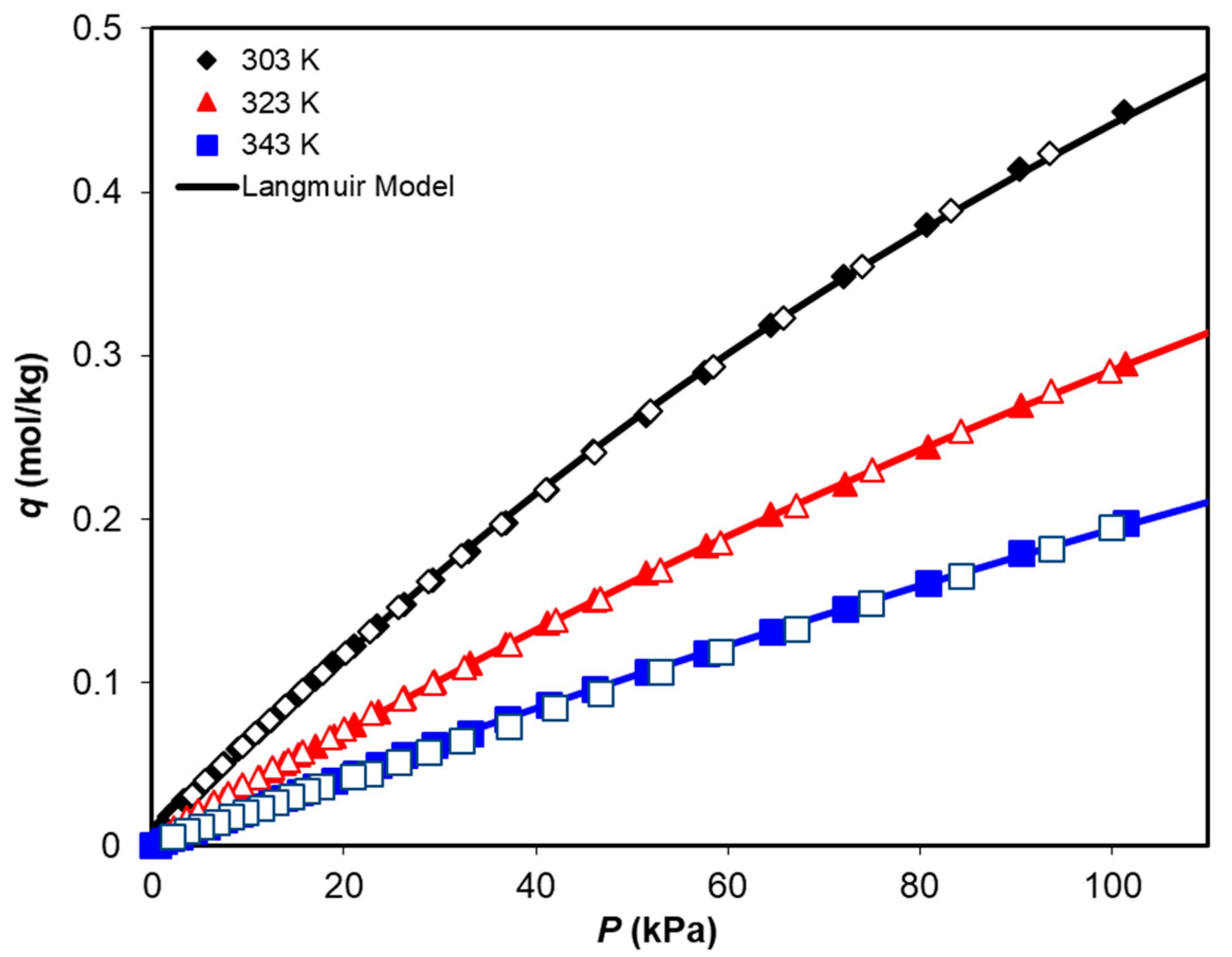
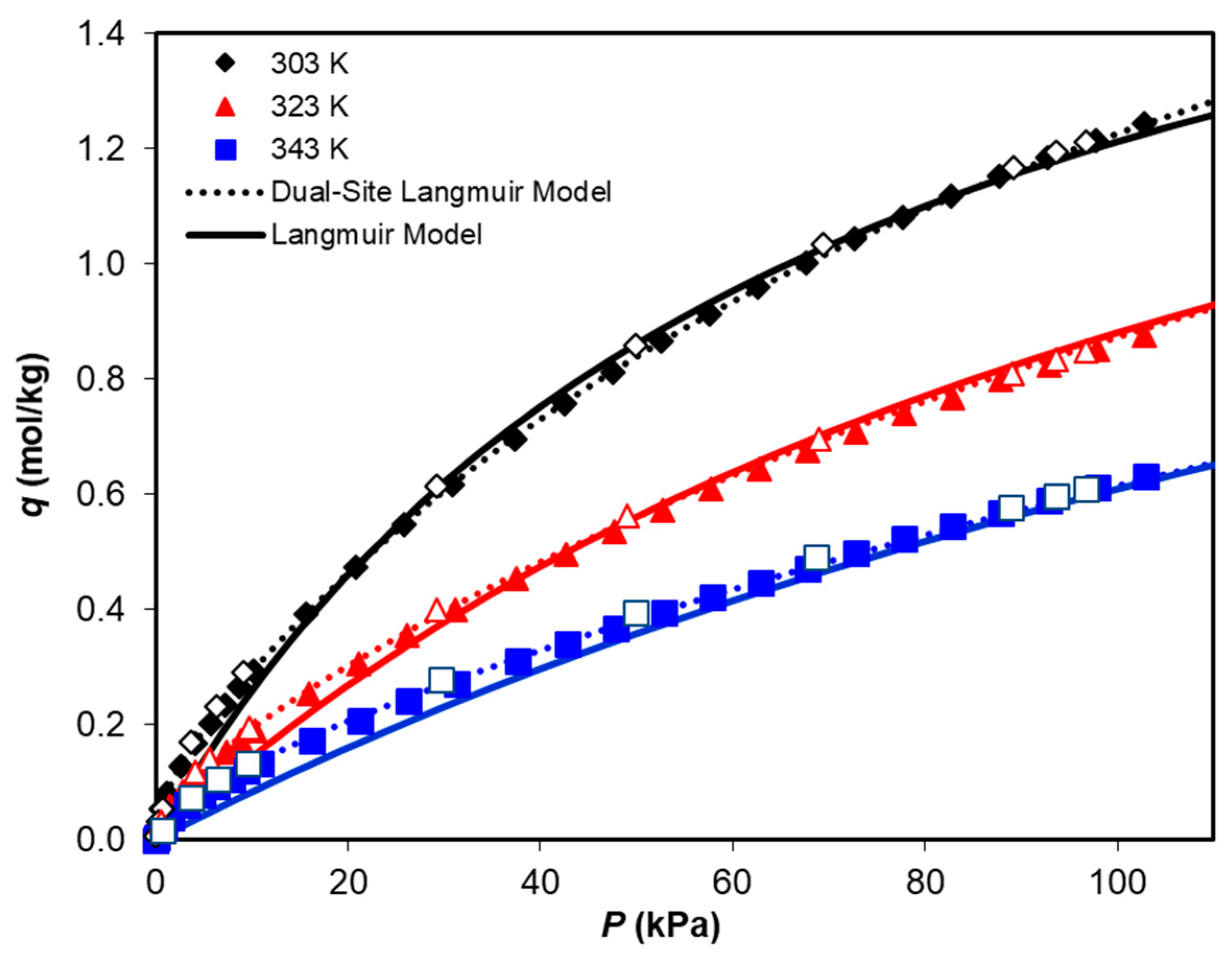
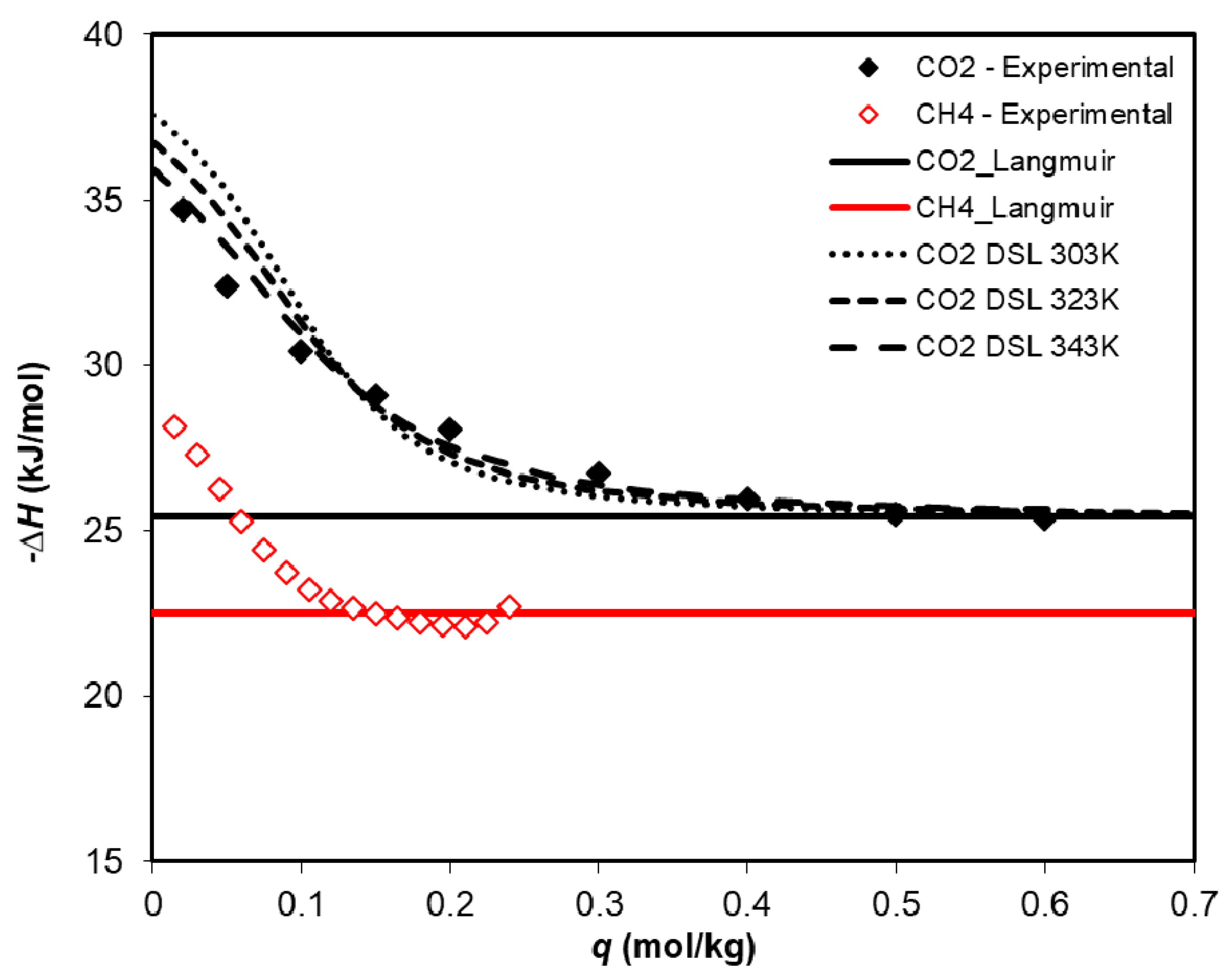
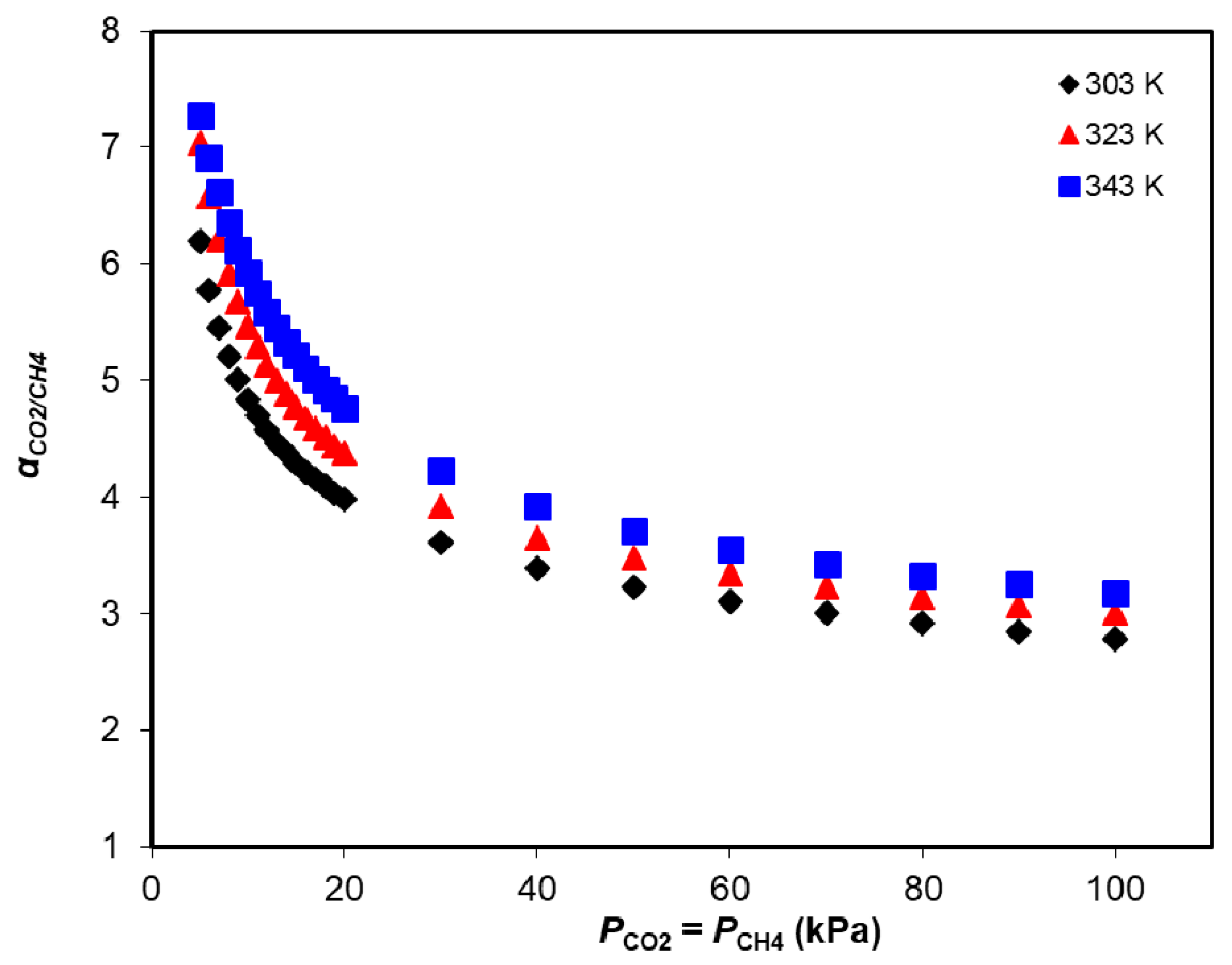
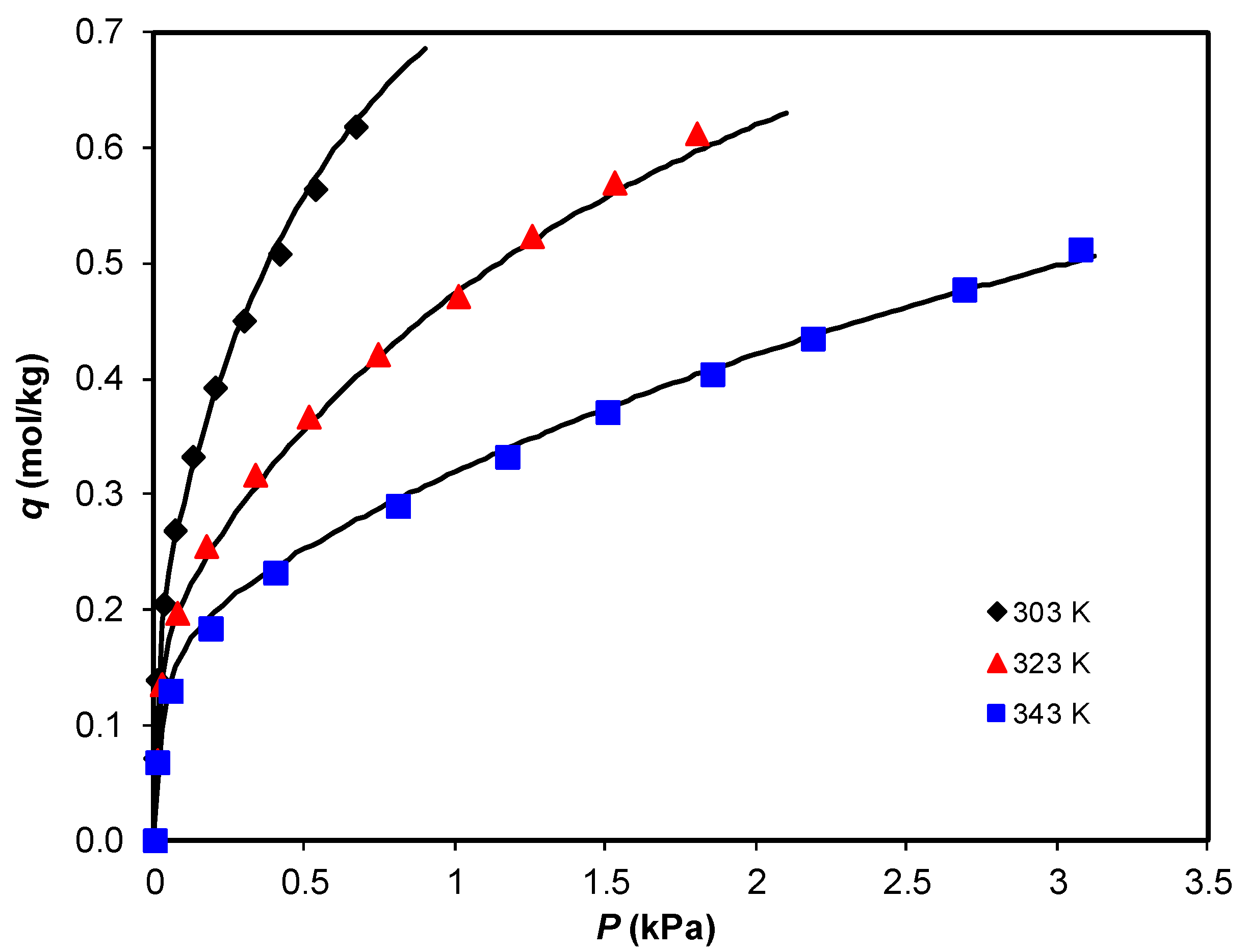
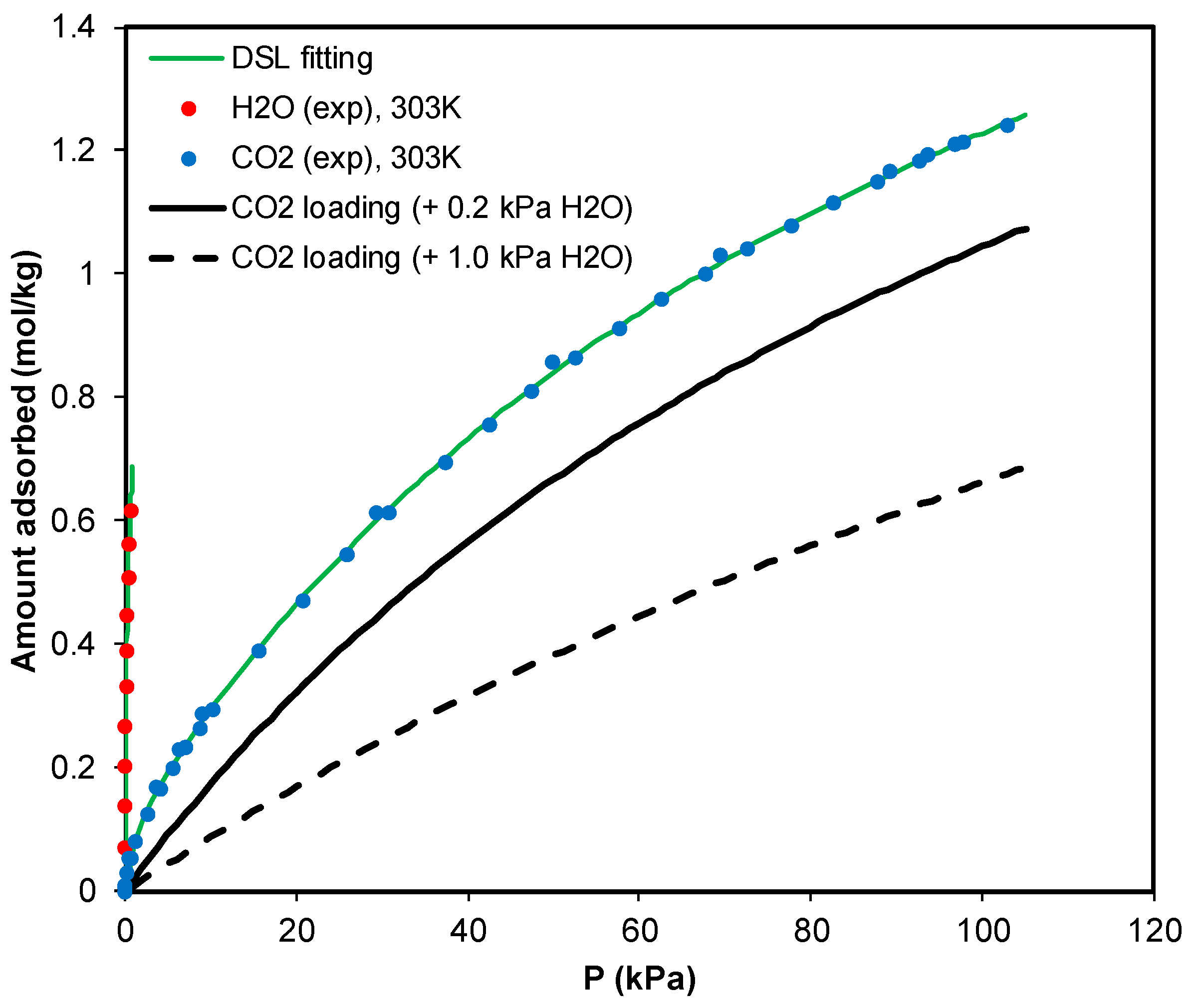
3.2. Adsorption Equilibrium of CH4, CO2, and H2O
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; Wiley-Interscience: New York, NY, USA, 1984. [Google Scholar]
- Yang, R.T. Gas Separation by Adsorption Processes; Butterworth Publishers: Boston, MA, USA, 1987. [Google Scholar]
- Perego, C.; Villa, P. Catalyst preparation methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Masala, A.; Vitillo, J.G.; Mondino, G.; Martra, G.; Blom, R.; Grande, C.A.; Bordiga, S. Conductive ZSM-5-Based Adsorbent for CO2 Capture: Active Phase vs Monolith. Ind. Eng. Chem. Res. 2017, 56, 8485–8498. [Google Scholar] [CrossRef]
- Omojola, T.; Cherkasov, N.; Rebrov, E.V.; Lukyanov, D.B.; Perera, S.P. Zeolite minilith: A unique structured catalyst for the methanol to gasoline process. Chem. Eng. Process. 2018, 131, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Armor, J.N.; Farris, T.S. Simultaneous exchange and extrusion of metal exchanged zeolites. Appl. Catal. A 1994, 114, L187–L190. [Google Scholar] [CrossRef]
- Schwarz, S.; Kojima, M.; O’Connor, C.T. Effect of stirring, extrusion and pelletisation on high pressure propene oligomerisation and xylene isomerisation over ZSM-5. Appl. Catal. 1991, 68, 81–96. [Google Scholar] [CrossRef]
