Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
2.2. Microreactors Test Bench for Screening Reaction Conditions
2.3. Hydrothermal Reaction Conditions
2.4. Characterization of Liquid Products
3. Results and Discussion
3.1. Lignin-Rich Stream Characterization
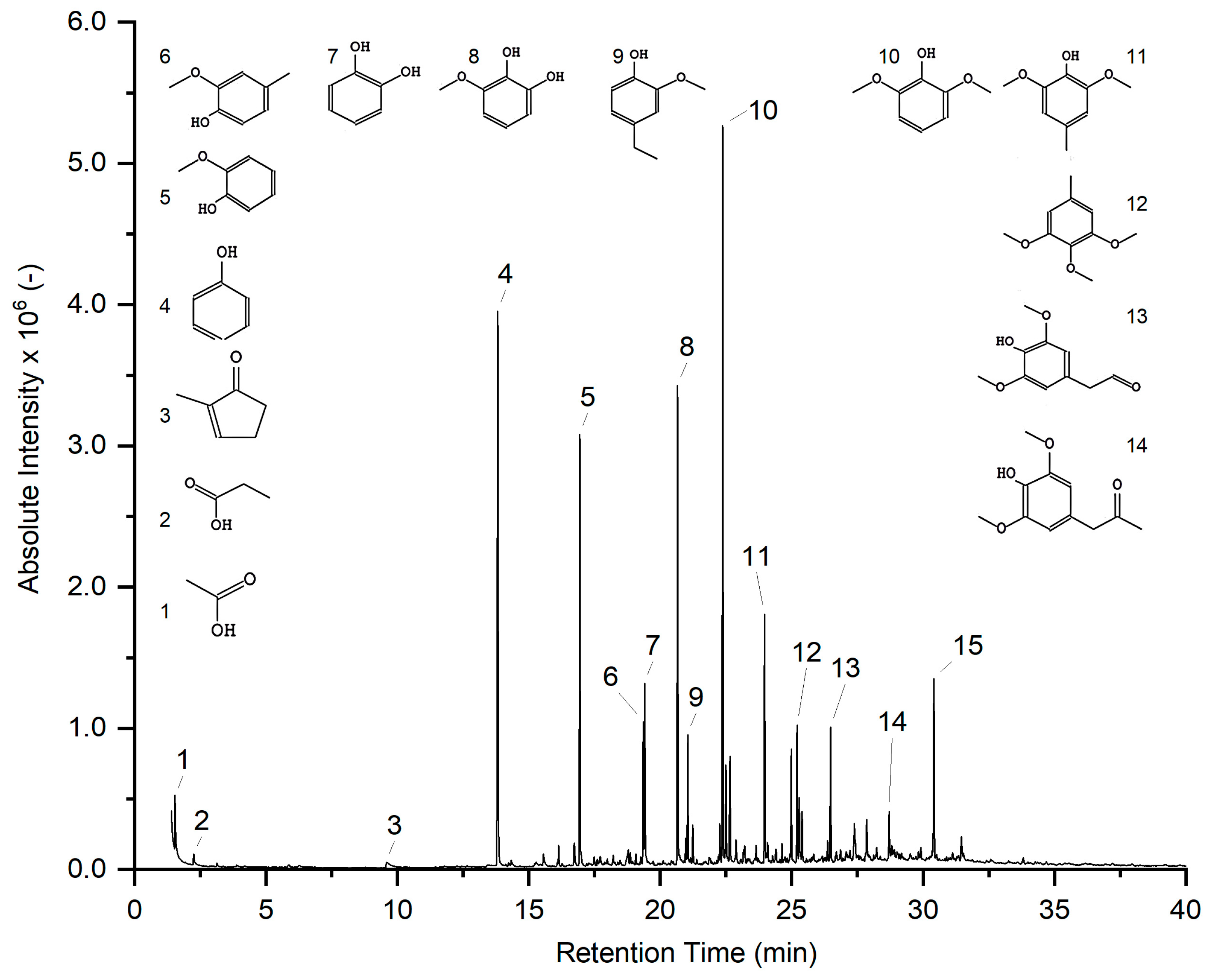
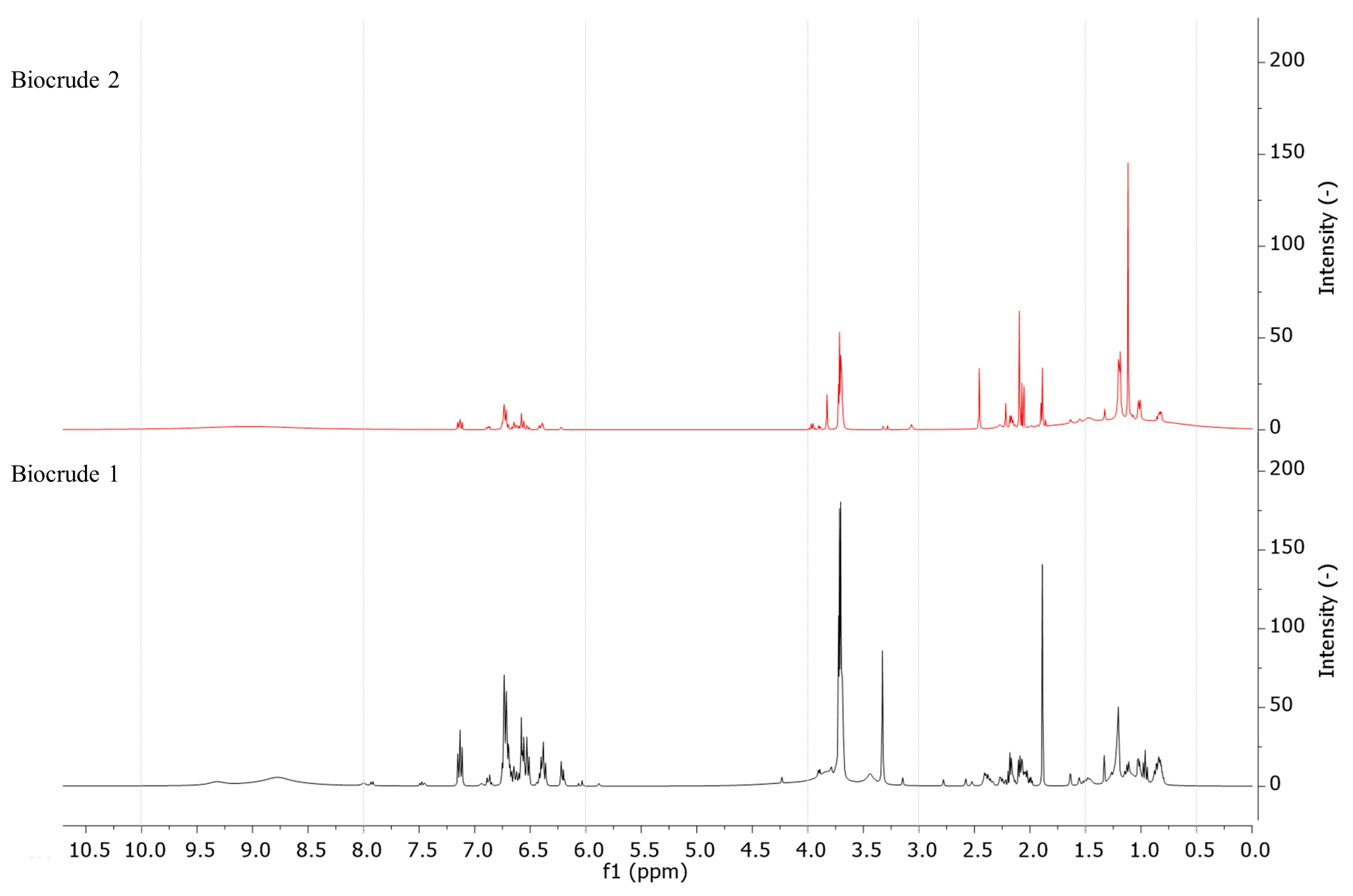
3.2. Analysis of HTL Products
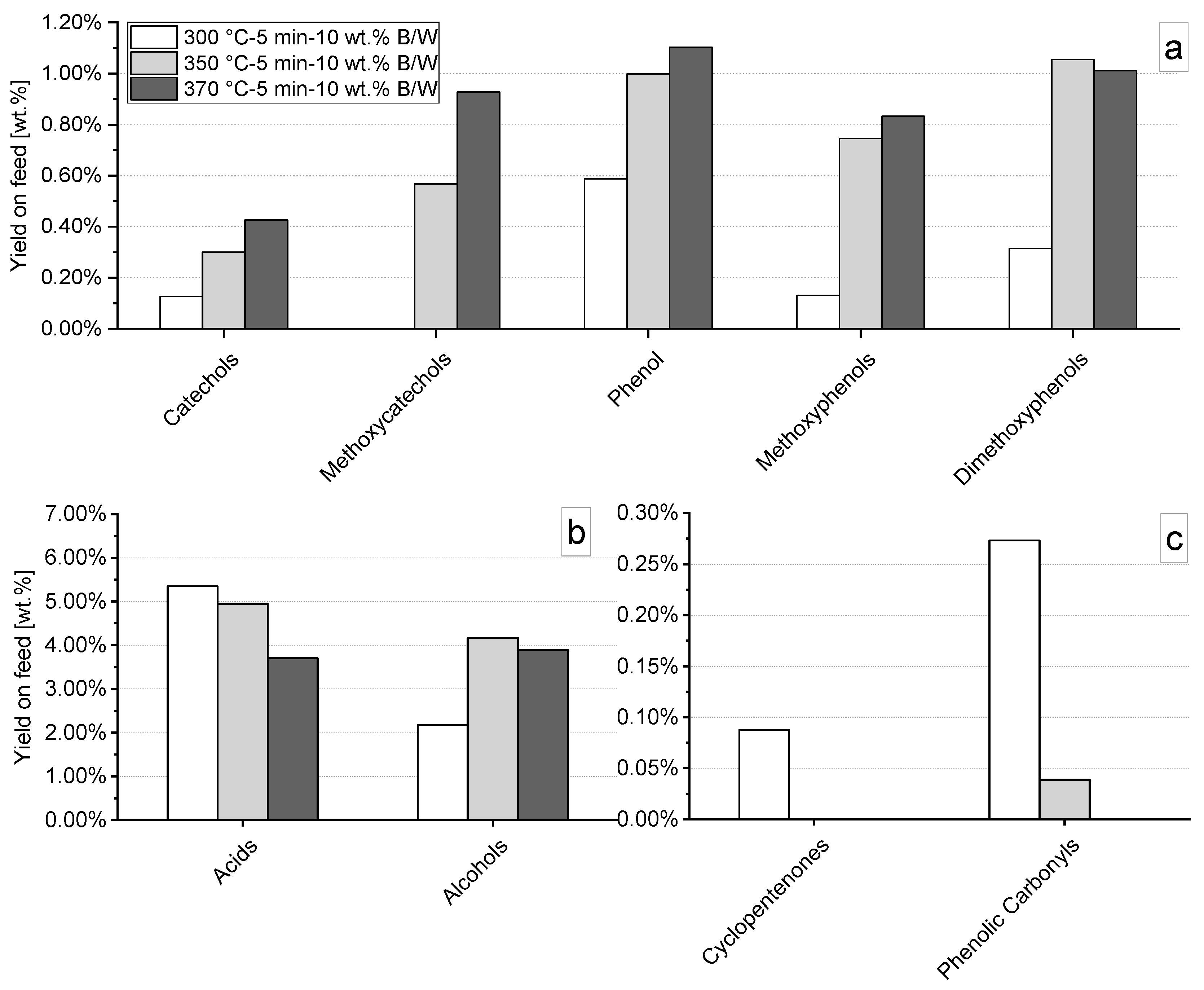
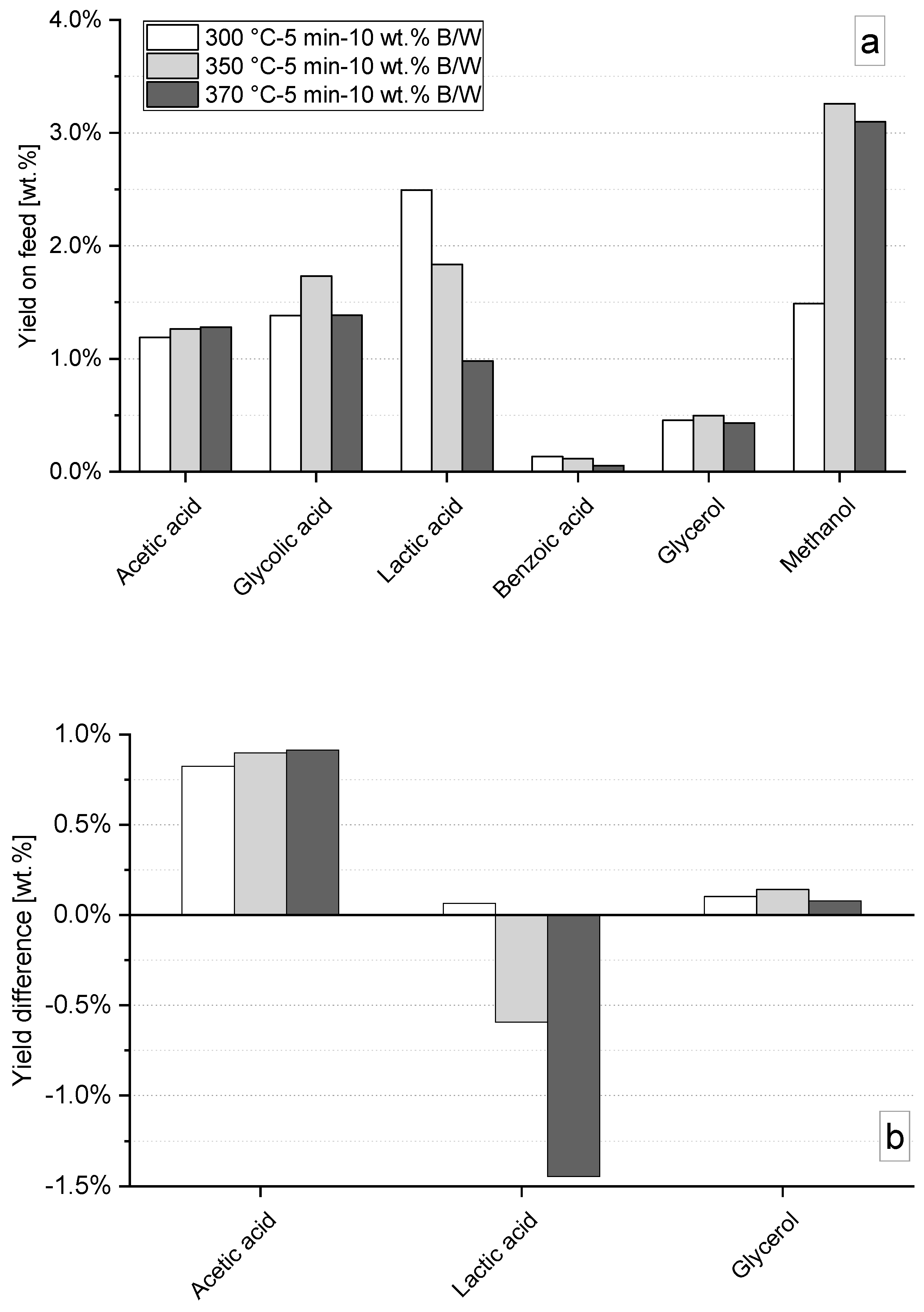
3.3. Hydrothermal Liquefaction in Absence of Catalysts: Influence of Temperature and Time
3.4. Influence of Catalytic Additives in the Process
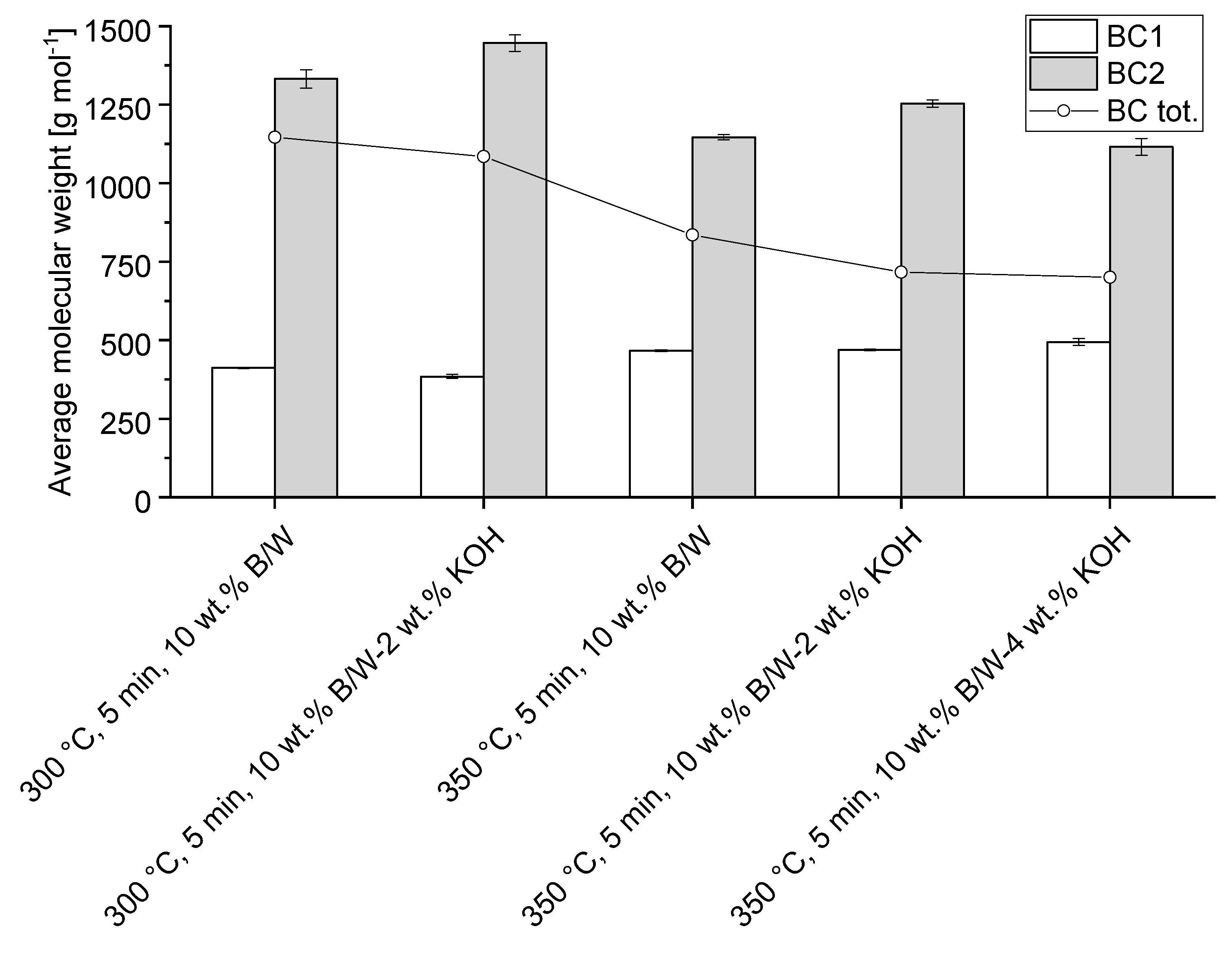
3.4.1. Effect of pH Control Using Strong Basis
3.4.2. Effect of Supercritical CO2
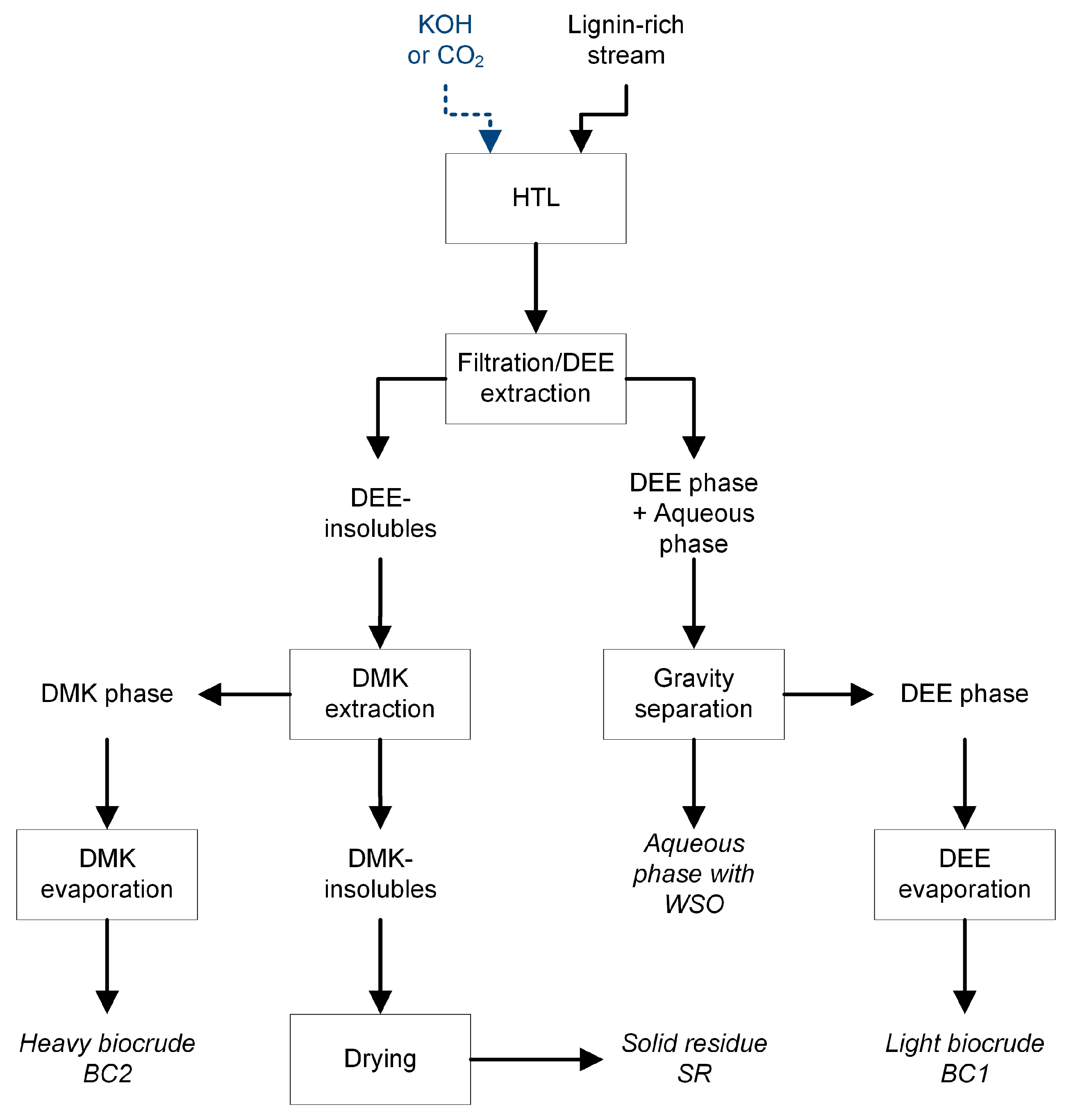
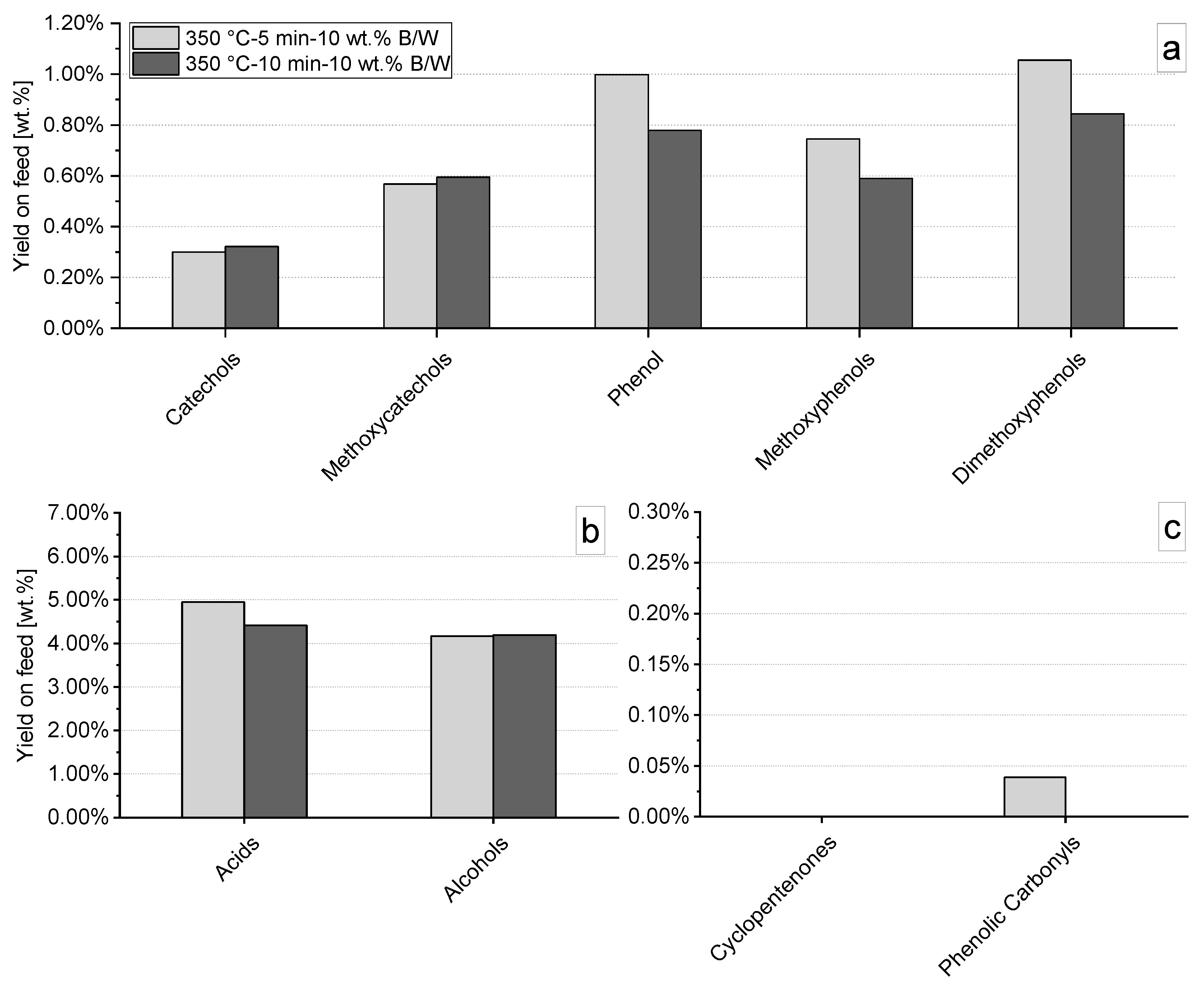
3.5. Compound Distribution among HTL Fractions: Influence of Biocrude Extraction Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Schuler, J.; Hornung, U.; Kruse, A.; Dahmen, N.; Sauer, J. Hydrothermal Liquefaction of Lignin. J. Biomater. Nanobiotechnol. 2017, 8, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoĝlu, M.Ö.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Chudziak, C.; Alberts, G.; Bauen, A. Ramp Up of Lignocellulosic Ethanol in Europe to 2030 Final Report; E4tech: London, UK, 2017. [Google Scholar]
- Biermann, C.J. Pulping Fundamentals. In Handbook of Pulping and Papermaking; Biermann, C.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 55–100. ISBN 9780120973620. [Google Scholar]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Solimene, R.; Cammarota, A.; Chirone, R.; Leoni, P.; Rossi, N.; Salatino, P. Devolatilization and Fragmentation of Solid Lignin-rich Residues from Bioethanol Production in Lab-scale Fluidized Bed Reactors. Chem. Eng. Trans 2016, 50, 79–84. [Google Scholar]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, S.; Leitch, M.; Xu, C.C. Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol. Bioresour. Technol. 2010, 101, 9308–9313. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.E.; Vamling, L.; Olausson, L.; Andersson, S.I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.M.; Djajadi, D.T.; Torri, C.; Rasmussen, H.B.; Madsen, R.B.; Venturini, E.; Vassura, I.; Becker, J.; Iversen, B.B.; Meyer, A.S.; et al. Hydrothermal Liquefaction of Enzymatic Hydrolysis Lignin: Biomass Pretreatment Severity Affects Lignin Valorization. ACS Sustain. Chem. Eng. 2018, 6, 5940–5949. [Google Scholar] [CrossRef]
- Katahira, R.; Mittal, A.; McKinney, K.; Chen, X.; Tucker, M.P.; Johnson, D.K.; Beckham, G.T. Base-Catalyzed Depolymerization of Biorefinery Lignins. ACS Sustain. Chem. Eng. 2016, 4, 1474–1486. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Jensen, A.; Madsen, L.R.; Larsen, F.H.; Felby, C.; Jensen, A.D. Noncatalytic Direct Liquefaction of Biorefinery Lignin by Ethanol. Energy Fuels 2017, 31, 7223–7233. [Google Scholar] [CrossRef] [Green Version]
- Belkheiri, T.; Mattsson, C.; Andersson, S.I.; Olausson, L.; Åmand, L.E.; Theliander, H.; Vamling, L. Effect of pH on Kraft Lignin Depolymerisation in Subcritical Water. Energy Fuels 2016, 30, 4916–4924. [Google Scholar] [CrossRef]
- Xu, Y.; Isom, L.; Hanna, M.A. Adding value to carbon dioxide from ethanol fermentations. Bioresour. Technol. 2010, 101, 3311–3319. [Google Scholar] [CrossRef]
- McAloon, A.; Taylor, F.; Yee, W.; Regional, E.; Ibsen, K.; Wooley, R.; Biotechnology, N. Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks; NERL: Golden, CO, USA, 2000. [Google Scholar]
