Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Procedures
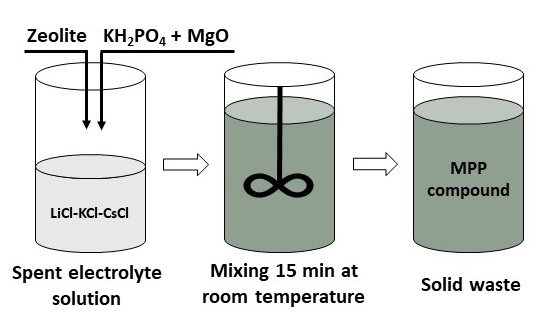
2.2. Methods
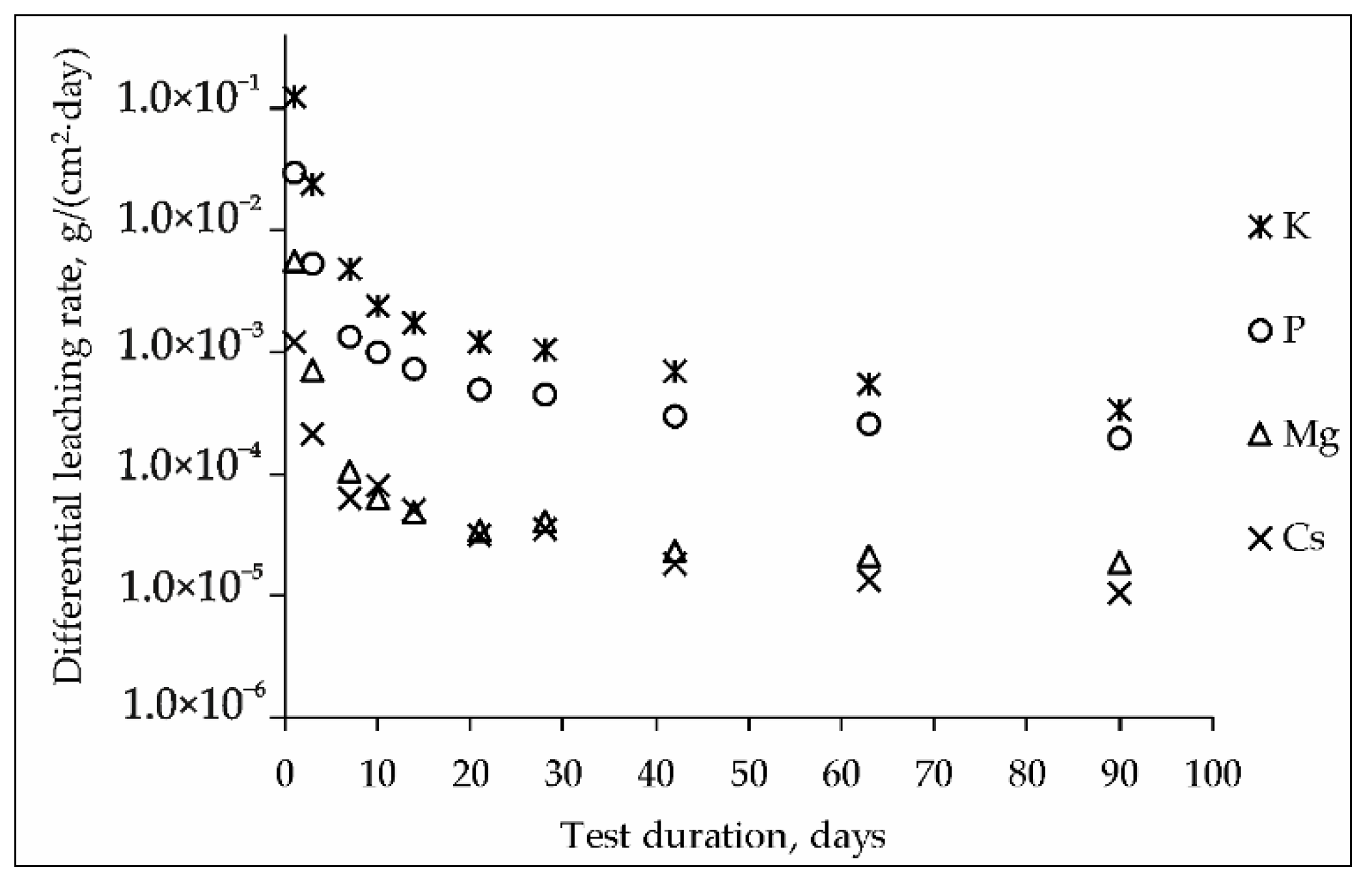
3. Results and Discussion
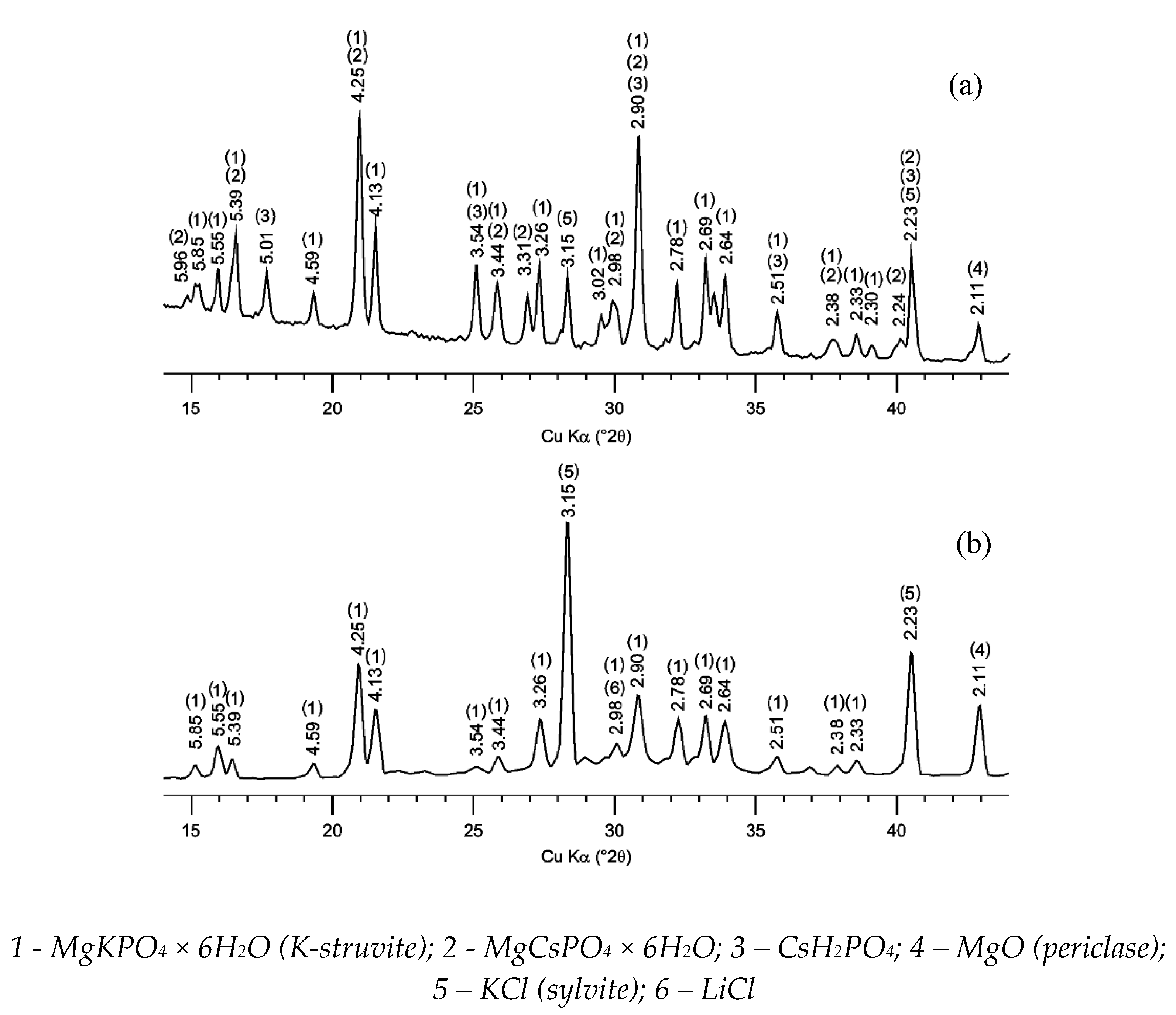
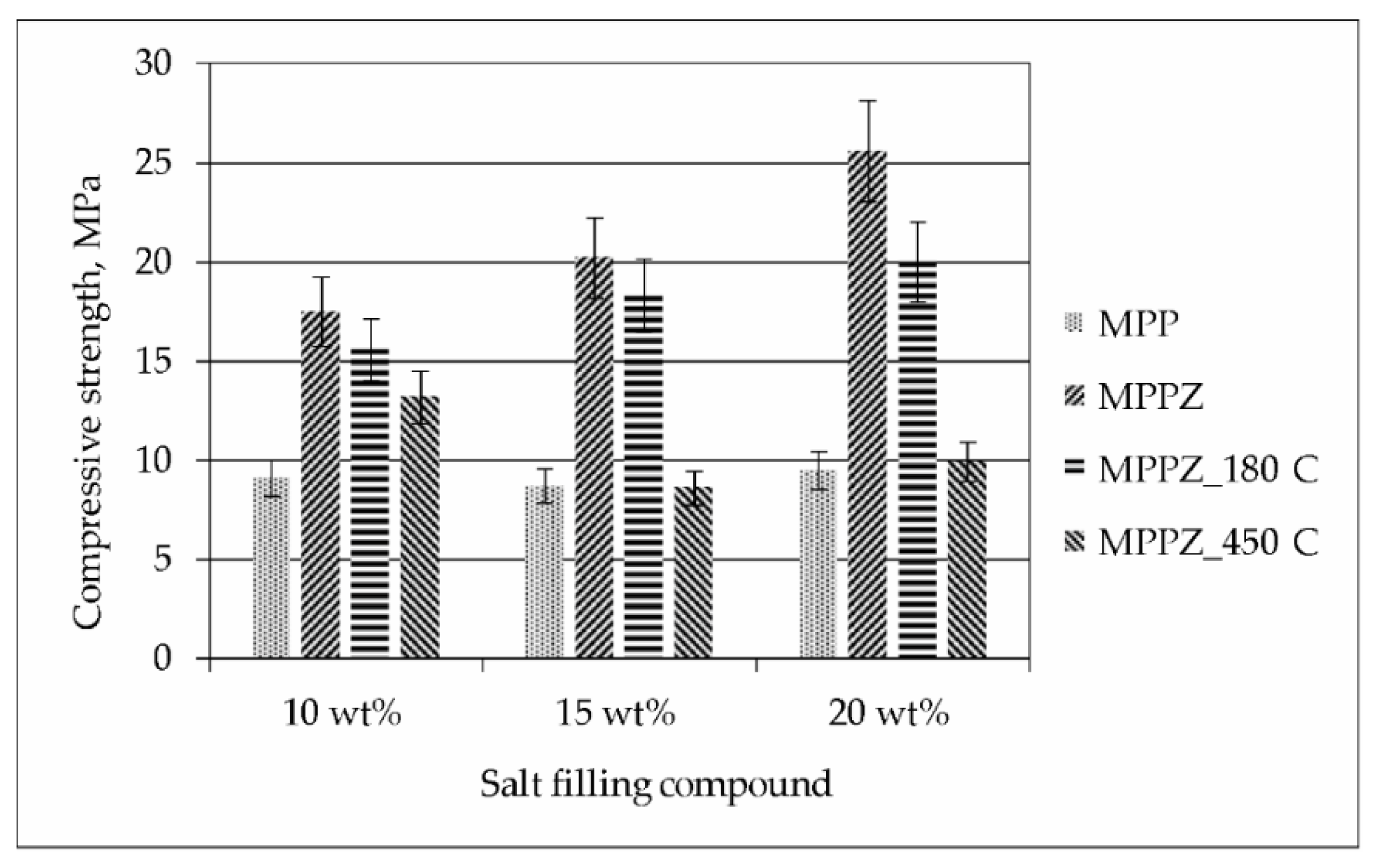
3.1. Characterization of the MPP Compound Containing Chloride
3.2. Characterization of MPP Compound Containing LiCl-KCl-CsCl and Zeolite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I.; Veselov, S.N.; et al. PH process as a technology for reprocessing mixed uranium-plutonium fuel from BREST-OD-300 reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Lizin, A.A.; Tomilin, S.V.; Gnevashov, O.E.; Lukinykh, A.N.; Orlova, A.I. Orthophosphates of langbeinite structure for immobilization of alkali metal cations of salt wastes from pyrochemical processes. Radiochemistry 2012, 54, 542–548. [Google Scholar] [CrossRef]
- Vance, E.R.; Davis, J.; Olufson, K.; Chironi, I.; Karatchevtseva, I.; Farnan, I. Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. J. Nucl. Mater. 2012, 420, 396–404. [Google Scholar] [CrossRef]
- Joseph, K.; Asuvathraman, R.; Raja Madhavan, R.; Jena, H.; Govindan Kutty, K.V.; Vasudeva Rao, P.R. Studies on Novel Matrices for High Level Waste from Fast Reactor Fuel Reprocessing. Energy Procedia 2011, 7, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Riley, B.J.; Kroll, J.O.; Peterson, J.A.; Pierce, D.A.; Ebert, W.L.; Williams, B.D.; Snyder Michelle, M.V.; Frank, S.M.; George, J.L.; Kruska, K. Assessment of lead tellurite glass for immobilizing electrochemical salt wastes from used nuclear fuel reprocessing. J. Nucl. Mater. 