- Grande, C.A.; Kvamsdal, H.; Mondino, G.; Blom, R. Development of Moving Bed Temperature Swing Adsorption (MBTSA) Process for Post-combustion CO2 Capture: Initial Benchmarking in a NGCC Context. Energy Procedia 2017, 114, 2203–2210. [Google Scholar] [CrossRef]
- Hou, J.; Sapnik, A.F.; Bennett, T.D. Metal-organic framework gels and monoliths. Chem. Sci. 2020, 11, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Kusgens, P.; Zgaverdea, A.; Fritz, H.G.; Siegle, S.; Kaskel, S. Metal-Organic Frameworks in Monolithic Structures. J. Am. Ceram. Soc. 2010, 93, 2476–2479. [Google Scholar] [CrossRef]
- Grande, C.A.; Águeda, V.I.; Spjelkavik, A.; Blom, R. An efficient recipe for formulation of metal-organic Frameworks. Chem. Eng. Sci. 2015, 124, 154–158. [Google Scholar] [CrossRef]
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Manufacturing of metal-organic framework monoliths and their application in CO2 adsorption. Microporous Mesoporous Mater. 2015, 214, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Tang, M. Effects of process factors on extrusion of hierarchically porous ZSM-5 zeolite. Powder Technol. 2019, 352, 79–90. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.; Liu, M.; Zhang, A.; Guo, X. A facile strategy to prepare shaped ZSM-5 catalysts with enhanced para-xylene selectivity and stability for toluene methylation: The effect of in situ modification by attapulgite. Molecules 2019, 24, 3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernando, H.; Ochoa-Fernandez, C.; Shamzhy, M.; Moreno, I.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Cejka, J.; Serrano, D.P. The crucial role of clay binders in the performance of ZSM-5 based materials for biomass catalytic pyrolysis. Catal. Sci. Technol. 2019, 9, 789–802. [Google Scholar] [CrossRef]
- Delgado, J.A.; Águeda, V.I.; Uguina, M.A.; Brea, P.; Grande, C.A. Comparison and evaluation of agglomerated MOFs in biohydrogen purification by means of pressure swing adsorption (PSA). Chem. Eng. J. 2017, 326, 117–129. [Google Scholar] [CrossRef]
- Zhang, H.P.; Li, W.; Xiao, W.D. Attrition Resistant Catalyst of Direct Dimethyl Ether Synthesis. Appl. Mech. Mater. 2012, 174, 97–101. [Google Scholar] [CrossRef]
- Marigo, M.; Cairns, D.L.; Bowen, J.; Ingram, A.; Stitt, E.H. Relationship between single and bulk mechanical properties for zeolite ZSM5 spray-dried particles. Particuology 2014, 14, 130–138. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Mousavi, B.; Ghadamyari, M.; Heynderickx, P.M.; Zhuiykov, S.; Yusubov, M.S.; Verpoort, F. Spray drying of zeolitic imidazolate frameworks: Investigation of crystal formation and properties. CrystEngComm 2018, 20, 3601–3608. [Google Scholar] [CrossRef]