- Restrepo-Valencia, S.; Walter, A. Techno-economic assessment of bio-energy with carbon capture and storage systems in a typical sugarcane mill in Brazil. Energies 2019, 12, 1129. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of cardanol and phenol from bio-oils obtained through vacuum pyrolysis of biomass using supercritical fluid extraction. Energy 2011, 36, 1535–1542. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Montesantos, N.; Pedersen, T.H.; Nielsen, R.P.; Rosendahl, L.; Maschietti, M. Supercritical carbon dioxide fractionation of bio-crude produced by hydrothermal liquefaction of pinewood. J. Supercrit. Fluids 2019, 149, 97–109. [Google Scholar] [CrossRef]
- Montesantos, N.; Pedersen, T.H.; Nielsen, R.P.; Rosendahl, L.A.; Maschietti, M. High-temperature extraction of lignocellulosic bio-crude by supercritical carbon dioxide. Chem. Eng. Trans. 2019, 74, 799–804. [Google Scholar]
- Numan-Al-Mobin, A.M.; Kolla, P.; Dixon, D.; Smirnova, A. Effect of water–carbon dioxide ratio on the selectivity of phenolic compounds produced from alkali lignin in sub- and supercritical fluid mixtures. Fuel 2016, 185, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Herng, Y.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M. Optimization of hydrothermal liquefaction of palm kernel shell and consideration of supercritical carbon dioxide mediation effect. J. Supercrit. Fluids 2018, 133, 640–646. [Google Scholar]
- Miliotti, E.; Dell’Orco, S.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Lignocellulosic Ethanol Biorefinery: Valorization of Lignin-Rich Stream through Hydrothermal Liquefaction. Energies 2019, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Technical Report NREL/TP-510-42623; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Miliotti, E.; Casini, D.; Lotti, G.; Pennazzi, S.; Rizzo, A.M.; Chiaramonti, D. Hydrothermal Carbonization of Digestate: Characterization of solid and liquid products. In Proceedings of the TC Biomass, Gas Technology Institute, Chicago, IL, USA, 27 June 2017. [Google Scholar]
