Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery
Abstract
1. Introduction
2. Lignocellulosic Bio-Oils
2.1. Lignocellulosic Feedstocks
2.2. Lignocellulosic Bio-Oils
2.3. Valorization
2.3.1. Fuel Upgrading
2.3.2. Production of Green Chemicals
3. sCO2 Separation of Bio-Oils
3.1. sCO2 Basics
3.2. sCO2 Extraction of Lignocelulosic Bio-Oils
3.3. Experimental Conditions, Extraction Yields, and Vapor Phase Loadings
3.4. Physical and Chemical Properties of sCO2 Extracts
3.5. Chemical Composition sCO2 Extracts
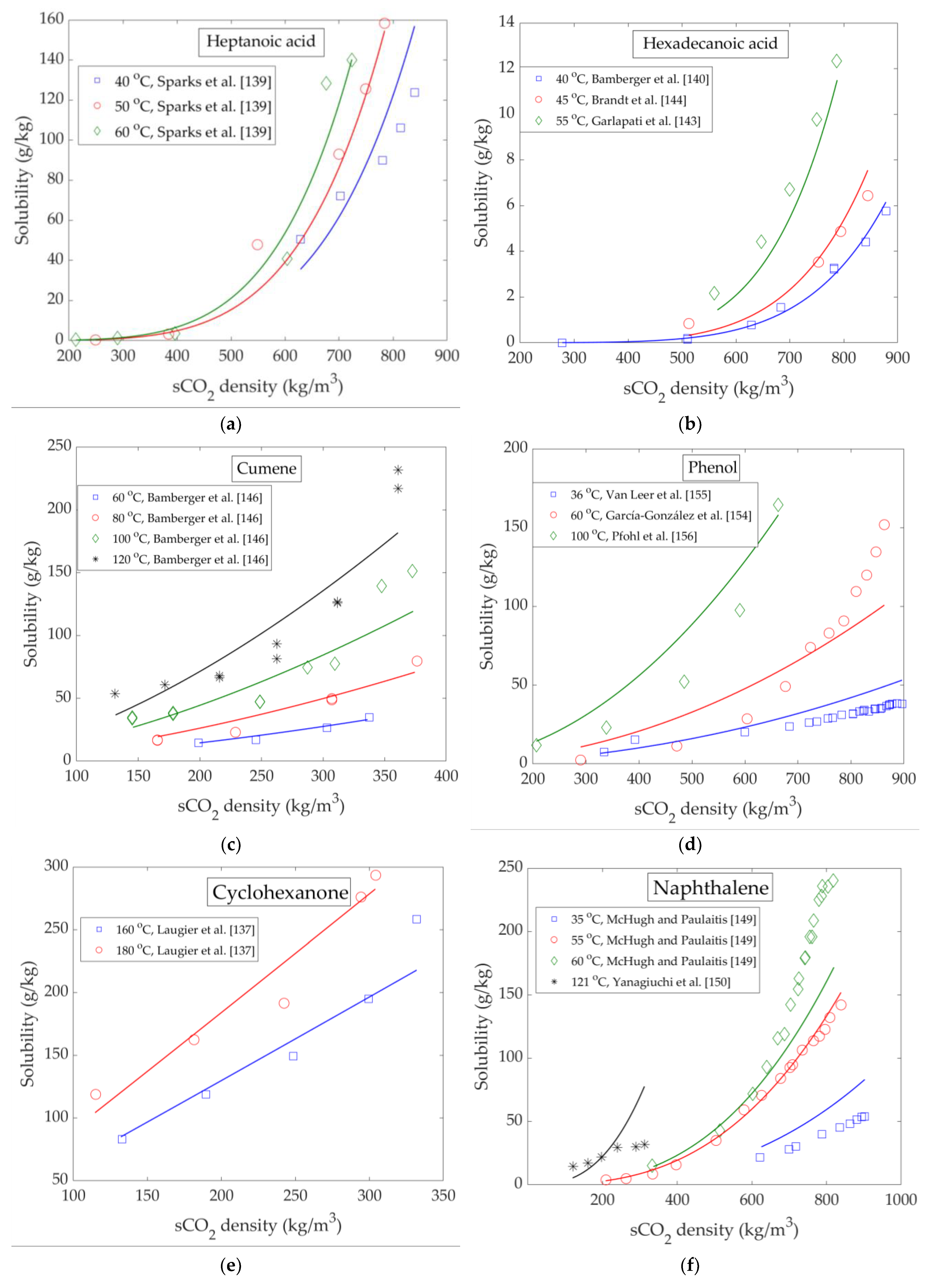
4. High Pressure Solubility Data and Modelling
- : solubility of the solute in the sCO2-rich phase (grams of solute per liters of pure solvent at the P, T conditions of interest);
- : density of the pure solvent (g/L) at the P, T conditions of interest;
- : association number (i.e., number of CO2 molecules associated with one solute molecule);
- a: constant connected to the enthalpy of solvation and vaporization;
- : temperature (K);
- : constant connected to the molecular weights of the solvent and the solute.
Binary Phase Equilibrium Data of LC Bio-Oil Components and sCO2
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Outlook 2019. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 17 December 2019).
- IEA. World Energy Statistics 2019. IEA: Paris, France, 2019. Available online: https://www.iea.org/reports/world-energy-statistics-2019 (accessed on 1 September 2019).
- Kosinkova, J.; Doshi, A.; Maire, J.; Ristovski, Z.; Brown, R.; Rainey, T.J. Measuring the regional availability of biomass for biofuels and the potential for microalgae. Renew. Sustain. Energy Rev. 2015, 49, 1271–1285. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Biofuels and the Environment: Second Triennial Report to Congress; US Environmental Protection Agency: Washington, DC, USA, 2018.
- IEA Renewables 2019. Available online: https://www.iea.org/reports/renewables-2019 (accessed on 17 December 2019).
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent advances in production and upgrading of bio-oil from biomass: A critical overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Hoffmann, J.; Jensen, C.U.; Rosendahl, L.A. Co-processing potential of HTL bio-crude at petroleum refineries-Part 1: Fractional distillation and characterization. Fuel 2016, 165, 526–535. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Jensen, C.U.; Sandström, L.; Rosendahl, L.A. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude. Appl. Energy 2017, 202, 408–419. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Boateng, A.A. Distillation and isolation of commodity chemicals from bio-oil made by tail-gas reactive pyrolysis. ACS Sustain. Chem. Eng. 2014, 2, 2042–2052. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 2013, 112, 60–73. [Google Scholar] [CrossRef]
- Taghipour, A.; Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of fractional distillation to improve hydrothermal liquefaction biocrude characteristics; future outlook and prospects. Renew. Sustain. Energy Rev. 2019, 115, 109355. [Google Scholar] [CrossRef]
- Hu, H.S.; Wu, Y.L.; Yang, M.D. Fractionation of bio-oil produced from hydrothermal liquefaction of microalgae by liquid-liquid extraction. Biomass Bioenergy 2018, 108, 487–500. [Google Scholar] [CrossRef]
- Bjelić, S.; Yu, J.; Iversen, B.B.; Glasius, M.; Biller, P. Detailed investigation into the asphaltene fraction of hydrothermal liquefaction derived bio-crude and hydrotreated bio-crudes. Energy and Fuels 2018, 32, 3579–3587. [Google Scholar] [CrossRef]
- Brunner, G. Counter-current separations. J. Supercrit. Fluids 2009, 47, 574–582. [Google Scholar] [CrossRef]
- Nielsen, R.P.; Valsecchi, R.; Strandgaard, M.; Maschietti, M. Experimental study on fluid phase equilibria of hydroxyl-terminated perfluoropolyether oligomers and supercritical carbon dioxide. J. Supercrit. Fluids 2015, 101, 124–130. [Google Scholar] [CrossRef]
- Speight, J.G. Nonthermal methods of recovery. In Enhanced Recovery Methods for Heavy Oil and Tar Sands; Elsevier: Austin, TX, USA, 2009; pp. 185–220. ISBN 9780127999883. [Google Scholar]
- Kummamuru, B. Global Bioenergy Statistics; World Bioenergy Association: Stockholm, Sweden, 2017. [Google Scholar]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global Wastewater and Sludge Production, Treatment and Use. In Wastewater: Economic Asset in an Urbanizing World; Drechsel, P., Qadir, M., Wichelns, D., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 15–38. ISBN 978-94-017-9545-6. [Google Scholar]
- Lignin Products Global Market Size, Sales Data 2017-2022 & Applications in Animal Feed Industry. Available online: https://www.orbisresearch.com/contacts/request-sample/218258 (accessed on 13 May 2019).
