Fabrication and Characterization of Cu2ZnSnSe4 Thin-Film Solar Cells using a Single-Stage Co-Evaporation Method: Effects of Film Growth Temperatures on Device Performances
Abstract
:1. Introduction
2. Experimental Details
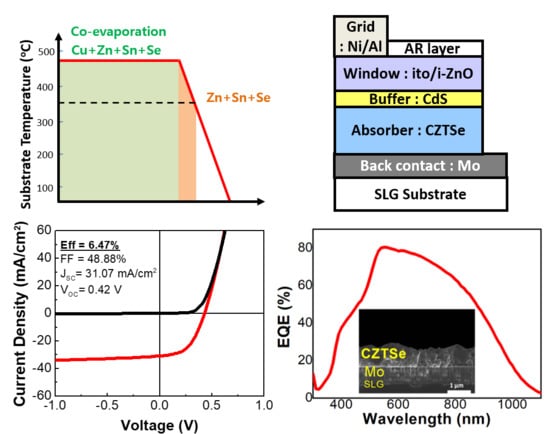
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakhiyi, B.; Labrèche, F.; Zayed, J. The photovoltaic industry on the path to a sustainable future—Environmental and occupational health issues. Environ. Int. 2014, 73, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Jain, P. Greenhouse effect and climate change: Scientific basis and overview. Renew. Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Kibria, M.T.; Ahammed, A.; Sony, S.M.; Hossain, F.; Islam, S. A Review: Comparative studies on different generation solar cells technology. In Proceedings of the 5th International Conference on Environmental Aspects of Bangladesh, Dhaka, Bangladesh, 5–6 September 2014; pp. 51–53. [Google Scholar]
- Ranabhat, K.; Patrikeev, L.; Antal’evna-Revina, A.; Andrianov, K.; Lapshinsky, V.; Sofronova, E. An introduction to solar cell technology. J. Appl. Eng. Sci. 2016, 14, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Philips, D.; Warmuth, W. Photovoltaics Report; Fraunhofer Institute for Solar Energy System: Freiburg, Germany, 2019; pp. 1–43. [Google Scholar]
- Płaczek-Popko, E. Top PV market solar cells 2016. Opto-Electron. Rev. 2017, 25, 55–64. [Google Scholar]
- Shin, S.W.; Han, J.H.; Park, C.Y.; Moholkar, A.V.; Lee, J.Y.; Kim, J.H. Quaternary Cu2ZnSnS4 nanocrystals: Facile and low cost synthesis by microwave-assisted solution method. J. Alloy. Compd. 2012, 516, 96–101. [Google Scholar] [CrossRef]
- Reddy, V.R.M.; Gedi, S.; Pejjai, B.; Park, C. Perspectives on SnSe-based thin film solar cells: A comprehensive review. J. Mater. Sci. Mater. Electron. 2016, 27, 5491–5508. [Google Scholar] [CrossRef]
- Siebentritt, S.; Schorr, S. Kesterites—A challenging material for solar cells. Prog. Photovolt. Res. Appl. 2012, 20, 512–519. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Feng, Y.; Chen, M.; Li, W.; Zhong, G.-H.; Lu, Y.; Yang, C. Preparation of Cu2ZnSn(SxSe1−x)4 solar cells with two step sulfurization. Sol. Energy 2020, 197, 73–77. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W. Solar cell efficiency tables (version 54). Prog. Photovolt. Res. Appl. 2019, 27, 565–575. [Google Scholar] [CrossRef]
- Repins, I.; Beall, C.; Vora, N.; DeHart, C.; Kuciauskas, D.; Dippo, P.; To, B.; Mann, J.; Hsu, W.-C.; Goodrich, A. Co-evaporated Cu2ZnSnSe4 films and devices. Sol. Energy Mater. Sol. Cells 2012, 101, 154–159. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Cai, H.; Chen, B.; Yao, L.; Yang, M.; Chen, S.; Huang, Z. The effects of SnS2 secondary phases on Cu2ZnSnS4 solar cells: A promising mechanical exfoliation method for its removal. J. Mater. Chem. A 2018, 6, 2995–3004. [Google Scholar] [CrossRef]
- Song, X.; Ji, X.; Li, M.; Lin, W.; Luo, X.; Zhang, H. A review on development prospect of CZTS based thin film solar cells. Int. J. Photoenergy 2014, 1–11. [Google Scholar] [CrossRef]
- Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; He, Q.; Zhou, Z.; Sun, G.; Sun, Y.; Chang, L.-B. CZTSe solar cells prepared by electrodeposition of Cu/Sn/Zn stack layer followed by selenization at low Se pressure. Nanoscale Res. Lett. 2014, 9, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.; Gwak, J.; Yun, J.H.; Ahn, S.; Nam, D.; Cheong, H.; Ahn, S.; Cho, A.; Shin, K.; Yoon, K. Cu2ZnSnSe4 thin film solar cells based on a single-step co-evaporation process. Thin Solid Film. 2013, 535, 52–56. [Google Scholar] [CrossRef]