- Beckner, M.; Ivey, M.L.; Phister, T.G. Microbial contamination of fuel ethanol fermentations. Lett. Appl. Microbiol. 2011, 53, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Li, M.; Bian, J.; Sun, R. Revealing the structure and distribution changes of Eucalyptus lignin during the hydrothermal and alkaline pretreatments. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: Influence of phenol and temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Sasaki Mitsuru, W.; Motonobu, G. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process. Process Intensif. 2008, 47, 1609–1619. [Google Scholar]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.A.; Cansell, F. Hydrothermal conversion of lignin compounds. A detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Lawson, J.J.R.; Klein, M.M.T. Influence of Water on Guaiacol Pyrolysis. Ind. Eng. Chem. Fundam. 1985, 24, 203–208. [Google Scholar] [CrossRef]
- Miller, J.E.; Evans, L.R.; Mudd, J.E.; Brown, K.A. Batch Microreactor Studies of Lignin Depolymerization by Bases. 2. Aqueous Solvents; Sandia National Labs.: Albuquerque, NM, USA, 2002. [Google Scholar]
- Porzio, G.F.; Prussi, M.; Chiaramonti, D.; Pari, L. Modelling lignocellulosic bioethanol from poplar: Estimation of the level of process integration, yield and potential for co-products. J. Clean. Prod. 2012, 34, 66–75. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Harnisch, F.; Schröder, U. Hydrothermal liquefaction of cellulose in subcritical water-the role of crystallinity on the cellulose reactivity. Rsc Adv. 2013, 3, 11035–11044. [Google Scholar] [CrossRef]
- Pedersen, T.H. HydroThermal Liquefaction of Biomass and Model Compounds. Ph.D. Thesis, Aalborg Universitet, Aalborg Universitetsforlag, Aalborg, Denmark, 2015. [Google Scholar]
- Pedersen, T.H.; Rosendahl, L.A. Production of fuel range oxygenates by supercritical hydrothermal liquefaction of lignocellulosic model systems. Biomass Bioenergy 2015, 83, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; NREL: Golden, CO, USA, 2002. [Google Scholar]