- Tian, X.; Fang, Z.; Smith, R.L.; Wu, Z.; Liu, M. Properties, chemical characteristics and application of lignin and its derivatives. In Production of Biofuels and Chemicals from Lignin; Fang, Z., Smith, R.L., Jr., Eds.; Springer: Singapore, 2016; pp. 3–33. ISBN 978-981-10-1965-4. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Miliotti, E.; Dell’Orco, S.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Lignocellulosic ethanol biorefinery: Valorization of lignin-rich stream through hydrothermal liquefaction. Energies 2019, 12, 723. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.P.; Mohamed, A.R. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- ECN.TNO Phyllis2, Database for Biomass and Waste. Available online: https://phyllis.nl/ (accessed on 18 November 2019).
- Oasmaa, A.; Van De Beld, B.; Saari, P.; Elliott, D.C.; Solantausta, Y. Norms, standards, and legislation for fast pyrolysis bio-oils from lignocellulosic biomass. Energy Fuels 2015, 29, 2471–2484. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.E.; Vamling, L.; Olausson, L.; Andersson, S.I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef]
- Feng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal liquefaction of barks into bio-crude - Effects of species and ash content/composition. Fuel 2014, 116, 214–220. [Google Scholar] [CrossRef]
- Haarlemmer, G.; Guizani, C.; Anouti, S.; Déniel, M.; Roubaud, A.; Valin, S. Analysis and comparison of bio-oils obtained by hydrothermal liquefaction and fast pyrolysis of beech wood. Fuel 2016, 174, 180–188. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Zhu, S.; Tian, F.; Xu, Y.; Zhu, C.; Dong, L. Synergistic hydrothermal liquefaction of wheat stalk with homogeneous and heterogeneous catalyst at low temperature. Bioresour. Technol. 2019, 278, 92–98. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Dumbre, D.K.; Srinivasan, M.P.; Griffin, G.J. Sub-supercritical liquefaction of sugarcane bagasse for production of bio-oil and char: Effect of two solvents. J. Environ. Chem. Eng. 2018, 6, 6589–6601. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.; Rosendahl, L. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- BTG-BTL Empyro Project. Available online: https://www.btg-btl.com/en/company/projects/empyro (accessed on 22 October 2019).
- Ensyn Côte-Nord. Available online: http://www.ensyn.com/quebec.html (accessed on 22 October 2019).
- Silva Green Fuel. Available online: https://www.statkraft.com/about-statkraft/Projects/norway/value-creation-tofte/silva-green-fuel/ (accessed on 22 October 2019).
- Elliott, D.C.; Oasmaa, A.; Meier, D.; Preto, F.; Bridgwater, A.V. Results of the IEA round robin on viscosity and aging of fast pyrolysis bio-oils: Long-Term tests and repeatability. Energy Fuels 2012, 26, 7362–7366. [Google Scholar] [CrossRef]
- Ong, B.H.Y.; Walmsley, T.G.; Atkins, M.J.; Walmsley, M.R.W. Hydrothermal liquefaction of Radiata Pine with Kraft black liquor for integrated biofuel production. J. Clean. Prod. 2018, 199, 737–750. [Google Scholar] [CrossRef]
- Jarvis, J.M.; Albrecht, K.O.; Billing, J.M.; Schmidt, A.J.; Hallen, R.T.; Schaub, T.M. Assessment of hydrotreatment for hydrothermal liquefaction biocrudes from sewage sludge, microalgae, and pine feedstocks. Energy Fuels 2018, 32, 8483–8493. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: the influence of the sodium and potassium fraction. Biomass Convers. Biorefinery 2018, 8, 585–595. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Supercritical carbon dioxide extraction of fast pyrolysis oil from softwood. J. Supercrit. Fluids 2017, 128, 6–17. [Google Scholar] [CrossRef]
- Leijenhorst, E.J.; Wolters, W.; Van De Beld, L.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111. [Google Scholar] [CrossRef]
- Sintamarean, I.M.; Grigoras, I.F.; Jensen, C.U.; Toor, S.S.; Pedersen, T.H.; Rosendahl, L.A. Two-stage alkaline hydrothermal liquefaction of wood to biocrude in a continuous bench-scale system. Biomass Convers. Biorefinery 2017, 7, 425–435. [Google Scholar] [CrossRef]
- Kosinkova, J.; Ramirez, J.A.; Ristovski, Z.D.; Brown, R.; Rainey, T.J. Physical and chemical stability of bagasse biocrude from liquefaction stored in real conditions. Energy Fuels 2016, 30, 10499–10504. [Google Scholar] [CrossRef]
- Elliott, D.C.; Meier, D.; Oasmaa, A.; Van De Beld, B.; Bridgwater, A.V.; Marklund, M. Results of the international energy agency round robin on fast pyrolysis bio-oil production. Energy Fuels 2017, 31, 5111–5119. [Google Scholar] [CrossRef]
- Speight, J.G. Petroleum Analysis. In The Chemistry and Technology of Petroleum; CRC Press: Boca Raton, FL, USA, 2006; pp. 274–314. ISBN 9780429118494. [Google Scholar]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Das, P.; Ganesh, A.; Wangikar, P. Influence of pretreatment for deashing of sugarcane bagasse on pyrolysis products. Biomass Bioenergy 2004, 27, 445–457. [Google Scholar] [CrossRef]
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Optimisation of bio-oil production by hydrothermal liquefaction of agro-industrial residues: Blackcurrant pomace (Ribes nigrum L.) as an example. Biomass Bioenergy 2016, 95, 273–285. [Google Scholar] [CrossRef]
- Speight, J.G. Chemical Composition. In The Chemistry and Technology of Petroleum; Chemical Industries; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781439873892. [Google Scholar]
- Ghorbannezhad, P.; Kool, F.; Rudi, H.; Ceylan, S. Sustainable production of value-added products from fast pyrolysis of palm shell residue in tandem micro-reactor and pilot plant. Renew. Energy 2020, 145, 663–670. [Google Scholar] [CrossRef]
- Gómez-Monedero, B.; Bimbela, F.; Arauzo, J.; Faria, J.; Ruiz, M.P. Pyrolysis of red eucalyptus, camelina straw, and wheat straw in an ablative reactor. Energy Fuels 2015, 29, 1766–1775. [Google Scholar] [CrossRef]
- Hernando, H.; Jiménez-Sánchez, S.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Assessing biomass catalytic pyrolysis in terms of deoxygenation pathways and energy yields for the efficient production of advanced biofuels. Catal. Sci. Technol. 2016, 6, 2829–2843. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R.; et al. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Montesantos, N.; Nielsen, R.P.; Maschietti, M. Upgrading of nondewatered nondemetallized lignocellulosic biocrude from hydrothermal liquefaction using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2020, accepted. [Google Scholar] [CrossRef]
- Elliott, D.C.; Oasmaa, A.; Preto, F.; Meier, D.; Bridgwater, A.V. Results of the IEA round robin on viscosity and stability of fast pyrolysis bio-oils. Energy Fuels 2012, 26, 3769–3776. [Google Scholar] [CrossRef]
- Jensen, C.U.; Rodriguez Guerrero, J.K.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Fundamentals of HydrofactionTM: Renewable crude oil from woody biomass. Biomass Convers. Biorefinery 2017, 7, 495–509. [Google Scholar] [CrossRef]
- Jarvis, J.M.; Billing, J.M.; Hallen, R.T.; Schmidt, A.J.; Schaub, T.M. Hydrothermal liquefaction biocrude compositions compared to petroleum crude and shale oil. Energy Fuels 2017, 31, 2896–2906. [Google Scholar] [CrossRef]
- Madsen, R.B.; Bernberg, R.Z.K.; Biller, P.; Becker, J.; Iversen, B.B.; Glasius, M. Hydrothermal co-liquefaction of biomasses-quantitative analysis of bio-crude and aqueous phase composition. Sustain. Energy Fuels 2017, 1, 789–805. [Google Scholar] [CrossRef]
- Christensen, E.D.; Chupka, G.M.; Luecke, J.; Smurthwaite, T.; Alleman, T.L.; Iisa, K.; Franz, J.A.; Elliott, D.C.; McCormick, R.L. Analysis of oxygenated compounds in hydrotreated biomass fast pyrolysis oil distillate fractions. Energy Fuels 2011, 25, 5462–5471. [Google Scholar] [CrossRef]
- Montesantos, N.; Pedersen, T.H.; Nielsen, R.P.; Rosendahl, L.; Maschietti, M. Supercritical carbon dioxide fractionation of bio-crude produced by hydrothermal liquefaction of pinewood. J. Supercrit. Fluids 2019, 149, 97–109. [Google Scholar] [CrossRef]
- ASTM International. ASTM D664-17a. Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Jensen, C.U.; Rosendahl, L.A.; Olofsson, G. Impact of nitrogenous alkaline agent on continuous HTL of lignocellulosic biomass and biocrude upgrading. Fuel Process. Technol. 2017, 159, 376–385. [Google Scholar] [CrossRef]