- Spjelkavik, A.I.; Aarti Divekar, S.; Didriksen, T.; Blom, R. Forming MOFs into Spheres by Use of Molecular Gastronomy Methods. Chem. Eur. J. 2014, 20, 8973–8978. [Google Scholar] [CrossRef]
- Lee, D.W.; Didriksen, R.; Olsbye, U.; Blom, R.; Grande, C.A. Shaping of metal-organic framework UiO-66 using alginates: Effect of operation variables. Sep. Purif. Technol. 2020, 235, 116182. [Google Scholar] [CrossRef]
- Finsy, V.; Ma, L.; Alaerts, L.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Separation of CO2/CH4 Mixtures with the MIL-53(Al) Metal–Organic Framework. Microporous Mesoporous Mater. 2009, 120, 221–227. [Google Scholar] [CrossRef]
- Peterson, G.W.; DeCoste, J.B.; Glover, T.G.; Huang, Y.; Jasuja, H.; Walton, K.S. Effects of pelletization pressure on the physical and chemical properties of the metal–organic frameworks Cu3(BTC)2 and UiO-66. Microporous Mesoporous Mater. 2013, 179, 48–53. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Antunes, C.L.; Garate, A.U.; Portela, A.F.; Plaza, M.G.; Mota, J.P.B.; Esteves, I.A.A.C. Binderless shaped metal-organic framework particles: Impact on carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 275, 111–121. [Google Scholar] [CrossRef]
- Nayak, N.; Vitorino, N.; Frade, J.R.; Kovalevsky, A.V.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Design of alumina monoliths by emulsion-gel casting: Understanding the monolith structure from a rheological approach. Mater. Des. 2018, 157, 119–129. [Google Scholar] [CrossRef]