- Villadsen, S.R.; Dithmer, L.; Forsberg, R.; Becker, J.; Rudolf, A.; Iversen, S.B.; Glasius, M. Development and Application of Chemical Analysis Methods for Investigation of Bio-Oils and Aqueous Phase from Hydrothermal Liquefaction of Biomass. Energy Fuels 2012, 26, 6988–6998. [Google Scholar] [CrossRef]
- Jensen, M.M.; Madsen, R.B.; Becker, J.; Iversen, B.B.; Glasius, M. Products of hydrothermal treatment of lignin and the importance of ortho-directed repolymerization reactions. J. Anal. Appl. Pyrolysis 2017, 126, 371–379. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Kumar, J.; Khan, A.A.; Krishna, B.B.; Bhaskar, T. Slow pyrolysis of prot, alkali and dealkaline lignins for production of chemicals. Bioresour. Technol. 2015, 213, 319–326. [Google Scholar] [CrossRef]
- Li, S.; Lundquist, K. A new method for the analysis of phenolic groups in lignins by 1H NMR spectrometry. Nord. Pulp Pap. Res. J. 1994, 09, 191–195. [Google Scholar] [CrossRef]
- Chu, S.; Subrahmanyam, A.V.; Huber, G.W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Cheng, S.; Wilks, C.; Yuan, Z.; Leitch, M.; Xu, C. Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water–ethanol co-solvent. Polym. Degrad. Stab. 2012, 97, 839–848. [Google Scholar] [CrossRef]
- Ingram, L.; Mohan, D.; Bricka, M.; Steele, P.; Strobel, D.; Crocker, D.; Mitchell, B.; Mohammad, J.; Cantrell, K.; Pittman, C.U.J. Pyrolysis of Wood and Bark in an Auger Reactor: Physical Properties and Chemical Analysis of the Produced Bio-oils. Energy Fuels 2008, 22, 614–625. [Google Scholar] [CrossRef]
- Ralph, S.; Ralph, J.; Landucci, L.L. NMR Database of Lignin and Cell Wall Model Compounds 2009. Available online: https://www.glbrc.org/databases_and_software/nmrdatabase/NMR_DataBase_2009_Complete.pdf (accessed on 27 February 2020).