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-oil valorization: A review. Renew. Sustain. Energy Rev. 2013, 23, 91–106. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Garcia-Perez, M. Quantification of bio-oil functional groups and evidences of the presence of pyrolytic humins. Energy Fuels 2016, 30, 6505–6524. [Google Scholar] [CrossRef]
- Madsen, R.B.; Anastasakis, K.; Biller, P.; Glasius, M. Rapid determination of water, total acid number, and phenolic content in bio-crude from hydrothermal liquefaction of biomass using FT-IR. Energy Fuels 2018, 32, 7660–7669. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Ferrell, J.R. Methods and challenges in the determination of molecular weight metrics of bio-oils. Energy Fuels 2018, 32, 8905–8920. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.H.; Choi, I.G.; Choi, J.W. Comprehensive characterization of hydrothermal liquefaction products obtained from woody biomass under various alkali catalyst concentrations. Environ. Technol. (United Kingdom) 2019, 40, 1657–1667. [Google Scholar] [CrossRef]
- Belkheiri, T.; Vamling, L.; Nguyen, T.D.H.; Maschietti, M.; Olausson, L.; Andersson, S.-I.; Åmand, L.-E.; Theliander, H. Kraft lignin depolymerization in near-critical water: effect of changing co-solvent. Cell Chem. Technol. 2014, 48, 813–818. [Google Scholar]
- Lyckeskog, H.N.; Mattsson, C.; Olausson, L.; Andersson, S.I.; Vamling, L.; Theliander, H. Thermal stability of low and high Mw fractions of bio-oil derived from lignin conversion in subcritical water. Biomass Convers. Biorefinery 2017, 7, 401–414. [Google Scholar] [CrossRef]
- Conrad, S.; Blajin, C.; Schulzke, T.; Deerberg, G. Comparison of fast pyrolysis bio-oils from straw and miscanthus. Environ. Prog. Sustain. Energy 2019, 38, e13287. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.A.; Prins, W. Effect of biomass ash in catalytic fast pyrolysis of pine wood. Appl. Catal. B Environ. 2015, 168–169, 203–211. [Google Scholar] [CrossRef]
- Doassans-Carrère, N.; Ferrasse, J.H.; Boutin, O.; Mauviel, G.; Lédé, J. Comparative study of biomass fast pyrolysis and direct liquefaction for bio-oils production: Products yield and characterizations. Energy Fuels 2014, 28, 5103–5111. [Google Scholar] [CrossRef]
- Azargohar, R.; Jacobson, K.L.; Powell, E.E.; Dalai, A.K. Evaluation of properties of fast pyrolysis products obtained, from Canadian waste biomass. J. Anal. Appl. Pyrolysis 2013, 104, 330–340. [Google Scholar] [CrossRef]
- Undri, A.; Abou-Zaid, M.; Briens, C.; Berruti, F.; Rosi, L.; Bartoli, M.; Frediani, M.; Frediani, P. A simple procedure for chromatographic analysis of bio-oils from pyrolysis. J. Anal. Appl. Pyrolysis 2015, 114, 208–221. [Google Scholar] [CrossRef]
- Nguyen Lyckeskog, H.; Mattsson, C.; Åmand, L.E.; Olausson, L.; Andersson, S.I.; Vamling, L.; Theliander, H. Storage stability of bio-oils derived from the catalytic conversion of softwood Kraft lignin in subcritical water. Energy Fuels 2016, 30, 3097–3106. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef]
- Aqsha, A.; Tijani, M.M.; Moghtaderi, B.; Mahinpey, N. Catalytic pyrolysis of straw biomasses (wheat, flax, oat and barley) and the comparison of their product yields. J. Anal. Appl. Pyrolysis 2017, 125, 201–208. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Extraction of value-added chemicals from pyrolysis liquids with supercritical carbon dioxide. J. Anal. Appl. Pyrolysis 2015, 113, 174–185. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers. Biorefinery 2014, 4, 157–191. [Google Scholar] [CrossRef]
- Chan, Y.H.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Liquefaction of palm kernel shell in sub- and supercritical water for bio-oil production. J. Energy Inst. 2018, 91, 721–732. [Google Scholar] [CrossRef]
- Kokayeff, P.; Zink, S.; Roxas, P. Hydrotreating in petroleum processing. In Handbook of Petroleum Processing; Treese, S.A., Pujadó, P.R., Jones, D.S.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 1995; pp. 363–434. ISBN 978-3-319-14529-7. [Google Scholar]
- Castello, D.; Haider, M.S.; Rosendahl, L.A. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energy 2019, 141, 420–430. [Google Scholar] [CrossRef]
- Jensen, C.U.; Hoffmann, J.; Rosendahl, L.A. Co-processing potential of HTL bio-crude at petroleum refineries. Part 2: A parametric hydrotreating study. Fuel 2016, 165, 536–543. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Hoffmann, J.; Pedersen, T.H.; Rosendahl, L.A. Near-critical and supercritical water and their applications for biorefineries. In Biofuels and Biorefineries; Fang, Z., Xu, C., Eds.; Biofuels and Biorefineries; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 373–400. ISBN 978-94-017-8922-6. [Google Scholar]
- Han, Y.; Gholizadeh, M.; Tran, C.-C.; Kaliaguine, S.; Li, C.-Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Furimsky, E.; Massoth, F.E. Deactivation of hydroprocessing catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar] [CrossRef]
- Mohamad, M.H.; Awang, R.; Yunus, W.Z.W. A Review of acetol: Application and production. Am. J. Appl. Sci. 2011, 8, 1135–1139. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Pradhan, S.; Brinson, B.E.; Scuseria, G.E.; Zhang, Z.C.; Wong, M.S. Ring-locking enables selective anhydrosugar synthesis from carbohydrate pyrolysis. Green Chem. 2016, 18, 5438–5447. [Google Scholar] [CrossRef]
- Rover, M.R.; Aui, A.; Wright, M.M.; Smith, R.G.; Brown, R.C. Production and purification of crystallized levoglucosan from pyrolysis of lignocellulosic biomass. Green Chem. 2019, 21, 5980–5989. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2004. [Google Scholar]
- Longley, C.J.; Fung, D.P.C. Potential Applications and Markets for Biomass-Derived Levoglucosan. In Advances in Thermochemical Biomass Conversion; Springer: Dordrecht, The Netherlands, 1993; pp. 1484–1494. ISBN 978-94-011-1336-6. [Google Scholar]
- What’s New in Phenol Production? Available online: https://www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/what-s-new-in-phenol-production-.html (accessed on 12 August 2019).
- Maqbool, W.; Hobson, P.; Dunn, K.; Doherty, W. Supercritical carbon dioxide separation of carboxylic scids and phenolics from bio-oil of lignocellulosic origin: Understanding bio-oil compositions, compound solubilities, and their fractionation. Ind. Eng. Chem. Res. 2017, 56, 3129–3144. [Google Scholar] [CrossRef]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 26, pp. 552–553. [Google Scholar]
- Naik, S.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Supercritical CO2 fractionation of bio-oil produced from wheat-hemlock biomass. Bioresour. Technol. 2010, 101, 7605–7613. [Google Scholar] [CrossRef] [PubMed]
- Vanilla and Vanillin Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019-2024. Available online: https://www.researchandmarkets.com/research/n4fxw5/global_vanilla?w=12 (accessed on 28 October 2019).
- Wu, X.F.; Zhou, Q.; Li, M.F.; Li, S.X.; Bian, J.; Peng, F. Conversion of poplar into bio-oil via subcritical hydrothermal liquefaction: Structure and antioxidant capacity. Bioresour. Technol. 2018, 270, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Sharma, B.K.; Marley, K.A.; Kunwar, B.; Murali, D.; Scott, J. Potential antioxidants for biodiesel from a softwood lignin pyrolyzate. Ind. Crops Prod. 2017, 109, 476–482. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Murali, D.; Marley, K.A.; Larson, R.A.; Doll, K.M.; Moser, B.R.; Scott, J.; Sharma, B.K. Antioxidants from slow pyrolysis bio-oil of birch wood: Application for biodiesel and biobased lubricants. ACS Sustain. Chem. Eng. 2016, 4, 1414–1421. [Google Scholar] [CrossRef]
- Qazi, S.S.; Li, D.; Briens, C.; Berruti, F.; Abou-Zaid, M.M. Antioxidant activity of the lignins derived from fluidized-bed fast pyrolysis. Molecules 2017, 22, 372. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Michels, A.; Blaisse, B.; Hoogschagen, J. The melting line of carbon dioxide up to 2800 atmospheres. Physica 1942, 9, 565–573. [Google Scholar] [CrossRef]
- Dortmund Data Bank. Available online: http://www.ddbst.com/en/EED/PCP/VAP_C1050.php (accessed on 29 October 2019).