- Lefevere, J.; Protasova, L.; Mullens, S.; Meynen, V. 3D-printing of hierarchical porous ZSM-5: The importance of the binder system. Mater. Des. 2017, 134, 331–341. [Google Scholar] [CrossRef]
- Lefevere, J.; Mullens, S.; Meynen, V. The impact of formulation and 3D-printing on the catalytic properties of ZSM-5 zeolite. Chem. Eng. J. 2018, 349, 260–268. [Google Scholar] [CrossRef]
- Couck, S.; Cousin-Saint-Remi, J.; Van der Perre, S.; Baron, G.V.; Minas, C.; Ruch, P.; Denayer, J.F.M. 3D-printed SAPO-34 monoliths for gas separation. Microporous Mesoporous Mater. 2018, 255, 185–191. [Google Scholar] [CrossRef]
- Lind, A.; Vistad, Ø.; Sunding, M.F.; Andreassen, K.A.; Cavka, J.H.; Grande, C.A. Multi-purpose structured catalysts designed and manufactured by 3D printing. Mater. Des. 2020, 187, 108377. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring Adsorbents and Catalysts by Processing of Porous Powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, J.P.; Campbell, L.E. Monolithic Catalyst Supports. In Advanced Materials in Catalysis; Burton, J.J., Garten, R.L., Eds.; Academic Press: New York, NY, USA, 1977; pp. 293–324. [Google Scholar]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous Mesoporous Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Calleja, G.; Pau, J.; Calles, J.A. Pure