Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Physico-Chemical Properties of Non-Precious Catalysts for Alkaline Electrolysis
2.2. Physico-Chemical Characterizations
2.3. Electrodes Preparation and Membrane-Electrode Assembly
2.4. Electrochemical Characterization in an Alkaline Electrolysis Single Cell
3. Results and Discussion
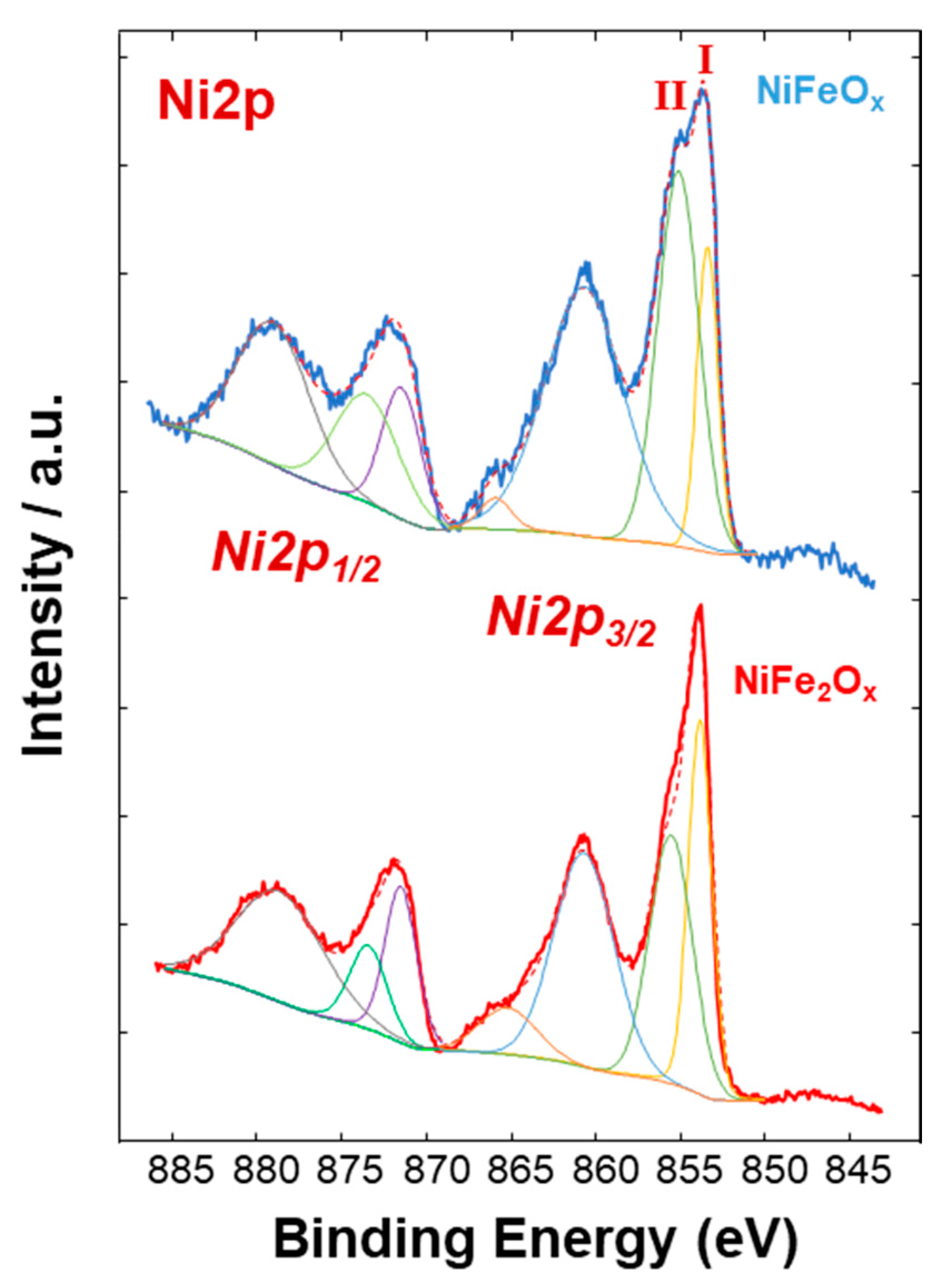
3.1. Physico-Chemical Properties of (CRM)-Free Catalysts for Alkaline Electrolysis
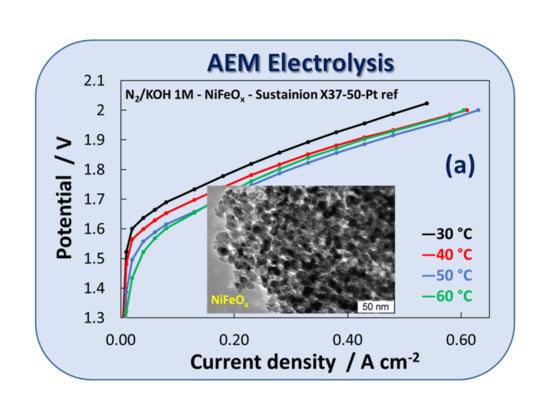
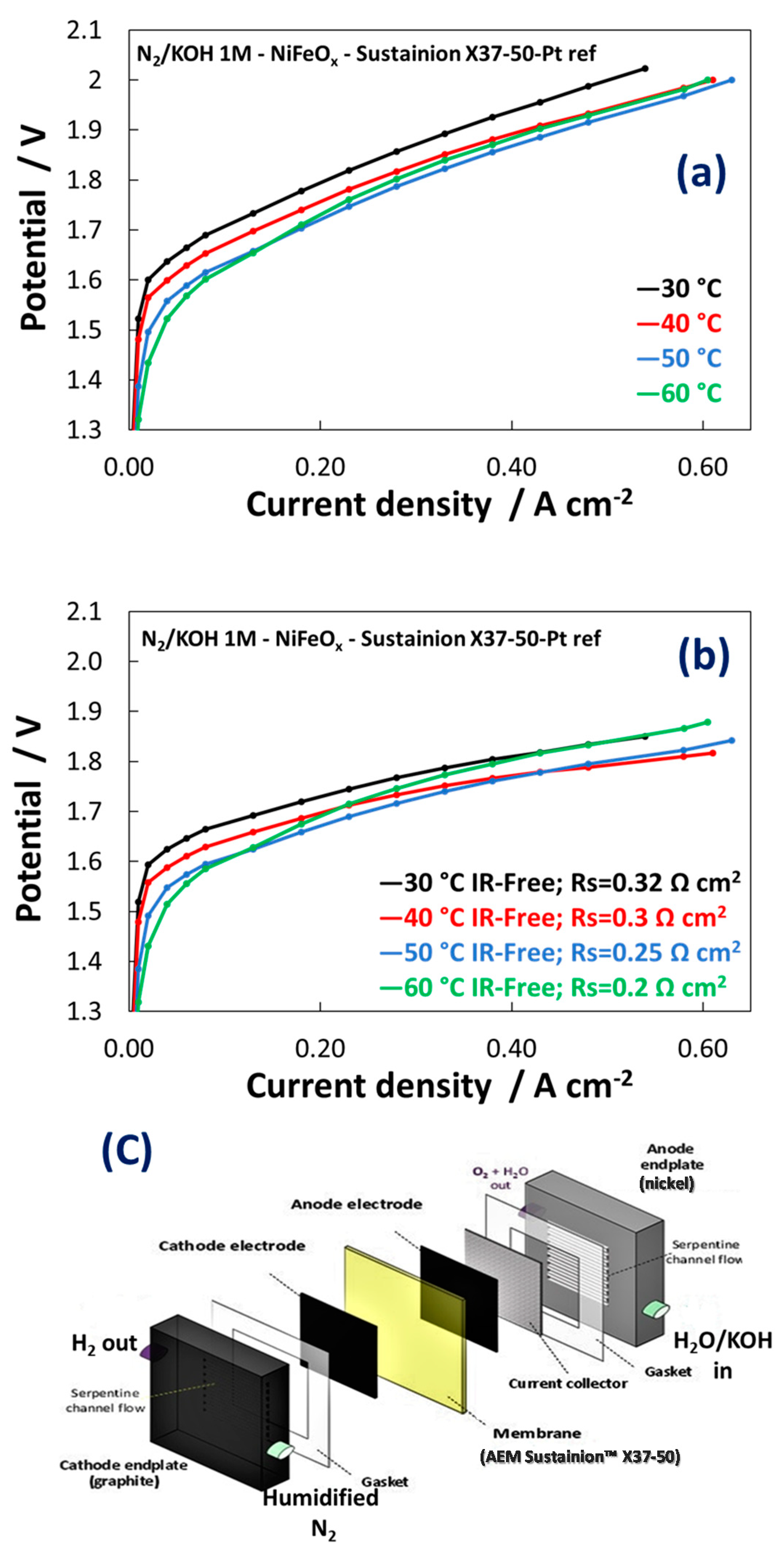
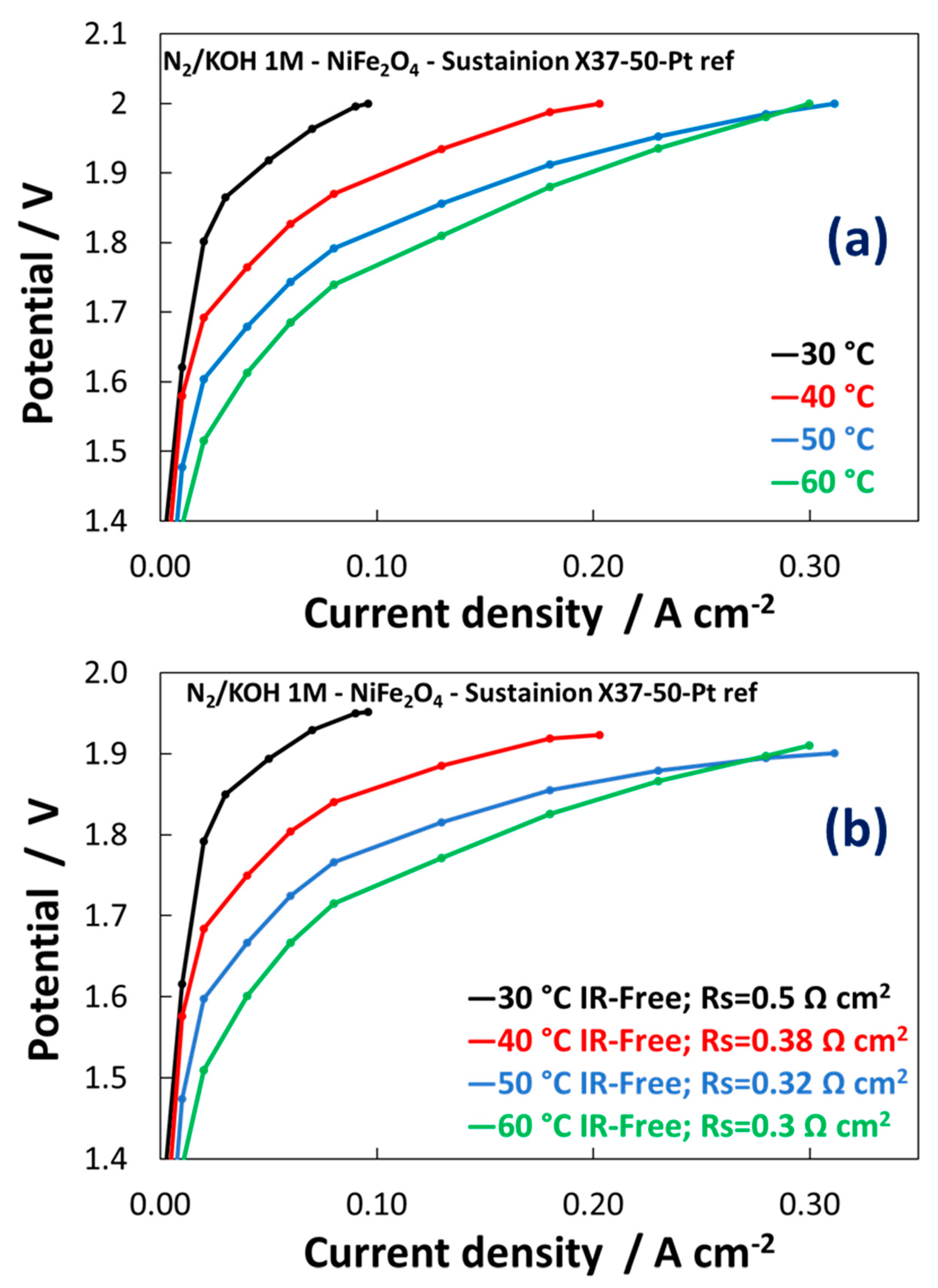
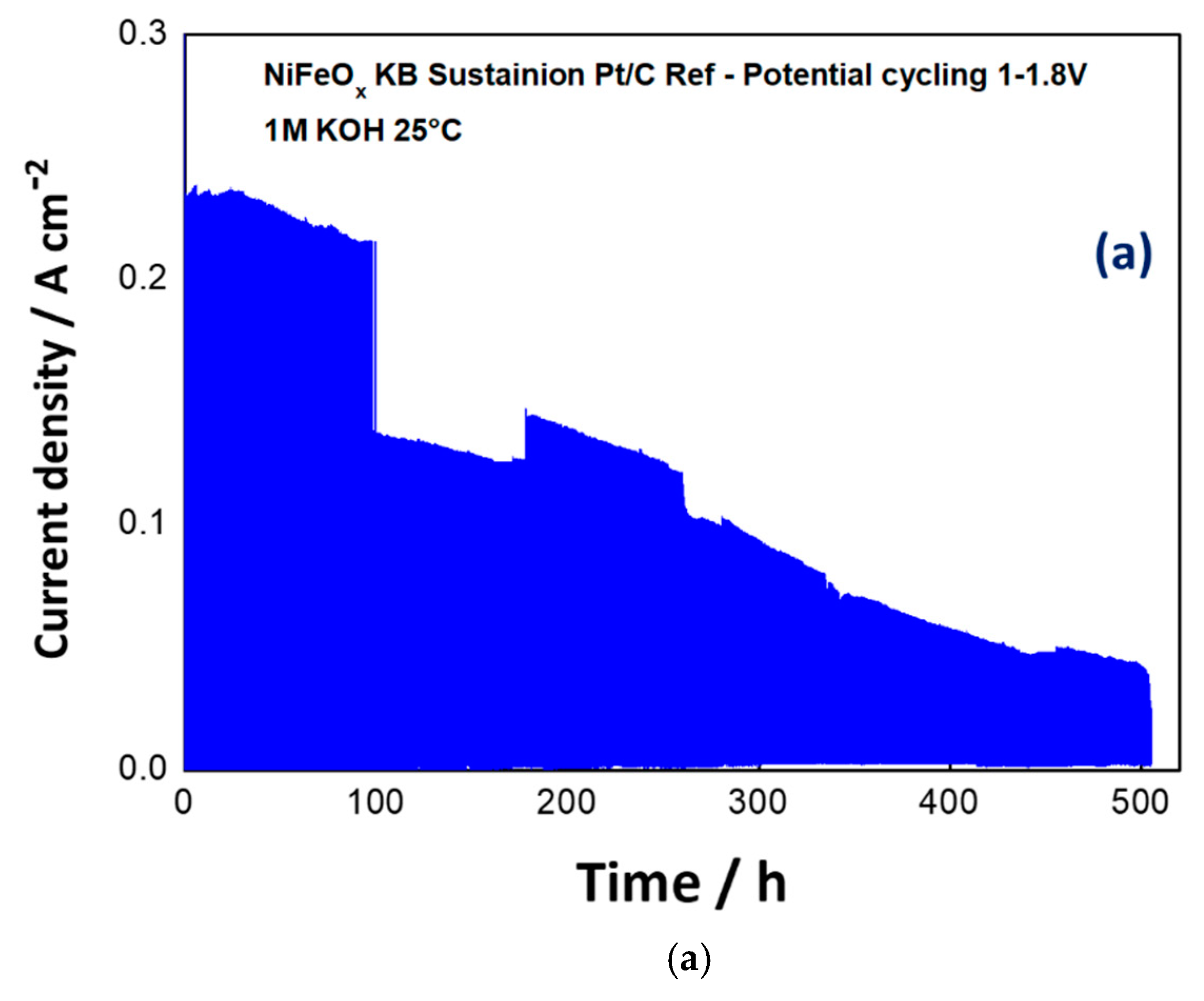
3.2. Electrochemical Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Holladay, J.; Hu, J.; King, D.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Santo, V.D.; Gallo, A.; Naldoni, A.; Guidotti, M.; Psaro, R. Bimetallic heterogeneous catalysts for hydrogen production. Catal. Today 2012, 197, 190–205. [Google Scholar] [CrossRef]
- Ferrero, D.; Lanzini, A.; Santarelli, M.; Leone, P. A comparative assessment on hydrogen production from low- and high-temperature electrolysis. Int. J. Hydrog. Energy 2013, 38, 3523–3536. [Google Scholar] [CrossRef]
- Faro, M.L.; Trocino, S.; Zignani, S.; Antonucci, V.; Aricò, A. Production of syngas by solid oxide electrolysis: A case study. Int. J. Hydrog. Energy 2017, 42, 27859–27865. [Google Scholar] [CrossRef]
- Dionigi, F.; Strasser, P. NiFe-Based (Oxy)hydroxide Catalysts for Oxygen Evolution Reaction in Non-Acidic Electrolytes. Adv. Energy Mater. 2016, 6, 1600621. [Google Scholar] [CrossRef]