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- McHugh, M.A.; Krukonis, V.J. Supercritical Fluid Extraction, 2nd ed.; Brenner, H., Ed.; Butterworth-Heinemann series in chemical engineering; Elsevier: Amsterdam, The Netherlands, 1994; ISBN 9780080518176. [Google Scholar]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide; Gupta, R.B., Shim, J.-J., Eds.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429122088. [Google Scholar]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- McHugh, M.A.; Krukonis, V.J. Processing Pharmaceuticals, Natural Products, Specialty Chemicals, and Waste Streams. In Supercritical Fluid Extraction; Butterworth-Heinemann: Oxford, UK, 1994; pp. 293–310. ISBN 9780080518176. [Google Scholar]
- Gironi, F.; Maschietti, M. Supercritical carbon dioxide fractionation of lemon oil by means of a batch process with an external reflux. J. Supercrit. Fluids 2005, 35, 227–234. [Google Scholar] [CrossRef]
- Maschietti, M.; Pedacchia, A. Supercritical carbon dioxide separation of fish oil ethyl esters by means of a continuous countercurrent process with an internal reflux. J. Supercrit. Fluids 2014, 86, 76–84. [Google Scholar] [CrossRef]
- Gironi, F.; Maschietti, M. Continuous countercurrent deterpenation of lemon essential oil by means of supercritical carbon dioxide: Experimental data and process modelling. Chem. Eng. Sci. 2008, 63, 651–661. [Google Scholar] [CrossRef]
- Gironi, F.; Maschietti, M. Separation of fish oils ethyl esters by means of supercritical carbon dioxide: Thermodynamic analysis and process modelling. Chem. Eng. Sci. 2006, 61, 5114–5126. [Google Scholar] [CrossRef]
- Riha, V.; Brunner, G. Separation of fish oil ethyl esters with supercritical carbon dioxide. J. Supercrit. Fluids 2000, 17, 55–64. [Google Scholar] [CrossRef]
- Osséo, L.S.; Caputo, G.; Gracia, I.; Reverchon, E. Continuous fractionation of used frying oil by supercritical CO2. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 879–885. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, J.Y.; Hong, S.A.; Lee, Y.W.; Kim, J. Supercritical CO2-purification of waste cooking oil for high-yield diesel-like hydrocarbons via catalytic hydrodeoxygenation. Fuel 2013, 111, 510–518. [Google Scholar] [CrossRef]
- Meyer, T. Extracting and upgrading heavy hydrocarbons using supercritical carbon dioxide 2011. UK Patent GB 2471862 A, 19 January 2011. [Google Scholar]
- Subramanian, A.; Floyd, R. Residuum oil supercritical extraction process 2011. US Patent 2011/0094937 A1, 28 April 2011. [Google Scholar]
- Mudraboyina, B.P.; Fu, D.; Jessop, P.G. Supercritical fluid rectification of lignin microwave-pyrolysis oil. Green Chem. 2015, 17, 169–172. [Google Scholar] [CrossRef]
- Montesantos, N.; Pedersen, T.H.; Nielsen, R.P.; Rosendahl, L.A.; Maschietti, M. High-temperature extraction of lignocellulosic bio-crude by supercritical carbon dioxide. Chem. Eng. Trans. 2019, 74, 799–804. [Google Scholar]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Chai, Y.H.; Uemura, Y.; Loh, S.K. Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: Evaluation and modeling of phenol solubility. Biomass Bioenergy 2018, 116, 106–112. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Loh, S.K. Fractionation of pyrolysis oil via supercritical carbon dioxide extraction: Optimization study using response surface methodology (RSM). Biomass Bioenergy 2017, 107, 155–163. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Comparison of supercritical CO2, liquid CO2, and solvent extraction of chemicals from a commercial slow pyrolysis liquid of beech wood. Biomass Bioenergy 2016, 85, 346–354. [Google Scholar] [CrossRef]
- Cheng, T.; Han, Y.; Zhang, Y.; Xu, C. Molecular composition of oxygenated compounds in fast pyrolysis bio-oil and its supercritical fluid extracts. Fuel 2016, 172, 49–57. [Google Scholar] [CrossRef]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of cardanol and phenol from bio-oils obtained through vacuum pyrolysis of biomass using supercritical fluid extraction. Energy 2011, 36, 1535–1542. [Google Scholar] [CrossRef]
- Rout, P.K.; Naik, M.K.; Naik, S.N.; Goud, V.V.; Das, L.M.; Dalai, A.K. Supercritical CO2 fractionation of bio-oil produced from mixed biomass of wheat and wood sawdust. Energy Fuels 2009, 23, 6181–6188. [Google Scholar] [CrossRef]