and Multicomponent Adsorption Equilibrium of Carbon Dioxide, Ethylene, and Propane on ZSM-5 Zeolites with Different Si/Al Ratios, J. Chem. Eng. Data 1998, 43, 994–1003. [Google Scholar] [CrossRef]
- Talesh, S.S.A.; Fatemi, S.; Hashemi, S.J.; Ghasemi, M. Effect of Si/Al Ratio on CO2—CH4 Adsorption and Selectivity in Synthesized SAPO-34. Sep. Sci. Technol. 2010, 45, 1295–1301. [Google Scholar] [CrossRef]
- Sarti, E.; Chenet, T.; Pasti, L.; Cavazzini, A.; Rodeghero, E.; Martucci, A. Effect of Silica Alumina Ratio and Thermal Treatment of Beta Zeolites on the Adsorption of Toluene from Aqueous Solutions. Minerals 2017, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Grande, C.A.; Cavenati, S.; Barcia, P.; Hammer, J.; Fritz, H.G.; Rodrigues, A.E. Adsorption of Propane and Propylene in Zeolite 4A Honeycomb Monolith. Chem. Eng. Sci. 2006, 61, 3053–3067. [Google Scholar] [CrossRef]
- Ohlin, L.; Bazin, P.; Thibault-Starzyk, F.; Hedlund, J.; Grahn, M. Adsorption of CO2, CH4, and H2O in Zeolite ZSM-5 Studied Using In Situ ATR-FTIR Spectroscopy. J. Phys. Chem. C 2013, 117, 16972–16982. [Google Scholar] [CrossRef]
- Grande, C.A.; Mondino, G.; Lind, A.; Vistad, Ø.; Akporiaye, D. Selective Removal of CH4 from CH4/CO/H2 Mixtures. In Small-Scale Gas to Liquid Fuel Synthesis; Kanellopoulos, N., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Heymans, N.; Alban, B.; Moreau, S.; De