- Redinger, A.; Siebentritt, S. Coevaporation of Cu2ZnSnSe4 thin films. Appl. Phys. Lett. 2010, 97, 092111. [Google Scholar] [CrossRef]
- Repins, I.L.; Romero, M.J.; Li, J.V.; Wei, S.-H.; Kuciauskas, D.; Jiang, C.-S.; Beall, C.; DeHart, C.; Mann, J.; Hsu, W.-C. Kesterite successes, ongoing work, and challenges: A perspective from vacuum deposition. IEEE J. Photovolt. 2012, 3, 439–445. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, I.; Cho, Y.; Shin, D.; Song, S.; Ahn, S.K.; Eo, Y.-J.; Cho, A.; Jung, C.; Jo, W. Mechanisms of extrinsic alkali incorporation in CIGS solar cells on flexible polyimide elucidated by nanoscale and quantitative analyses. Nano Energy 2019, 67, 104201. [Google Scholar] [CrossRef]
- Schwarz, T.; Cojocaru-Mirédin, O.; Choi, P.; Mousel, M.; Redinger, A.; Siebentritt, S.; Raabe, D. Atom probe study of Cu2ZnSnSe4 thin-films prepared by co-evaporation and post-deposition annealing. Appl. Phys. Lett. 2013, 102, 042101. [Google Scholar] [CrossRef]
- Colombara, D.; Robert, E.V.C.; Crossay, A.; Taylor, A.; Guennou, M.; Arasimowicz, M.; Malaquias, J.C.B.; Djemour, R.; Dale, P.J. Quantification of surface ZnSe in Cu2ZnSnSe4-based solar cells by analysis of the spectral response. Sol. Energy Mater. Sol. Cells 2014, 123, 220–227. [Google Scholar] [CrossRef]
- Ko, B.-S.; Kim, D.-H.; Hwang, D.-K.; Lee, S.-J.; Kim, J.S. Effects of the annealing temperature on the properties of sulfur-graded Cu2ZnSn(S,Se)4 thin films grown by a modified two-step process. J. Ind. Eng. Chem. 2020, 82, 406–412. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ogawa, H.; Nakashima, M.; Naoi, H.; Araki, H.; Jimbo, K.; Katagiri, H.; Sasano, J.; Izaki, M. Fabrication of Cu2ZnSn(S,Se)4 thin-film solar cells by sulfurization using Cu2ZnSnSe4, NaF and KF compounds. Jpn. J. Appl. Phys. 2020, 59, SGGF11. [Google Scholar] [CrossRef]
- Son, D.-H.; Kim, Y.-I.; Kim, S.-H.; Nam, D.; Cheong, H.; Kang, J.-K.; Yang, K.-J.; Kim, D.-H. Effects of S and Se contents on the physical and photovoltaic properties of Cu2ZnSn(SX,Se1−X)4 thin films: Achieving a PCE of 9.47%. J. Mater. Chem. A 2019, 7, 22986–22995. [Google Scholar] [CrossRef]
- Katırcı, R.; Walls, J.M. Optimisation of the CZTSe thin film composition obtained by a sequential electrodeposition process. Surf. Eng. 2019, 35, 854–860. [Google Scholar] [CrossRef]
- Dumcenco, D.; Huang, Y.-S. The vibrational properties study of kesterite Cu2ZnSnS4 single crystals by using polarization dependent Raman spectroscopy. Opt. Mater. 2013, 35, 419–425. [Google Scholar] [CrossRef]
- Kodigala, S.R. Thin Film Solar Cells from Earth Abundant Materials: Growth and Characterization of Cu2ZnSn(SSe)4 Thin Films and Their Solar Cells, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 173. [Google Scholar]
- Lai, F.-I.; Yang, J.-F.; Wei, Y.-L.; Kuo, S.-Y. High quality sustainable Cu2ZnSnSe4 (CZTSe) absorber layers in highly efficient CZTSe solar cells. Green Chem. 2017, 19, 795–802. [Google Scholar] [CrossRef]
- Zhang, R.; Cho, S.; Lim, D.G.; Hu, X.; Stach, E.A.; Handwerker, C.A.; Agrawal, R. Metal–metal chalcogenide molecular precursors to binary, ternary, and quaternary metal chalcogenide thin films for electronic devices. Chem. Commun. 2016, 52, 5007–5010. [Google Scholar] [CrossRef]