- Wang, J.; Cui, H.; Wei, S.; Zhuo, S.; Wang, L.; Li, Z.; Yi, W. Separation of biomass pyrolysis oil by supercritical CO2 extraction. Smart Grid Renew. Energy 2010, 01, 98–107. [Google Scholar] [CrossRef]
- ISO 8217:2017. Petroleum Products-Fuels (class F)-Specifications of Marine Fuels; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Pedersen, K.S.; Christensen, P.L.; Shaikh, J.A. Phase Behavior of Petroleum Reservoir Fluids, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429110306. [Google Scholar]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Dohrn, R.; Brunner, G. High-pressure fluid-phase equilibria: Experimental methods and systems investigated (1988–1993). Fluid Phase Equilib. 1995, 106, 213–282. [Google Scholar] [CrossRef]
- Laugier, S.; Richon, D. High-pressure vapor-liquid equilibria of two binary systems: Carbon dioxide + cyclohexanol and carbon dioxide + cyclohexanone. J. Chem. Eng. Data 1997, 42, 155–159. [Google Scholar] [CrossRef]
- Jing, Y.; Hou, Y.; Wu, W.; Liu, W.; Zhang, B. Solubility of 5-Hydroxymethylfurfural in supercritical carbon dioxide with and without ethanol as cosolvent at (314.1 to 343.2) K. J. Chem. Eng. Data 2011, 56, 298–302. [Google Scholar] [CrossRef]
- Sparks, D.L.; Hernandez, R.; Estévez, L.A.; Holmes, W.E.; French, W.T. Solubility of small-chain fatty acids in supercritical carbon dioxide. AIChE Annu. Meet. Conf. Proc. 2008, 55, 4922–4927. [Google Scholar]
- Bamberger, T.; Erickson, J.C.; Cooney, C.L.; Kumar, S.K. Measurement and model prediction of solubilities of pure fatty acids, pure triglycerides, and mixtures of triglycerides in supercritical carbon dioxide. J. Chem. Eng. Data 1988, 33, 327–333. [Google Scholar] [CrossRef]
- Maheshwari, P.; Nikolov, Z.L.; White, T.M.; Hartel, R. Solubility of fatty acids in supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1992, 69, 1069–1076. [Google Scholar] [CrossRef]
- Iwai, Y.; Fukuda, T.; Koga, Y.; Arai, Y. Solubilities of myristic acid, palmitic acid, and cetyl alcohol in supercritical carbon dioxide at 35 °C. J. Chem. Eng. Data 1991, 36, 430–432. [Google Scholar] [CrossRef]
- Garlapati, C.; Madras, G. Solubilities of palmitic and stearic fatty acids in supercritical carbon dioxide. J. Chem. Thermodyn. 2010, 42, 193–197. [Google Scholar] [CrossRef]
- Brandt, L.; Elizalde-Solis, O.; Galicia-Luna, L.A.; Gmehling, J. Solubility and density measurements of palmitic acid in supercritical carbon dioxide + alcohol mixtures. Fluid Phase Equilib. 2010, 289, 72–79. [Google Scholar] [CrossRef]
- Schwarz, C.E.; Knoetze, J.H. Phase equilibrium measurements of long chain acids in supercritical carbon dioxide. J. Supercrit. Fluids 2012, 66, 36–48. [Google Scholar] [CrossRef]
- Bamberger, A.; Schmelzer, J.; Walther, D.; Maurer, G. High-pressure vapour-liquid equilibria in binary mixtures of carbon dioxide and benzene compounds: experimental data for mixtures with ethylbenzene, isopropylbenzene, 1,2,4-trimethylbenzene, 1,3,5-trimethylbenzene, ethenylbenzene and isopropenylbenzene, and their correlation with the generalized Bender and Skjold-Jorgensen’s group contribution equation of state. Fluid Phase Equilib. 1994, 97, 167–189. [Google Scholar] [CrossRef]
- Jennings, D.W.; Schucker, R.C. Comparison of high-pressure vapor-liquid equilibria of mixtures of CO2 or propane with nonane and C9 alkylbenzenes. J. Chem. Eng. Data 1996, 41, 831–838. [Google Scholar] [CrossRef]
- Phiong, H.S.; Lucien, F.P. Volumetric expansion and vapour-liquid equilibria of α-methylstyrene and cumene with carbon dioxide at elevated pressure. J. Supercrit. Fluids 2003, 25, 99–107. [Google Scholar] [CrossRef]
- McHugh, M.; Paulaitls, M.E. Solid solubilities of naphthalene and biphenyl in supercritical carbon dioxide. J. Chem. Eng. Data 1980, 25, 326–329. [Google Scholar] [CrossRef]
- Yanagiuchi, M.; Ueda, T.; Matsubara, K.; Inomata, H.; Arai, K.; Saito, S. Fundamental investigation on supercritical extraction of coal-derived aromatic compounds. J. Supercrit. Fluids 1991, 4, 145–151. [Google Scholar] [CrossRef]
- Wells, P.A.; Chaplin, R.P.; Foster, N.R. Solubility of phenylacetic acid and vanillan in supercritical carbon dioxide. J. Supercrit. Fluids 1990, 3, 8–14. [Google Scholar] [CrossRef]