Weireld, G. Experimental and theoretical study of the adsorption of pure molecules and binary systems containing methane, carbon monoxide, carbon dioxide and nitrogen. Application to the syngas generation. Chem. Eng. Sci. 2011, 66, 3850–3858. [Google Scholar] [CrossRef]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric heats of adsorption and adsorption isotherms 2. O2, N2, Ar, CO2, CH4, C2H6 and SF6 on NaX, H-ZSM-5 and Na-ZSM-5 zeolites. Langmuir 1996, 12, 5896–5904. [Google Scholar] [CrossRef]
- Mathias, P.M.; Kumar, R.; Moyer, J.D.; Schork, J.M.; Srinivasan, S.R.; Auvil, S.R.; Talu, O. Correlation of Multicomponent Gas Adsorption by the Dual-Site Langmuir Model. Application to Nitrogen/Oxygen Adsorption on 5A-Zeolite. Ind. Eng. Chem. Res. 1996, 35, 2477–2483. [Google Scholar] [CrossRef]
- Olson, D.H.; Haag, W.O.; Borghard, W.S. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Microporous Mesoporous Mater. 2000, 35, 435–446. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Ugliengo, P. Thermodynamic Study of Water Adsorption in High-Silica Zeolites. J. Phys. Chem. B 2006, 110, 14849–14859. [Google Scholar] [CrossRef]
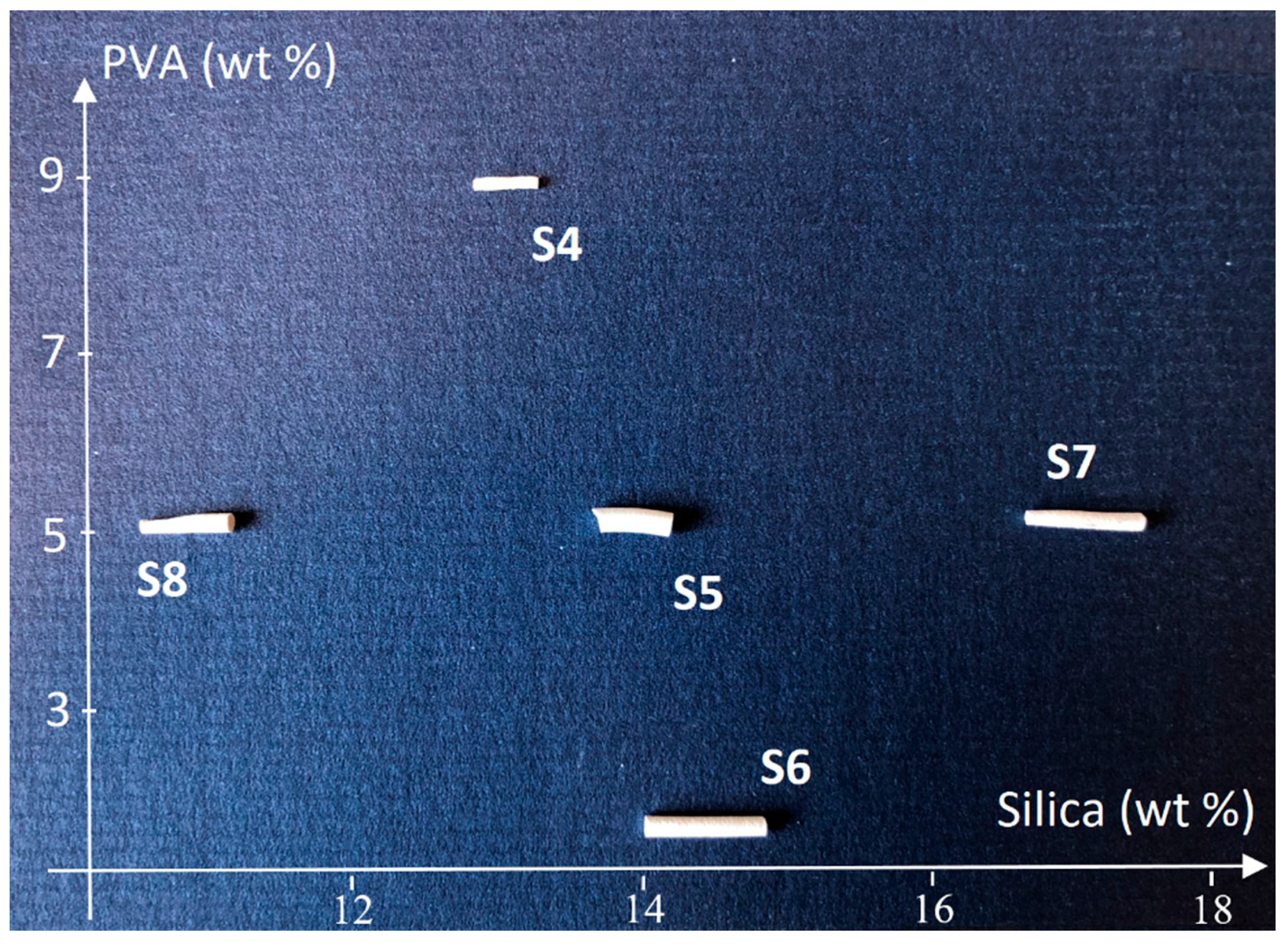
| Sample | Preparation | ZSM-5 (wt %) | PVA (wt %) | Silica (wt %) | Density (g/cm3) |
|---|---|---|---|---|---|
| 4 | Open cycle | 77.4 | 9.0 | 13.6 | N.A. |