- Schalenbach, M.; Tjarks, G.; Carmo, M.; Lueke, W.; Mueller, M.; Stolten, D. Acidic or Alkaline? Towards a New Perspective on the Efficiency of Water Electrolysis. J. Electrochem. Soc. 2016, 163, F3197–F3208. [Google Scholar] [CrossRef] [Green Version]
- Bandal, H.; Jadhav, V.H.; Kim, H. Facile synthesis of bicontinuous Ni3Fe alloy for efficient electrocatalytic oxygen evolution reaction. J. Alloy. Compd. 2017, 726, 875–884. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Kaczur, J.; Masel, R. An Alkaline Water Electrolyzer with Sustainion™ Membranes: 1 A/cm² at 1.9V with Base Metal Catalysts. ECS Trans. 2017, 77, 71–73. [Google Scholar] [CrossRef]
- Hwang, G.-J.; Gil, B.-M.; Ryu, C.-H. Preparation of the electrode using NiFe 2 O 4 powder for the alkaline water electrolysis. J. Ind. Eng. Chem. 2017, 48, 242–248. [Google Scholar] [CrossRef]
- Phillips, R.; Gannon, W.J.F.; Dunnill, C.W. Chapter 2: Alkaline electrolysers. In RSC Energy and Environment Series; Royal Society of Chemistry (RSC): London, UK, 2020; Volume 2020-January, pp. 28–58. [Google Scholar]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Takenaka, H.; Torikai, E.; Kawami, Y.; Wakabayashi, N. Solid polymer electrolyte water electrolysis. Int. J. Hydrog. Energy 1982, 7, 397–403. [Google Scholar] [CrossRef]
- Millet, P.; Andolfatto, F.; Durand, R. Design and performance of a solid polymer electrolyte water electrolyzer. Int. J. Hydrog. Energy 1996, 21, 87–93. [Google Scholar] [CrossRef]
- Leroy, R. Industrial water electrolysis: Present and future. Int. J. Hydrog. Energy 1983, 8, 401–417. [Google Scholar] [CrossRef]