- Schmitt, W.J.; Reid, R.C. Solubility of monofunctional organic solids in chemically diverse supercritical fluids. J. Chem. Eng. Data 1986, 31, 204–212. [Google Scholar] [CrossRef]
- Kurnlk, R.T.; Holla, S.J.; Reid, R.C. Solubility of solids in supercritical carbon dioxide and ethylene. J. Chem. Eng. Data 1981, 26, 47–51. [Google Scholar] [CrossRef]
- García-González, J.; Molina, M.J.; Rodríguez, F.; Mirada, F. Solubilities of phenol and pyrocatechol in supercritical carbon dioxide. J. Chem. Eng. Data 2001, 46, 918–921. [Google Scholar] [CrossRef]
- Van Leer, R.A.; Paulaitis, M.E. Solubilities of phenol and chlorinated phenols in supercritical carbon dioxide. J. Chem. Eng. Data 1980, 25, 257–259. [Google Scholar] [CrossRef]
- Pfohl, O.; Brunner, G. Two-and three-phase equilibria in systems containing benzene derivatives, carbon dioxide, and water at 373.15 K and 10–30 MPa. Fluid Phase Equilibria 1997, 141, 179–206. [Google Scholar] [CrossRef]
- Yamini, Y.; Fat’Hi, M.R.; Alizadeh, N.; Shamsipur, M. Solubility of dihydroxybenzene isomers in supercritical carbon dioxide. Fluid Phase Equilibria 1998, 152, 299–305. [Google Scholar] [CrossRef]
- Škerget, M.; Čretnik, L.; Knez, Ž.; Škrinjar, M. Influence of the aromatic ring substituents on phase equilibria of vanillins in binary systems with CO2. Fluid Phase Equilibria 2005, 231, 11–19. [Google Scholar] [CrossRef]
- Liu, J.; Kim, Y.; McHugh, M.A. Phase behavior of the vanillin-CO2 system at high pressures. J. Supercrit. Fluids 2006, 39, 201–205. [Google Scholar] [CrossRef]
- Lee, M.J.; Kou, C.F.; Cheng, J.W.; Lin, H.M. Vapor-liquid equilibria for binary mixtures of carbon dioxide with 1,2-dimethoxybenzene, 2-methoxyphenol, or p-cresol at elevated pressures. Fluid Phase Equilibria 1999, 162, 211–224. [Google Scholar] [CrossRef]
- Pfohl, O.; Pagel, A.; Brunner, G. Phase equilibria in systems containing o-cresol, p-cresol, carbon dioxide, and ethanol at 323.15-473.15 K and 10-35 MPa. Fluid Phase Equilibria 1999, 157, 53–79. [Google Scholar] [CrossRef]
- Lee, R.J.; Chao, K.C. Extraction of 1-methylnaphthalene and m-cresol with supercritical carbon dioxide and ethane. Fluid Phase Equilibria 1988, 43, 329–340. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
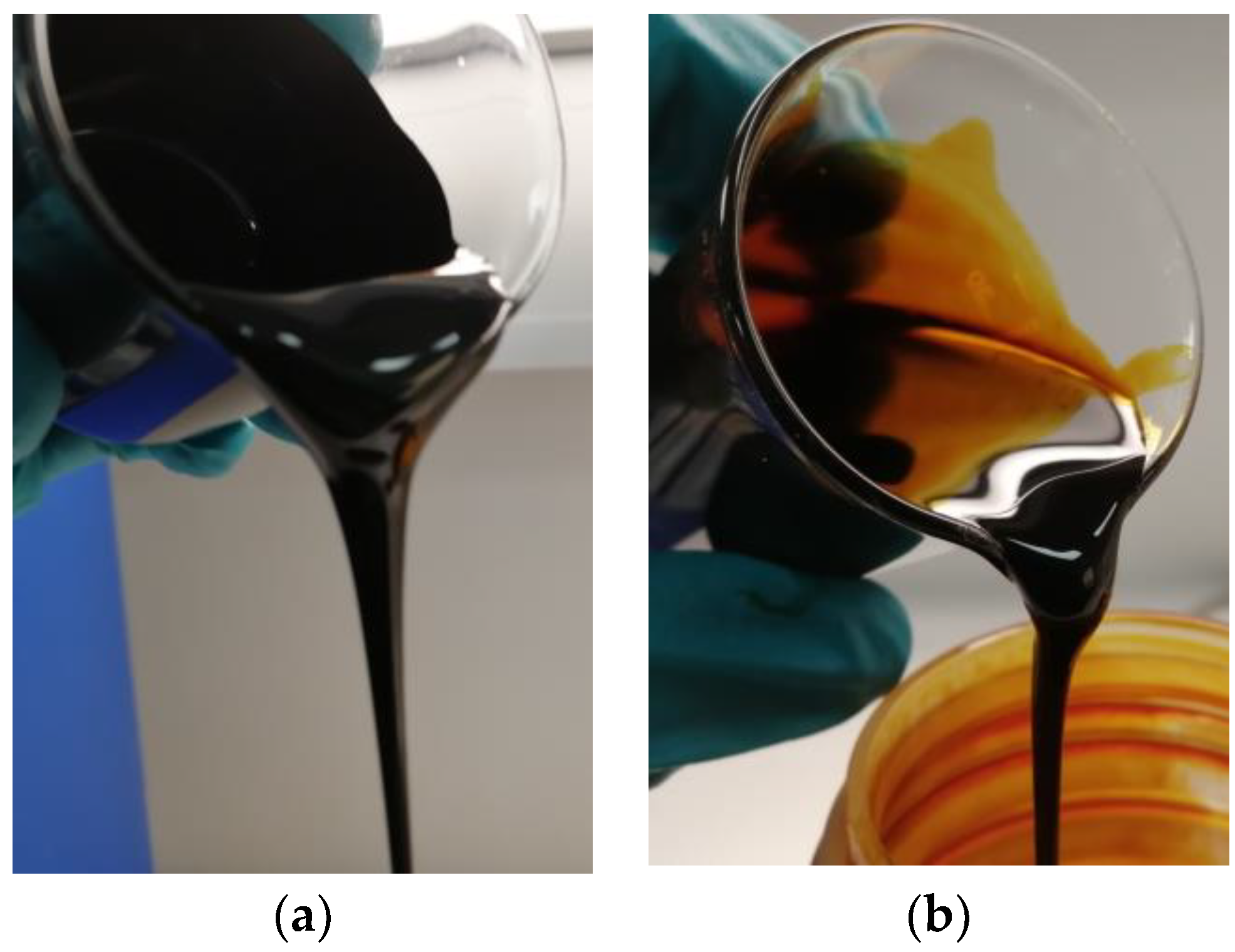
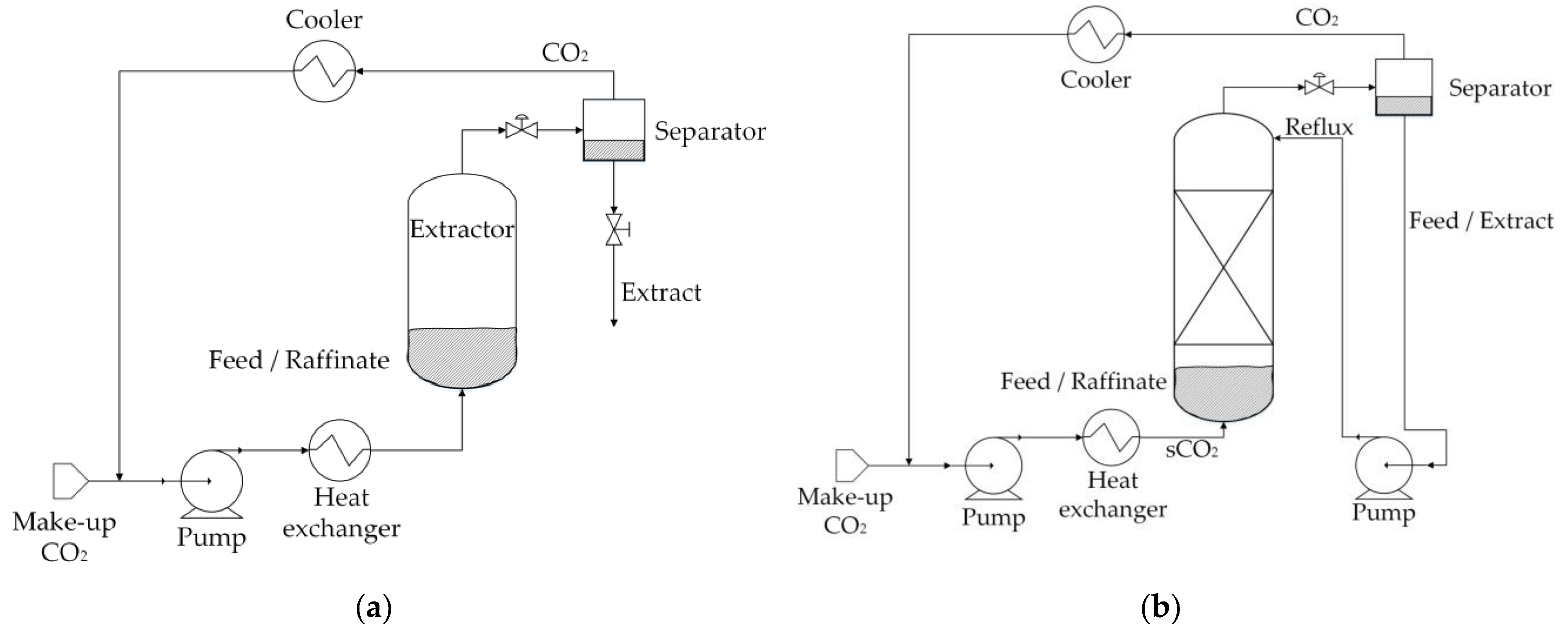
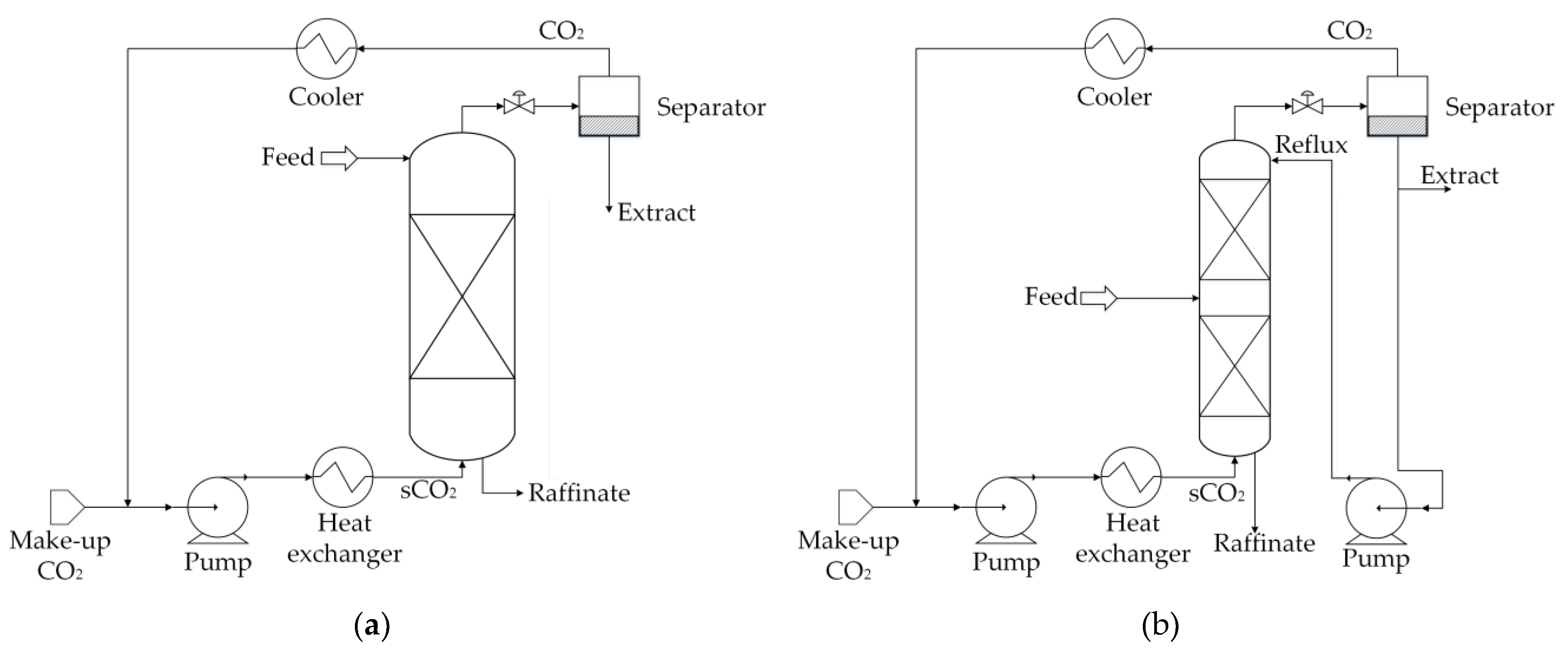
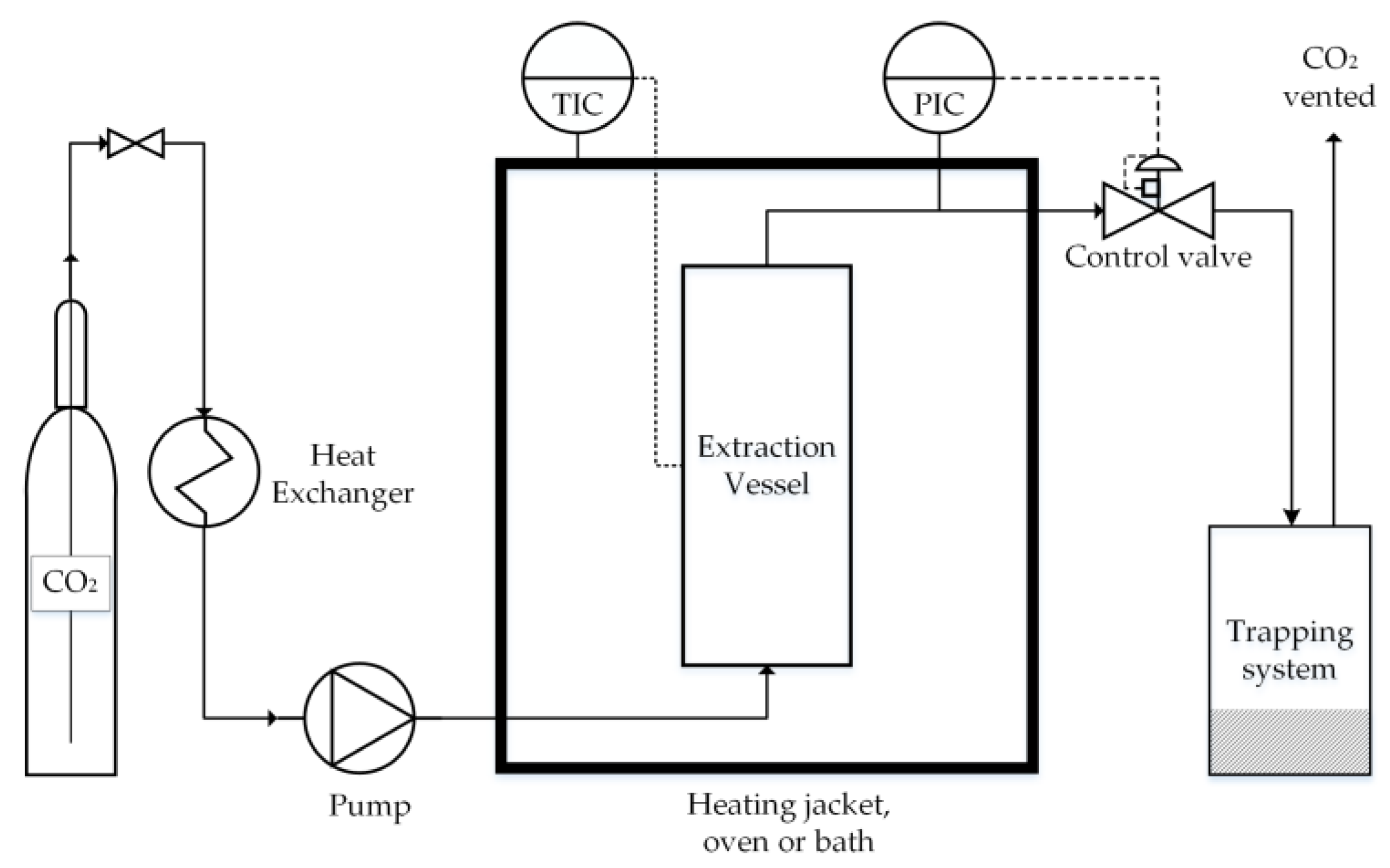





| Residual Biomass Type | Forestry | Agricultural | Industrial | Municipal | ||
|---|---|---|---|---|---|---|
| Poplar | Pine | Sugarcane Bagasse | Wheat Straw | Kraft Lignin | Sewage Sludge1 | |
| Cellulose | 41–49 | 38–50 | 34–42 | 29 –52 | 0–1 | - |
| Hemicellulose | 17–33 | 18–30 | 19–43 | 11–39 | 0–1 | - |
| Lignin | 18–32 | 23–28 | 19–21 | 8–30 | 90 | - |
| Ash | 0–2 | 0–6 | 2–12 | 1–14 | 1–2 | 26–55 |
| Biomass Type | Industrial Residue | Softwood | Hardwood | Energy Crops | Agricultural Residues |
|---|---|---|---|---|---|
| Kraft Lignin | Pine Bark | Beech | Wheat Stalk | Sugarcane Bagasse | |
| Water (wt%) | 32.6 | NA | 8.7 | 10.5 | NA |
| Oxygen (wt%) | 26 | 42.13 | 44.5 | 47.9 | 52.5 |
| H/C | 1.04 | 1.38 | 1.38 | 1.53 | 2.00 |
| O/C | 0.31 | 0.64 | 0.69 | 0.79 | 1.00 |
| Ash | 0.8 | 1.07 | 0.8 | NA | NA |
| HHV (MJ/kg) | 27.67 | 20.21 | 19.21 | 17.81 | 16.41 |
| Reference | [28] | [29] | [30] | [31] | [32] |
| Process | Pretreatment | Temperature | Pressure | Bio-Oil Yield |
|---|---|---|---|---|
| Pyrolysis | Drying, size reduction | 500–600 °C | Atmospheric | up to 75 wt% |
| HTL | Size reduction | 250–450 °C | 100–350 bar | up to 75 wt% |
| Biomass Type | Industrial Residue | Softwood | Hardwood | Energy Crop | Agricultural Residue | |||
|---|---|---|---|---|---|---|---|---|
| Kraft Lignin | Palm Shell | Pine Bark | Beech | Eucalyptus | Wheat Stalk | Wheat Straw | Sugarcane Bagasse | |
| Process | HTL | Pyrolysis | HTL | HTL | Pyrolysis | HTL | Pyrolysis | HTL |
| Oxygen (wt%) | 21 | 33 | 28.3 | 27.3 | 23.9 | 18.8 | 40.0 | 36.3 |
| H/C | 1.11 | 1.74 | 1.2 | 1.19 | 1.08 | 1.29 | 1.35 | 1.64 |
| O/C | 0.23 | 0.43 | 0.33 | 0.30 | 0.26 | 0.20 | 0.56 | 0.49 |
| HHV (MJ/kg) | 31.7 | 27 | 27.41 | 28.31 | 29.2 | 32.41 | 21.9 | 24.81 |
| Reference | [28] | [54] | [29] | [30] | [55] | [31] | [56] | [32] |
| Chemical Class | Pyrolysis [43] | HTL [58] | MW | Carbon Number | Examples |
|---|---|---|---|---|---|