2017, 495, 405–420. [Google Scholar] [CrossRef]
- Choi, J.; Um, W.; Choung, S. Development of iron phosphate ceramic waste form to immobilize radioactive waste solution. J. Nucl. Mater. 2014, 452, 16–23. [Google Scholar] [CrossRef]
- Giacobbo, F.; Da Ros, M.; Macerata, E.; Mariani, M.; Giola, M.; De Angelis, G.; Capone, M.; Fedeli, C. An experimental study on Sodalite and SAP matrices for immobilization of spent chloride salt waste. J. Nucl. Mater. 2018, 499, 512–527. [Google Scholar] [CrossRef]
- Cho, I.-H.; Park, H.-S.; Lee, K.-R.; Choi, J.-H.; Kim, I.-T.; Hur, J.M.; Lee, Y.-S. Treatment of radioactive waste salt by using synthetic silica-based phosphate composite for de-chlorination and solidification. J. Nucl. Mater. 2017, 493, 388–397. [Google Scholar] [CrossRef]
- Leturcq, G.; Grandjean, A.; Rigaud, D.; Perouty, P.; Charlot, M. Immobilization of fission products arising from pyrometallurgical reprocessing in chloride media. J. Nucl. Mater. 2005, 347, 1–11. [Google Scholar] [CrossRef]
- Lepry, W.C.; Riley, B.J.; Crum, J.V.; Rodriguez, C.P.; Pierce, D.A. Solution-based approaches for making high-density sodalite waste forms to immobilize spent electrochemical salts. J. Nucl. Mater. 2013, 442, 350–359. [Google Scholar] [CrossRef]