- Lu, P.W.T.; Srinivasan, S. Advances in water electrolysis technology with emphasis on use of the solid polymer electrolyte. J. Appl. Electrochem. 1979, 9, 269–283. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Busacca, C.; Zignani, S.C.; Di Blasi, A.; Di Blasi, O.; Faro, M.L.; Antonucci, V.; Aricò, A. Electrospun NiMn2O4 and NiCo2O4 spinel oxides supported on carbon nanofibers as electrocatalysts for the oxygen evolution reaction in an anion exchange membrane-based electrolysis cell. Int. J. Hydrog. Energy 2019, 44, 20987–20996. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Améduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.; Varcoe, J.R. Alkaline anion-exchange radiation-grafted membranes for possible electrochemical application in fuel cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-H.; Maes, A.M.; Vandiver, M.A.; Versek, C.; Seifert, S.; Tuominen, M.; Liberatore, M.W.; Coughlin, E.B. Synthesis and structure-conductivity relationship of polystyrene- block -poly(vinyl benzyl trimethylammonium) for alkaline anion exchange membrane fuel cells. J. Polym. Sci. Part B: Polym. Phys. 2012, 51, 1751–1760. [Google Scholar] [CrossRef]
- Morandi, C.; Peach, R.; Krieg, H.; Kerres, J. Novel imidazolium-functionalized anion-exchange polymer PBI blend membranes. J. Membr. Sci. 2015, 476, 256–263. [Google Scholar] [CrossRef]
- Aili, D.; Kraglund, M.R.; Tavacoli, J.; Chatzichristodoulou, C.; Jensen, J.O. Polysulfone-polyvinylpyrrolidone blend membranes as electrolytes in alkaline water electrolysis. J. Membr. Sci. 2020, 598, 117674. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, B.-N.; Nam, S.Y. Synthesis and characterization of PEEK containing imidazole for anion exchange membrane fuel cell. Int. J. Hydrog. Energy 2017, 42, 23759–23767. [Google Scholar] [CrossRef]