| 5 | Closed cycle | 81.0 | 5.0 | 14.0 | 1.17 |
| 6 | Open cycle | 83.7 | 2.0 | 14.3 | 1.03 |
| 7 | Closed cycle | 78.0 | 5.0 | 17.0 | 0.98 |
| 8 | Closed cycle | 84.0 | 5.0 | 11.0 | 1.14 |
| 16 | Closed cycle | 80.0 | 5.0 | 15.0 | 1.13 |
| ZSM-5 Sample | ABET (m2/g) | Micropore Volume (cm3/g) |
|---|---|---|
| Powder | 341 | 0.305 |
| Extrudates (Sample 5) | 318 | 0.260 |
| Langmuir Model | ||||||
|---|---|---|---|---|---|---|
| qmax (mol/kg) | KA0 (kPa−1) | −ΔH (J/mol) | ||||
| CO2 | 2.04 | 3.79 × 10−7 | 26,600 | |||
| CH4 | 1.47 | 5.70 × 10−7 | 22,500 | |||
| Dual-Site Langmuir Model | ||||||
| qmax1 (mol/kg) | K10 (kPa−1) | −ΔH1 (J/mol) | qmax2 (mol/kg) | K20 (kPa−1) | −ΔH2 (J/mol) | |
| CO2 | 2.360 | 3.77 × 10−7 | 25,417 | 0.106 | 1.39 × 10−7 | 39,886 |
| H2O | 0.869 | 2.55 × 10−8 | 45,071 | 0.187 | 7.91 × 10−5 | 37,394 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Grande, C. Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder. Energies 2020, 13, 1201. https://doi.org/10.3390/en13051201
Almeida A, Ribeiro RPPL, Mota JPB, Grande C. Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder. Energies. 2020; 13(5):1201. https://doi.org/10.3390/en13051201
Chicago/Turabian StyleAlmeida, Ana, Rui P. P. L. Ribeiro, José P. B. Mota, and Carlos Grande. 2020. "Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder" Energies 13, no. 5: 1201. https://doi.org/10.3390/en13051201