| Ketones | Up to 8 wt% | Up to 0.5 wt% | 74–124 | C3–C10 | Hydroxyacetone Cyclopenten-1-one, 2- Cyclopentanone, 2,5-dimethyl |
| Phenols | Up to 0.1 wt% | Up to 0.3 wt% | 94–122 | C6–C8 | Phenol o-Cresol 4-Ethylphenol |
| Guaiacols | Up to 0.5 wt% | Up to 0.6 wt% | 124–178 | C7–C10 | Guaiacol Eugenol Creosol |
| Benzenediols | - | Up to 1.7 wt% | 110–124 | C6–C8 | Catechol 4-Ethylcatechol |
| Short chain fatty acids1 | Up to 5 wt% | Up to 0.2 wt% | 60–144 | C2–C8 | Acetic acid Octanoic acid |
| Long chain fatty acids | - | Up to 3.8 wt% | 172–284 | C10–C19 | Decanoic acid Octadecanoic acid |
| Aromatic acids | - | Up to 1.8 wt% | 152–300 | C8–C20 | Dehydroabietic acid Benzeneacetic acid, 3-hydroxy |
| Furans | Up to 0.5 wt% | - | 84–132 | C4–C8 | Furfural Furanone, 2(5H)- |
| Aldehydes | Up to 8 wt% | - | 60–152 | C2–C8 | Glycolaldehyde Benzaldehyde, 3-hydroxy-4-methyl- |
| Esters | - | - | 130–296 | C6–C19 | Benzoic acid, 4-methoxy-, methyl ester Furoic acid methylester |
| Sugars | Up to 10 wt% | - | 132–144 | C5–C6 | Levoglucosan 2,3-Anhydro-d-galactosan |
| Benzenes | - | Up to 1 wt% | 92–134 | C7–C10 | o-Cymene Toluene |
| Polyaromatic hydrocarbons | - | Up to 9 wt% | 128–234 | C10–C18 | Naphthalene Retene |
| Feedstock | Thermochemical Process | Extractor Volume (cm3) | Year | Ref. |
|---|---|---|---|---|
| Pine | HTL | 178 | 2020 | [58] |
| Pine | HTL | 178 | 2019 | [127] |
| Pine | HTL | 178 | 2019 | [64] |
| Palm kernel shell | Slow pyrolysis | 50 | 2018 | [128] |
| Pine | Fast pyrolysis | 640 | 2017 | [43] |
| Palm kernel shell | Slow pyrolysis | 50 | 2017 | [129] |
| Beech | Slow pyrolysis | 640 | 2016 | [130] |
| Red pine | Fast pyrolysis | 25 | 2016 | [131] |
| Kraft lignin | Microwave pyrolysis | 1601 | 2015 | [126] |
| Beech | Slow pyrolysis and fast pyrolysis | 600 | 2015 | [84] |
| Sugarcane bagasse and cashew shells | Pyrolysis | - | 2011 | [132] |
| Wheat-hemlock | Fast pyrolysis | - | 2010 | [103] |
| Wheat-sawdust | Fast pyrolysis | - | 2009 | [133] |
| P (bar) | T (°C) | CO2 Density (kg/m3) | F (g) | CO2 Flow (g/min) | S/F (g/g) | Y (wt%) | VPL (g/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 330–450 | 80–150 | 531–851 | 49–54 | 4.7–5.9 | 30.0–36.7 | 44.1–53.4 | 5.9–99.7 | [58] |
| 247–448 | 120 | 500–730 | 28.0–30.8 | 4.8–6.9 | 40.6–50.9 | 33.9–48.9 | 2.7–46.5 | [127] |
| 112–400 | 40–120 | 548–882 | 40.9–51.1 | 3.2–7.0 | 12.8–85.5 | 17.1–41.8 | 13.1–36.5 | [64] |
| 300–400 | 50–70 | 788–923 | 1.8–2.4 | 3.1–3.8 | 78.7–116.3 | 4.7–12.0 | 0.4–1.0 | [128] |
| 100–300 | 60–80 | 221–830 | 1001 | 8.3 1 | 30 1 | 0.1–14.3 | 0.03–4.8 | [43] |
| 150–400 | 33–66 | 691–961 | 2.5 | 1.1–8.3 | 26.6–199.0 | 4.2–30.4 | 0.9–2.2 | [129] |
| 200 | 60 | 723 | 40–80 | 8.3 | 37.5–75.0 | 23.4–40.9 | 3.1–10.9 | [130] |
| 100–300 | 50 | 384–870 | 1.0 | 0.4–0.9 | 56.3 | 71.1 2 | 30.6 3 | [131] |
| 80–100 | 35 (45–95) 4 | 490–700 | 2–5 | 2–10 | 40–100 | 11–31 | 2.2–5.1 | [126] |
| 150–250 | 60 | 603–786 | 80 | 10 | 45 | 7.3–41.4 | 1.6–11.5 | [84] |
| 120–300 | 50 | 510–870 | 100 | 11.7–20 | 21–36 | 9–15 | 4.2–4.3 | [132] |
| 100–300 | 40 | 628–910 | 50 | 40 | 288 | 46 | 0.7–2.16 | [103] |
| 250–300 | 45 | 857–890 | 50 | 30 | 288 | 45 | 0.7–2.8 | [133] |
| Number | Class | MW Range | Key Components | Carbon Number |
|---|---|---|---|---|
| 1 | Ketones | 74–124 | Acetol | 3 |
| Acetylacetone | 5 | |||
| 2-pentanone | 5 | |||
| Propan-2-one. 1-acetyloxy- | 5 | |||
| 2-Cyclopenten-1-one, 2,3-dimethyl- | 7 | |||
| 2 | Phenols | 94–122 | Phenol | 6 |
| o-cresol | 7 | |||
| m-cresol | 7 | |||
| p-cresol | 7 | |||
| 2,5-Dimethylphenol (p-xylenol) | 8 | |||
| 3 | Guaiacols | 124–178 | Guaiacol | 7 |
| 4-methyl guaiacol | 8 | |||
| 4-ethyl guaiacol | 8 | |||
| 4-propyl guaiacol | 8 | |||
| Eugenol | 10 | |||
| Isoeugenol | 10 | |||
| Creosol | 8 | |||
| Vanillin | 8 | |||
| 4 | Benzenediols | 110–124 | 1,2-Benzenediol | 6 |
| 5 | Aldehydes | 60–152 | Glycolaldehyde | 2 |
| 6 | Esters | 130–296 | Pentanoic acid. 4-oxo-. methylester | 6 |
| 7 | Furans | 84–126 | Furfural | 5 |
| 5-Hydroxymethylfurfural | 6 | |||
| 8 | Syringols | 154 | Syringol | 8 |
| 9 | Short chain fatty acids | 60–144 | Acetic acid | 2 |
| Propionic acid | 3 | |||
| Hexanoic acid | 6 | |||
| 10 | Long chain fatty acids | 256–284 | n-Hexadecanoic acid | 16 |
| n-Octadecanoic acid | 18 | |||