- Davydova, E.S.; Mukerjee, S.; Jaouen, F.; Dekel, D.R. Electrocatalysts for Hydrogen Oxidation Reaction in Alkaline Electrolytes. ACS Catal. 2018, 8, 6665–6690. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Narducci, R.; Pasquini, L.; Pelzer, K.; Knauth, P. Anion-conducting ionomers: Study of type of functionalizing amine and macromolecular cross-linking. Int. J. Hydrog. Energy 2014, 39, 14039–14049. [Google Scholar] [CrossRef]
- Di Blasi, A.; D’Urso, C.; Baglio, V.; Antonucci, V.; Arico, A.S.; Ornelas, R.; Matteucci, F.; Orozco, G.; Beltran, D.; Meas, Y.; et al. Preparation and evaluation of RuO2-IrO2, IrO2-Pt and IrO2-Ta2O5 catalysts for the oxygen evolution reaction in an SPE electrolyzer. J. Appl. Electrochem. 2008, 39, 191–196. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Benrabaa, R.; Boukhlouf, H.; Löfberg, A.; Rubbens, A.; Rose-Noëlle, V.; Bordes-Richard, E.; Barama, A. Nickel ferrite spinel as catalyst precursor in the dry reforming of methane: Synthesis, characterization and catalytic properties. J. Nat. Gas Chem. 2012, 21, 595–604. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Alonso, F.; Adán, C.; Rojas, S.; Peña, M.A.; Jose, F. Ni/Fe electrodes prepared by electrodeposition method over different substrates for oxygen evolution reaction in alkaline medium. Int. J. Hydrog. Energy 2014, 39, 5204–5212. [Google Scholar] [CrossRef]