- Djemour, R.; Redinger, A.; Mousel, M.; Gütay, L.; Fontané, X.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Siebentritt, S. The three A symmetry Raman modes of kesterite in Cu2ZnSnSe4. Opt. Express 2013, 21, A695–A703. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Fairbrother, A.; Fontané, X.; Jawhari, T.; Izquierdo-Roca, V.; Saucedo, E.; Pérez-Rodríguez, A. Multiwavelength excitation Raman scattering study of polycrystalline kesterite Cu2ZnSnS4 thin films. Appl. Phys. Lett. 2014, 104, 021901. [Google Scholar] [CrossRef] [Green Version]
- López-Marino, S.; Sanchez, Y.; Espindola-Rodriguez, M.; Alcobé, X.; Xie, H.; Neuschitzer, M.; Becerril, I.; Giraldo, S.; Dimitrievska, M.; Placidi, M. Alkali doping strategies for flexible and light-weight Cu2ZnSnSe4 solar cells. J. Mater. Chem. A 2016, 4, 1895–1907. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Han, Q.; Jiang, C.; Song, T.B.; Chen, H.; Meng, L.; Zhou, H.; Yang, Y. Efficiency enhancement of Cu2ZnSn(S,Se)4 solar cells via alkali metals doping. Adv. Energy Mater. 2016, 6, 1502386. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Zhuang, D.; Zhang, N.; Yu, X.; Wei, Y.; Lyu, X.; Ren, G.; Wang, C.; Hu, L. The effect of Rb doping on CZTSSe solar cells. Sol. Energy 2019, 187, 269–273. [Google Scholar] [CrossRef]
- Wanda, M.D.; Ouédraogo, S.; Ndjaka, J. Theoretical analysis of minority carrier lifetime and Cd-free buffer layers on the CZTS based solar cell performances. Optik 2019, 183, 284–293. [Google Scholar] [CrossRef]
- Cui, X.; Sun, K.; Huang, J.; Yun, J.S.; Lee, C.-Y.; Yan, C.; Sun, H.; Zhang, Y.; Xue, C.; Eder, K. Cd-Free Cu2ZnSnS4 solar cell with an efficiency greater than 10% enabled by Al2O3 passivation layers. Energy Environ. Sci. 2019, 12, 2751–2764. [Google Scholar] [CrossRef]
- Rana, T.R.; Kim, S.; Kim, J.; Kim, K.; Yun, J.H. A Cd-reduced hybrid buffer layer of CdS/Zn(O,S) for environmentally friendly CIGS solar cells. Sustain. Energy Fuels 2017, 1, 1981–1990. [Google Scholar] [CrossRef]






| Sample | Effi. (%) | VOC (V) | Jsc (mA/cm2) | Fill Factor FF (%) | RSh (Ώ) | RS (Ώ) | A | J0 (mA/cm2) | Voc deficit (mV) |
|---|---|---|---|---|---|---|---|---|---|
| 480 °C | 6.08 | 0.425 | 30.77 | 46.48 | 4166.7 | 2.02 | 3.11 | 1.8 × 10−4 | 805 |
| 440 °C | 3.60 | 0.363 | 22.84 | 43.47 | 109.9 | 1.98 | 1.34 | 3.9 × 10−3 | 827 |
| 380 °C | 1.36 | 0.274 | 17.71 | 28.15 | 45.23 | 2.11 | 3.77 | 1.4 × 10−3 | 873 |
| Sample | NA (cm3) | NIF (cm3) | NB (cm3) | Depletion Width (nm) |
|---|---|---|---|---|
| 480 °C | 1.0 × 1016 | 3.0 × 1015 | 1.6 × 1016 | 119 |
| 440 °C | 9.2 × 1016 | 2.2 × 1016 | 3.7 × 1016 | 64.7 |
| 380 °C | 5.6 × 1016 | 1.5 × 1017 | 1.3 × 1016 | 70.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehan, M.; Jeon, H.; Cho, Y.; Cho, A.; Kim, K.; Cho, J.-S.; Yun, J.H.; Ahn, S.; Gwak, J.; Shin, D. Fabrication and Characterization of Cu2ZnSnSe4 Thin-Film Solar Cells using a Single-Stage Co-Evaporation Method: Effects of Film Growth Temperatures on Device Performances. Energies 2020, 13, 1316. https://doi.org/10.3390/en13061316
Rehan M, Jeon H, Cho Y, Cho A, Kim K, Cho J-S, Yun JH, Ahn S, Gwak J, Shin D. Fabrication and Characterization of Cu2ZnSnSe4 Thin-Film Solar Cells using a Single-Stage Co-Evaporation Method: Effects of Film Growth Temperatures on Device Performances. Energies. 2020; 13(6):1316. https://doi.org/10.3390/en13061316
Chicago/Turabian StyleRehan, Muhammad, Hyeonmin Jeon, Yunae Cho, Ara Cho, Kihwan Kim, Jun-Sik Cho, Jae Ho Yun, Seungkyu Ahn, Jihye Gwak, and Donghyeop Shin. 2020. "Fabrication and Characterization of Cu2ZnSnSe4 Thin-Film Solar Cells using a Single-Stage Co-Evaporation Method: Effects of Film Growth Temperatures on Device Performances" Energies 13, no. 6: 1316. https://doi.org/10.3390/en13061316