- Mullen, C.A.; Strahan, G.D.; Boateng, A.A. Characterization of various fast-pyrolysis bio-oils by NMR spectroscopy. Energy Fuels 2009, 23, 2707–2718. [Google Scholar] [CrossRef]
- Chern, S.; Tu, H.; Materials, A. The Thermochemical Conversion of Lactic Acid in Subcritical and Supercritical Water. Eng. Technol. Int. J. Chem. Mol. 2017, 11, 98–102. [Google Scholar]
- Islam, M.N.; Taki, G.; Rana, M.; Park, J.H. Yield of Phenolic Monomers from Lignin Hydrothermolysis in Subcritical Water System. Ind. Eng. Chem. Res. 2018, 57, 4779–4784. [Google Scholar] [CrossRef]
- Singh, R.; Prakash, A.; Dhiman, S.K.; Balagurumurthy, B.; Arora, A.K.; Puri, S.K.; Bhaskar, T. Hydrothermal conversion of lignin to substituted phenols and aromatic ethers. Bioresour. Technol. 2014, 165, 319–322. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Złocińska, A. Hydrothermal decomposition of alkali lignin in sub- and supercritical water. Chem. Eng. J. 2012, 187, 410–414. [Google Scholar] [CrossRef]
- Wahyudiono; Kanetake, T.; Sasaki, M.; Goto, M. Decomposition of a Lignin Model Compound under Hydrothermal Conditions. Chem. Eng. Technol. 2007, 30, 1113–1122. [Google Scholar] [CrossRef]
- Jegers, H.E.; Klein, M.T. Primary and Secondary Lignin Pyrolysis Reaction Pathways. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 173–183. [Google Scholar] [CrossRef]
- Numan-Al-Mobin, A.M.; Voeller, K.; Bilek, H.; Kozliak, E.; Kubatova, A.; Raynie, D.; Dixon, D.; Smirnova, A. Selective Synthesis of Phenolic Compounds from Alkali Lignin in a Mixture of Sub- and Supercritical Fluids: Catalysis by CO2. Energy and Fuels 2016, 30, 2137–2143. [Google Scholar] [CrossRef]
- Tabasinejad, F.; Moore, R.G.; Mehta, S.A.; Van Fraassen, K.C.; Barzin, Y.; Rushing, J.A.; Newsham, K.E. Water solubility in supercritical methane, nitrogen, and carbon dioxide: Measurement and modeling from 422 to 483 K and pressures from 3.6 to 134 MPa. Ind. Eng. Chem. Res. 2011, 50, 4029–4041. [Google Scholar] [CrossRef]