- Huang, L.; Ge, X.; Dong, S. A facile conversion of a Ni/Fe coordination polymer to a robust electrocatalyst for the oxygen evolution reaction. RSC Adv. 2017, 7, 32819–32825. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Dang, L.; Shearer, M.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Fan, H.; Chen, W.; Chen, G.; Huang, J.; Song, C.; Du, Y.; Li, C.; Ostrikov, K.K. Plasma-heteroatom-doped Ni-V-Fe trimetallic phospho-nitride as high-performance bifunctional electrocatalyst. Appl. Catal. B: Environ. 2019, 268, 118440. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Z.; Alam, K.; Ni, Y.; Hernandez, F.C.R.; Yu, L.; Chen, S.; Ren, Z.; Wang, Z.; Bao, J. Trimetallic NiFeMo for Overall Electrochemical Water Splitting with a Low Cell Voltage. ACS Energy Lett. 2018, 3, 546–554. [Google Scholar] [CrossRef]
- Chanda, D.; Hnát, J.; Paidar, M.; Bouzek, K. Evolution of physicochemical and electrocatalytic properties of NiCo2O4 (AB2O4) spinel oxide with the effect of Fe substitution at the A site leading to efficient anodic O2 evolution in an alkaline environment. Int. J. Hydrog. Energy 2014, 39, 5713–5722. [Google Scholar] [CrossRef]
- Pebley, A.; Decolvenaere, E.; Pollock, T.; Gordon, M. Oxygen evolution on Fe-doped NiO electrocatalysts deposited: Via microplasma. Nanoscale 2017, 9, 15070–15082. [Google Scholar] [CrossRef]
- Paulraj, A.R.; Kiros, Y.; Göthelid, M.; Johansson, M.B. NiFeOx as a bifunctional electrocatalyst for oxygen reduction (OR) and evolution (OE) reaction in alkaline media. Catalysts 2018, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Lo Faro, M.; Frontera, P.; Antonucci, P.; Aricò, A.S. Ni-Cu based catalysts prepared by two different methods and their catalytic activity toward the ATR of methane. Chem. Eng. Res. Des. 2015, 93, 269–277. [Google Scholar] [CrossRef]