| 11 | Aromatic acids | 136 | Benzeneacetic acid, 3-hydroxy | 8 |
| 12 | Benzenes | 120 | o-Cumene | 10 |
| 13 | Polyaromatic hydrocarbons | 128 | Naphthalene | 10 |
| Component | T (°C) | P (bar) | ρ (kg/m3)1 | Method | Data | Ref. |
|---|---|---|---|---|---|---|
| Cyclohexanone | 160–180 | 90–220 | 115–332 | Analytical | VLE | [139] |
| 5-Hydroxymethylfurfural | 41–70 | 97–196 | 390–823 | Synthetic | Solubility | [140] |
| Heptanoic acid | 40–60 | 85–200 | 212–840 | Analytical | Solubility | [141] |
| Hexadecanoic acid | 40 | 80–248 | 278–878 | Analytical | Solubility | [142] |
| 35–55 | 138–414 | 610–977 | Analytical | Solubility | [143] | |
| 35 | 99–230 | 709–888 | Analytical | Solubility | [144] | |
| 35–55 | 128–226 | 560–885 | Analytical | Solubility | [145] | |
| 40–45 | 101–233 | 512–864 | Synthetic | Solubility | [146] | |
| 64–78 | 105–260 | 643–759 | Synthetic | VLE | [147] | |
| Cumene | 40–120 | 76–183 | 131–376 | Analytical | VLE | [148] |
| 70 | 87–116 | 197–324 | Analytical | VLE | [149] | |
| 50 | 80–88 | 220–270 | Analytical | VLE | [150] | |
| Naphthalene | 35–65 | 81–287 | 208–903 | Analytical | Solubility | [151] |
| 121–162 | 77–166 | 120–313 | Analytical | VLE | [152] | |
| Benzeneacetic acid | 35–45 | 90–200 | 381–852 | Analytical | Solubility | [153] |
| Benzoic acid | 35–70 | 101–364 | 252–958 | Analytical | Solubility | [154] |
| 45–65 | 120–280 | 384–878 | Analytical | Solubility | [155] | |
| Phenol | 60–90 | 100–350 | 203–863 | Analytical | Solubility | [156] |
| 36–60 | 79–249 | 334–897 | Analytical | Solubility | [157] | |
| 100 | 107–301 | 207–663 | Analytical | VLE | [158] | |
| Catechol | 60–90 | 100–350 | 203–863 | Analytical | Solubility | [156] |
| 35–65 | 122–405 | 396–974 | Analytical | Solubility | [159] | |
| Vanillin | 40–80 | 80–277 | 160–895 | Analytical | Solubility | [160] |
| 35–45 | 83–195 | 466–857 | Analytical | Solubility | [153] | |
| 68–136 | 216–1341 | 561–1115 | Synthetic | VLE | [161] | |
| Guaiacol | 50–120 | 80–200 | 128–784 | Analytical | VLE | [162] |
| o-Cresol | 100 | 104–263 | 199–610 | Analytical | VLE | [158] |
| 50–200 | 99–300 | 121–848 | Analytical | VLE | [163] | |
| m-Cresol | 35–55 | 80–240 | 204–895 | Analytical | VLE | [164] |
| 100 | 102–300 | 194–662 | Analytical | VLE | [158] | |
| p-Cresol | 50–200 | 100–348 | 123–898 | Analytical | VLE | [163] |
| 80–150 | 80–200 | 113–594 | Analytical | VLE | [162] | |
| 100 | 103–302 | 196–663 | Analytical | VLE | [158] |
| Regressed Parameter | Goodness of Fit | |||||
|---|---|---|---|---|---|---|
| Component | k | a (K) | b | R2 | ARD % | N |
| Cyclohexanone | 2.0216 | −3425.3 | 0.45405 | 0.984 | 2.0 | 10 |
| 5-Hydroxymethylfurfural | 4.0412 | −3263.6 | −15.267 | 0.973 | 9.0 | 19 |
| Heptanoic acid | 6.0527 | −3806.1 | −23.733 | 0.987 | 8.1 | 15 |
| Hexadecanoic acid | 7.5664 | −9042.3 | −20.706 | 0.950 | 19.0 | 62 |
| Cumene | 2.5852 | −3481.6 | −2.1835 | 0.963 | 9.3 | 41 |
| Naphthalene | 3.7852 | −4080.9 | −8.1975 | 0.940 | 14.7 | 63 |
| Benzeneacetic acid | 6.1072 | −10177 | −5.2314 | 0.986 | 4.9 | 24 |
| Benzoic acid | 5.2174 | −5860.4 | −14.636 | 0.986 | 13.4 | 53 |
| Phenol | 3.0544 | −3081.4 | 6.9331 | 0.729 | 10.8 | 73 |
| Catechol | 3.7457 | −3716.1 | −12.417 | 0.977 | 18.2 | 62 |
| Vanillin | 4.0675 | −4210.7 | −11.707 | 0.948 | 18.4 | 74 |
| Guaiacol | 4.0447 | −2597.8 | −14.042 | 0.965 | 21.0 | 13 |
| o-Cresol | 3.4937 | −3026.3 | −9.2413 | 0.867 | 13.1 | 16 |
| m-Cresol | 3.8196 | −2950.5 | −12.383 | 0.98 | 18.0 | 23 |
| p-Cresol | 3.2734 | −3441.3 | −7.5747 | 0.924 | 15.7 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesantos, N.; Maschietti, M. Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery. Energies 2020, 13, 1600. https://doi.org/10.3390/en13071600
Montesantos N, Maschietti M. Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery. Energies. 2020; 13(7):1600. https://doi.org/10.3390/en13071600
Chicago/Turabian StyleMontesantos, Nikolaos, and Marco Maschietti. 2020. "Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery" Energies 13, no. 7: 1600. https://doi.org/10.3390/en13071600
APA StyleMontesantos, N., & Maschietti, M. (2020). Supercritical Carbon Dioxide Extraction of Lignocellulosic Bio-Oils: The Potential of Fuel Upgrading and Chemical Recovery. Energies, 13(7), 1600. https://doi.org/10.3390/en13071600