- Takenouchi, S.; Kennedy, G.C. The Binary System H2O-CO2 at High Temperature and Pressures. Am. J. Sci. 1964, 262, 1055–1074. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Bocchini, S.; Rizzo, A.M.; Chiaramonti, D.; Pirone, R.; Bensaid, S. Aqueous phase reforming of the residual waters derived from lignin-rich hydrothermal liquefaction: Investigation of representative organic compounds and actual biorefinery streams. Catal. Today 2019. [Google Scholar] [CrossRef]
- Wilson, A.N.; Dutta, A.; Black, B.A.; Mukarakate, C.; Schaidle, J.A.; Michener, W.E.; Beckham, G.T.; Nimlos, M.R. Valorization of Aqueous Waste Streams from Thermochemical Biorefineries. Green Chem. 2019, 21, 4217–4230. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.D.; Lopez-Ruiz, J.A.; Zhu, Y.; Cooper, A.R.; Albrecht, K.O.; Dagle, R.A. Strategies to Valorize Hydrothermal Liquefaction-Derived Aqueous Phase into Fuels and Chemicals. ACS Sustainable Chem. Eng. 2019, 7, 19889–19901. [Google Scholar] [CrossRef]
- INCO. Corrosion Resistance of the Austenic Chromium-Nickel Stainless Steels in Chemical Environments; INCO: New York, NY, USA, 1963. [Google Scholar]

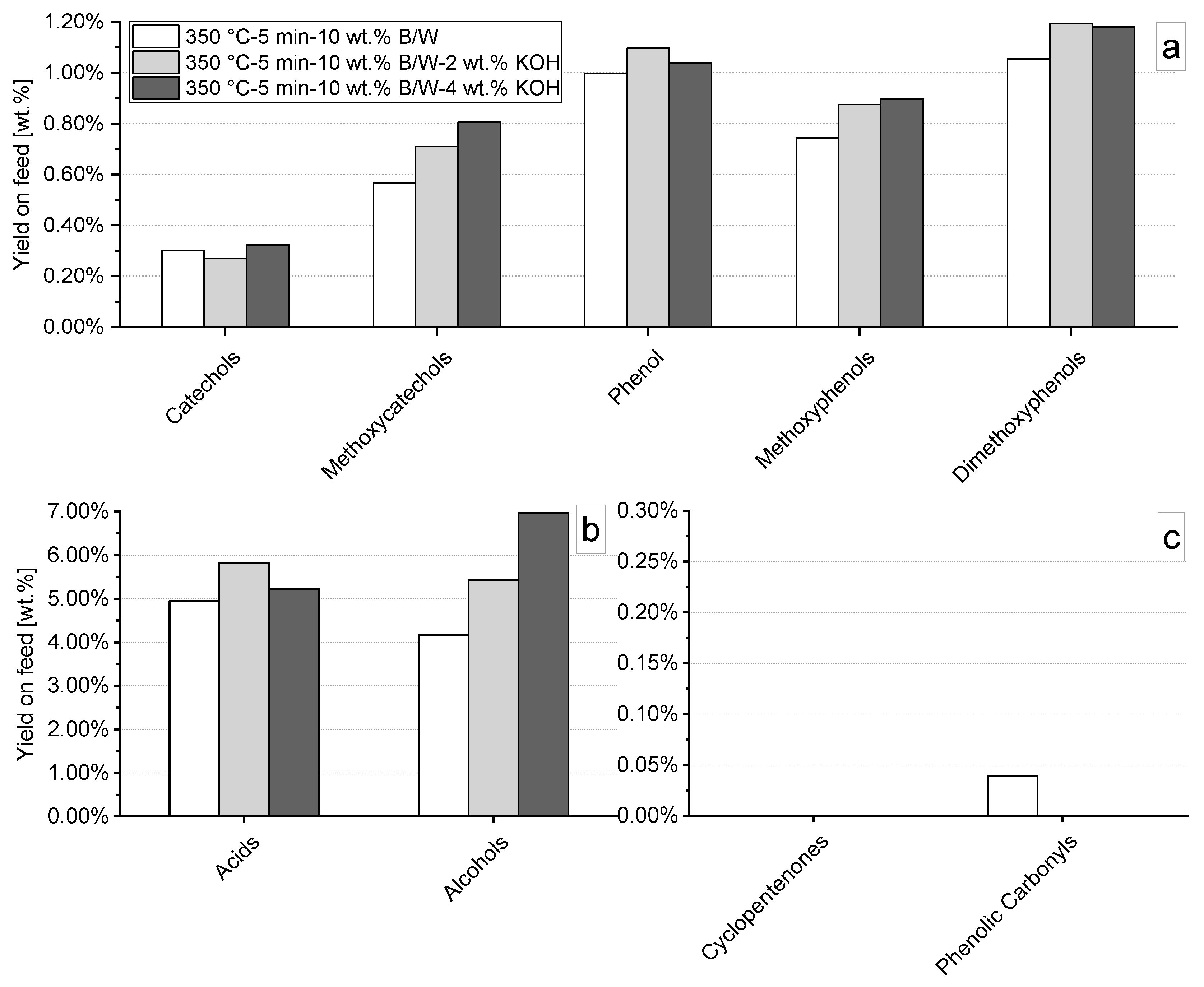
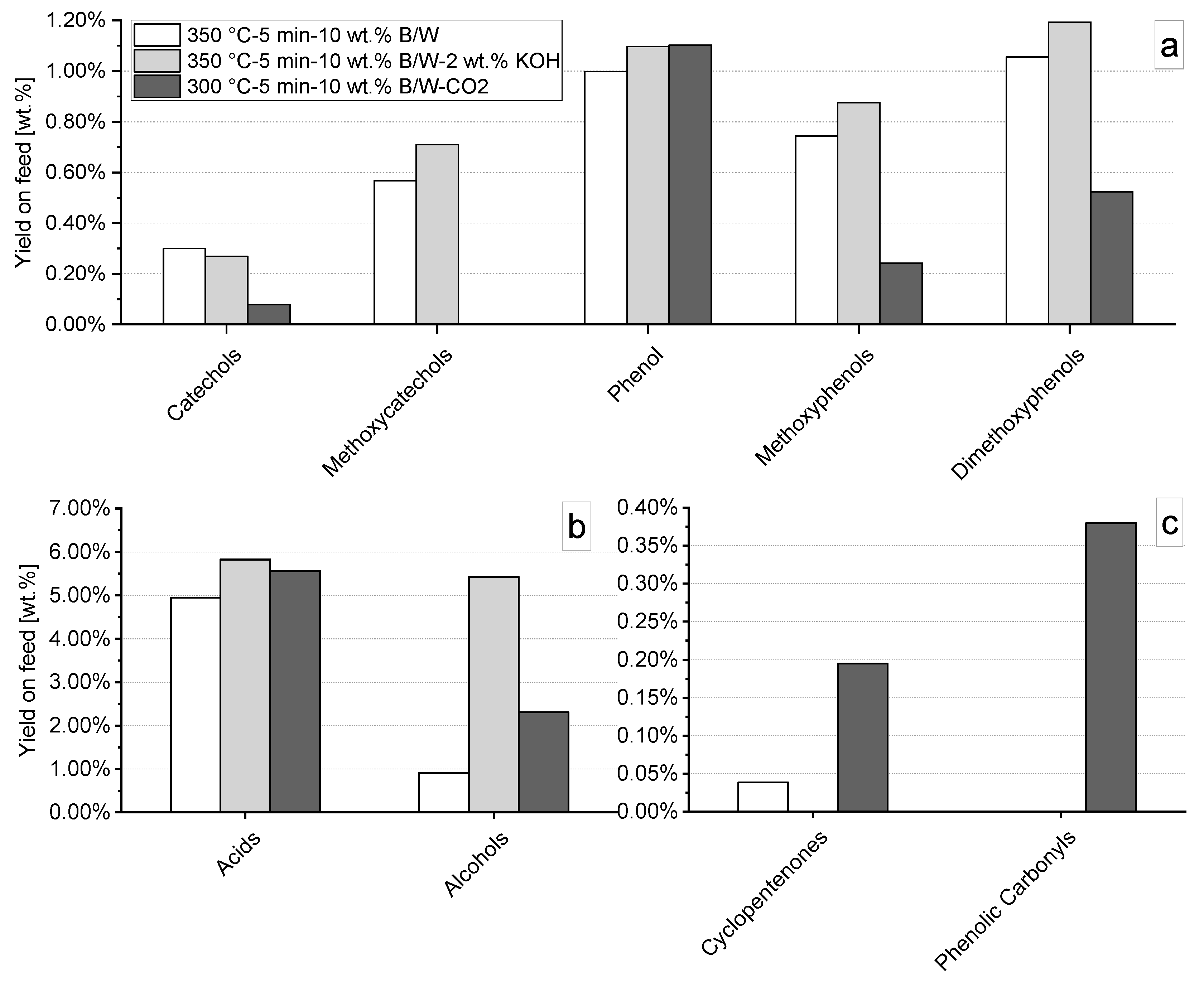
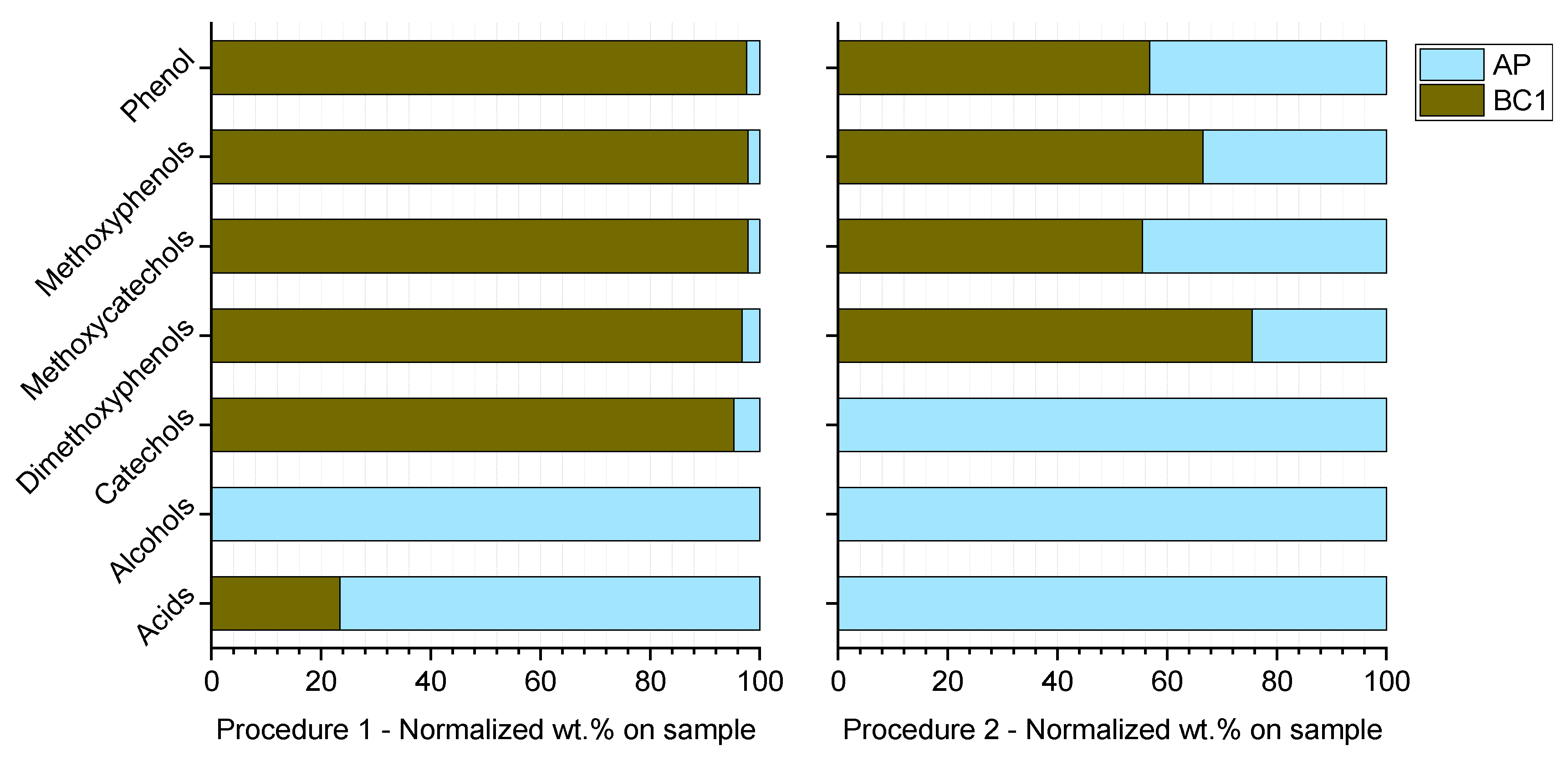
| Parameter | Value [-] wt. % (d.a.f.) |
|---|---|
| Acid insoluble lignin | 52.7 ± 4.99 |
| Acid soluble lignin | 0.253 ± 0.0216 |
| Total lignin | 53.0 ± 5.02 |
| Structural sugars | 35.8 ± 0.314 |
| Glucan | 30.7 ± 0.484 |
| XMG* | 4.94 ± 0.170 |
| Arabinan | 0.163 ± 0.0287 |
| Stream | pH of Control | pH @ 2 wt. % KOH | pH @ 4 wt. % KOH |
|---|---|---|---|
| feed | 4.6 | 8.0 | 10.0 |
| AP | 4.4 | 5.1 | 5.8 |
| Temp. [°C] | KOH [%] | BC1 | BC2 | SR | Gas | WSO + Loss * |
|---|---|---|---|---|---|---|
| 300 | - | 9.8% (1.6%) | 38.8% (11.5%) | 16.5% (3.0%) | 2.4% (0.7%) | 32.6% (10.5%) |
| 2 | 18.8% (0.7%) | 36.5% (2.6%) | 13.5% (0.7%) | 2.2% (n.d.) | 29.0% (2.6%) | |