- Zignani, S.C.; Antolini, E.; Gonzalez, E.R. Stability of Pt-Ni/C (1:1) and Pt/C electrocatalysts as cathode materials for polymer electrolyte fuel cells: Effect of ageing tests. J. Power Sources 2009, 191, 344–350. [Google Scholar] [CrossRef]
- Linares, J.J.; Zignani, S.C.; Rocha, T.A.; Gonzalez, E.R. Ethanol oxidation on a high temperature PBI-based DEFC using Pt/C, PtRu/C and Pt3Sn/C as catalysts. J. Appl. Electrochem. 2013, 43, 147–158. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landon, J.; Demeter, E.; Inoǧlu, N.; Keturakis, C.; Wachs, I.E.; Vasić, R.; Frenkel, A.I.; Kitchin, J.R. Spectroscopic characterization of mixed Fe-Ni oxide electrocatalysts for the oxygen evolution reaction in alkaline electrolytes. ACS Catal. 2012, 2, 1793–1801. [Google Scholar] [CrossRef]
- Fominykh, K.; Chernev, P.; Zaharieva, I.; Sicklinger, J.; Stefanic, G.; Döblinger, M.; Müller, A.; Pokharel, A.; Böcklein, S.; Scheu, C.; et al. Iron-doped nickel oxide nanocrystals as highly efficient electrocatalysts for alkaline water splitting. ACS Nano 2015, 9, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Q.; Liu, Y.R.; Hu, W.H.; Dong, B.; Li, X.; Shang, X.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Three dimensional nickel oxides/nickel structure by in situ electro-oxidation of nickel foam as robust electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2015, 359, 172–176. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Ling, T.; Vasileff, A.; Qiao, S.Z. S-NiFe2O4 ultra-small nanoparticle built nanosheets for efficient water splitting in alkaline and neutral pH. Nano Energy 2017, 40, 264–273. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, C.; Ai, L. Hierarchical iron nickel oxide architectures derived from metal-organic frameworks as efficient electrocatalysts for oxygen evolution reaction. Electrochimica Acta 2016, 208, 17–24. [Google Scholar] [CrossRef]
- Fang, J.; Hu, L.; Wang, M.; Gan, L.; Chen, C.; Jiang, Y.; Xiao, B.; Lai, Y.; Li, J. NiO-Fe2O3/carbon nanotubes composite as bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Lett. 2018, 218, 36–39. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, S.; Wei, G.; Niu, Y.; Guo, L.; Chen, Z. Investigation of Fe-based integrated electrodes for water oxidation in neutral and alkaline solutions. J. Phys. Chem. C 2019, 123, 12313–12320. [Google Scholar] [CrossRef]
- Pushkareva, I.V.; Pushkarev, A.S.; Grigoriev, S.A.; Modisha, P.; Bessarabov, D.G. Comparative study of anion exchange membranes for low-cost water electrolysis. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Arges, C.G.; Zhang, L. Anion exchange membranes’ evolution toward high hydroxide ion conductivity and alkaline resiliency. ACS Appl. Energy Mater. 2018, 1, 2991–3012. [Google Scholar] [CrossRef]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B: Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Li, N.; Leng, Y.; Hickner, M.A.; Wang, C.Y. Highly stable, anion conductive, comb-shaped copolymers for alkaline fuel cells. J. Am. Chem. Soc. 2013, 135, 10124–10133. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, M.; Manolova, M. Chapter 6: Alkaline anionic exchange membrane water electrolysers. In RSC Energy and Environment Series; Royal Society of Chemistry (RSC): London, UK, 2020; Volume 2020-January, pp. 180–252. [Google Scholar]