| 350 | - | 27.0% (6.7%) | 32.0% (6.4%) | 11.4% (0.9%) | 4.5% (0.8%) | 23.8% (5.4%) |
| 2 | 37.0% (1.4%) | 17.0% (0.5%) | 10.2% (0.7%) | 4.4% (0.6%) | 31.3% (1.1%) | |
| 4 | 35.5% (2.1%) | 17.7% (1.4%) | 9.3% (0.5%) | 2.2% (0.3%) | 35.4% (0.6%) | |
| 370 | - | 36.8% (1.7%) | 15.8% (3.1%) | 13.0% (0.9%) | 4.0% (0.1%) | 30.3% (2.3%) |
| 2 | 39.2% (0.5%) | 9.7% (1.9%) | 12.3% (0.0%) | 4.3% (0.1%) | 34.5% (2.0%) |
| Temp. [°C] | KOH [wt. %] | TOC—Concentration [mg l−1] | C—HPLC Detected WSO Concentration [mg l−1] |
|---|---|---|---|
| 300 | - | 4741 | 3405 |
| 2 | 5310 | 3859 | |
| 350 | - | 6648 | 3186 |
| 2 | 8823 | 3337 | |
| 4 | 10,981 | 4707 | |
| 370 | - | 5937 | 5608 |
| 2 | 8692 | 4513 |
| Temp. [°C] | Catalyst | BC1 | BC2 | SR | Gas | WSO + Loss * |
|---|---|---|---|---|---|---|
| 300 | - | 9.8% (1.6%) | 38.8% (11.5%) | 16.5% (3.0%) | 2.4% (0.7%) | 32.6% (10.5%) |
| 300 | 2 wt. % KOH | 18.8% (0.7%) | 36.5% (2.6%) | 13.5% (0.7%) | 2.2% (n.d.) | 29.0% (2.6%) |
| 300 | sCO2 | 15.5% (5.0%) | 38.1% (8.2%) | 16.2% (0.7%) | 3.3% (n.d.) | 26.9% (2.56%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Orco, S.; Miliotti, E.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase. Energies 2020, 13, 1241. https://doi.org/10.3390/en13051241
Dell’Orco S, Miliotti E, Lotti G, Rizzo AM, Rosi L, Chiaramonti D. Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase. Energies. 2020; 13(5):1241. https://doi.org/10.3390/en13051241
Chicago/Turabian StyleDell’Orco, Stefano, Edoardo Miliotti, Giulia Lotti, Andrea Maria Rizzo, Luca Rosi, and David Chiaramonti. 2020. "Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase" Energies 13, no. 5: 1241. https://doi.org/10.3390/en13051241
APA StyleDell’Orco, S., Miliotti, E., Lotti, G., Rizzo, A. M., Rosi, L., & Chiaramonti, D. (2020). Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase. Energies, 13(5), 1241. https://doi.org/10.3390/en13051241