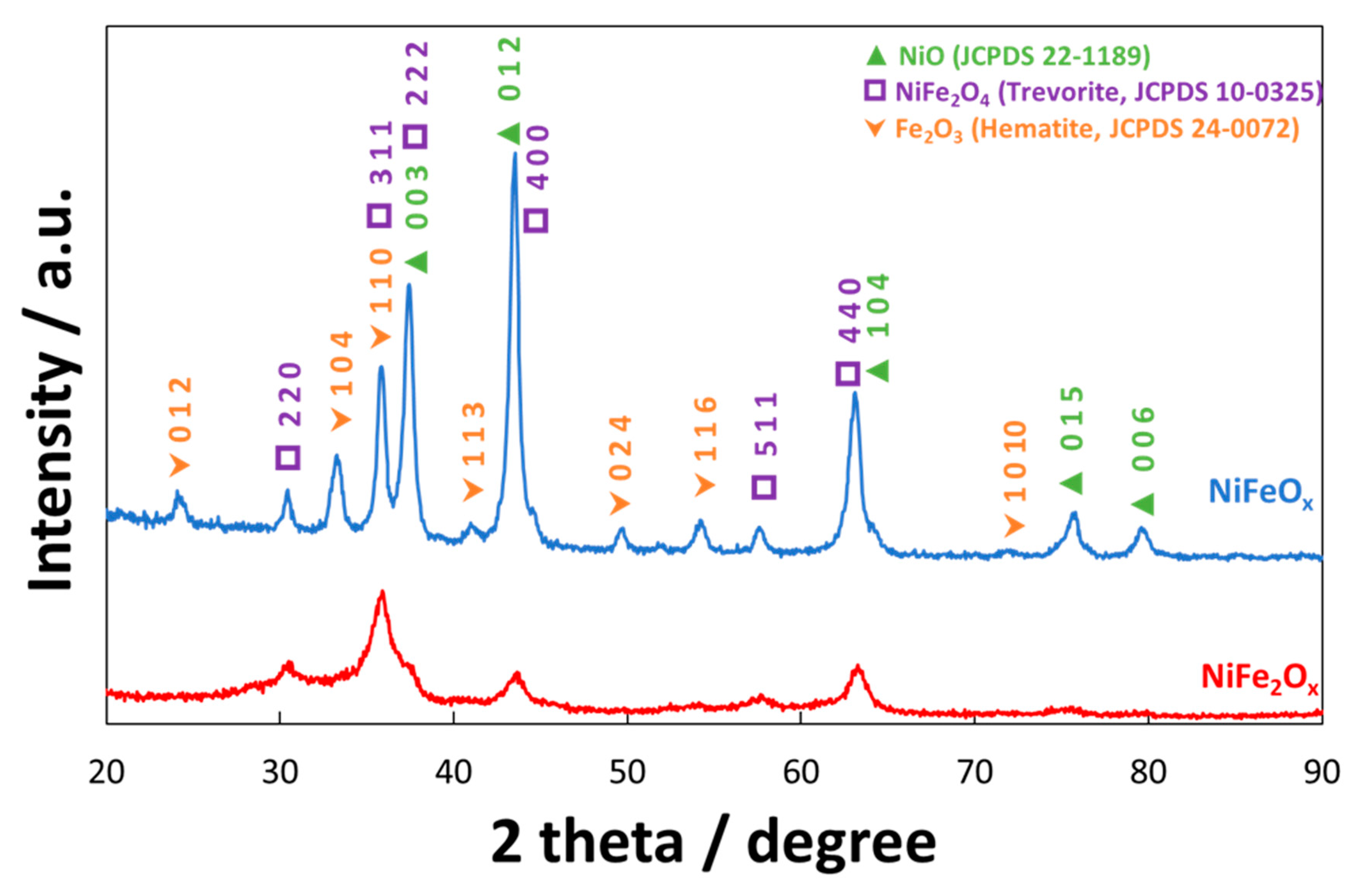

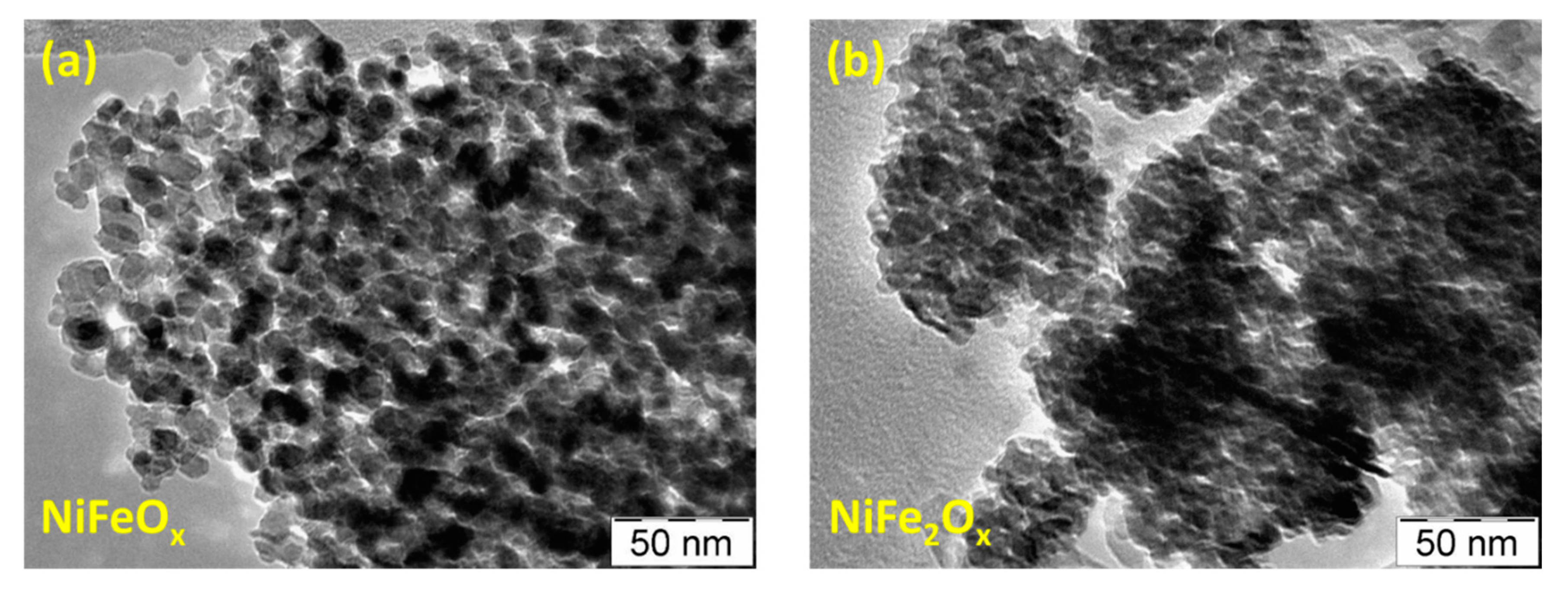
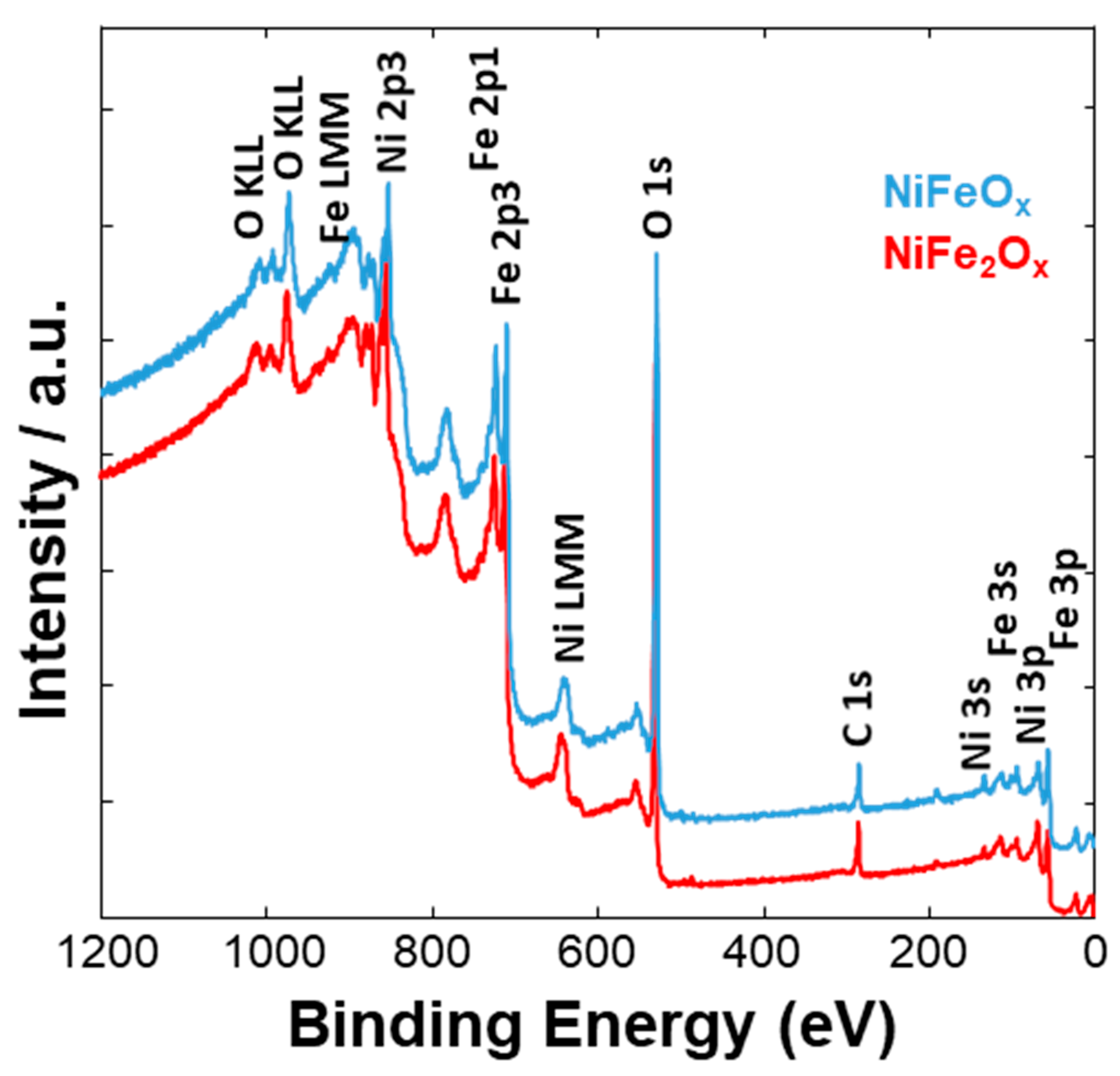


| Catalyst Formula | Concentration of Metal Oxide % | Crystallite Size (nm) XRD | Particle Size (nm) TEM | BET (m2 gr–1) |
|---|---|---|---|---|
| NiFeOx / KB | 70 | 12 | 10 | 50 |
| NiFe2Ox / KB | 70 | 5 | 5 | 141 |
| Catalyst Formula | Conditions | 30 °C A g−1NiFe @ 1.5 V | 40 °C A g−1NiFe @ 1.5 V | 50 °C A g−1NiFe @ 1.5 V | 60 °C A g−1NiFe @ 1.5 V |
|---|---|---|---|---|---|
| NiFeOx KB (70:30) | 1M KOH | 4 | 8 | 16 | 24 |
| NiFe2Ox KB (70:30) | 1M KOH | 4 | 4 | 4 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagna Zignani, S.; Lo Faro, M.; Trocino, S.; Aricò, A.S. Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis. Energies 2020, 13, 1720. https://doi.org/10.3390/en13071720
Campagna Zignani S, Lo Faro M, Trocino S, Aricò AS. Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis. Energies. 2020; 13(7):1720. https://doi.org/10.3390/en13071720
Chicago/Turabian StyleCampagna Zignani, Sabrina, Massimiliano Lo Faro, Stefano Trocino, and Antonino Salvatore Aricò. 2020. "Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis" Energies 13, no. 7: 1720. https://doi.org/10.3390/en13071720
APA StyleCampagna Zignani, S., Lo Faro, M., Trocino, S., & Aricò, A. S. (2020). Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis. Energies, 13(7), 1720. https://doi.org/10.3390/en13071720