- Jena, H.; Maji, B.K.; Asuvathraman, R.; Govindan Kutty, K.V. Synthesis and thermal characterization of glass bonded Ca-chloroapatite matrices for pyrochemical chloride waste immobilization. J. Non-Cryst. Solids 2012, 358, 1681–1686. [Google Scholar] [CrossRef]
- Jena, H.; Maji, B.K.; Asuvathraman, R.; Govindan Kutty, K.V. Effect of pyrochemical chloride waste loading on thermo-physical properties of borosilicate glass bonded Sr-chloroapatite composites. Mater. Chem. Phys. 2015, 162, 188–196. [Google Scholar] [CrossRef]
- Poluektov, P.P.; Schmidt, O.V.; Kascheev, V.A.; Ojovan, M.I. Modelling aqueous corrosion of nuclear waste phosphate glass. J. Nucl. Mater. 2017, 484, 357–366. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovny, S.I.; Myasoedov, B.F. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices. J. Nucl. Mater. 2009, 385, 189–192. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovnyi, S.I.; Wagh, A.S.; Maloney, M.D.; Myasoedov, B.F. Magnesium potassium phosphate matrices for immobilization of high-level liquid wastes. Radiochemistry 2009, 51, 65–72. [Google Scholar] [CrossRef]
- Shkuropatenko, V.A. High level wastes immobilization in ceramic and hydrated phosphate matrix. East Eur. J. Phys. 2016, 3, 49–60. [Google Scholar]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials 2018, 11, 976. [Google Scholar] [CrossRef] [Green Version]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Hydrolytic and thermal stability of magnesium potassium phosphate compound for immobilization of high level waste. J. Radioanal. Nucl. Chem. 2018, 318, 2401–2405. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Solidification of high level waste using magnesium potassium phosphate compound. Nucl. Eng. Technol. 2019, 51, 755–760. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Danilov, S.S.; Gromyak, I.N.; Myasoedov, B.F. Investigation of the leaching behavior of components of the magnesium potassium phosphate matrix after high salt radioactive waste immobilization. J. Radioanal. Nucl. Chem. 2018, 315, 481–486. [Google Scholar] [CrossRef]
- Sayenko, S.Y.; Shkuropatenko, V.A.; Dikiy, N.P.; Tarasov, R.V.; Ulybkina, K.A.; Surkov, O.Y.; Litvinenko, L.M. Clinoptilolite with cesium immobilization to potassium magnesium phosphate matrix. East Eur. J. Phys. 2017, 4, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Wagh, A.S.; Sayenko, S.Y.; Shkuropatenko, V.A.; Tarasov, R.V.; Dykiy, M.P.; Svitlychniy, Y.O.; Virych, V.D.; Ulybkina, Е.А. Experimental study on cesium immobilization in struvite structures. J. Hazard. Mater. 2016, 302, 241–249. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4∙6H2O, the potassium equivalent of struvite—A new mineral. Eur. J. Mineral. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Radioactive Waste Immobilization: Phase Composition, Structure, and Physicochemical and Hydrolytic Durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. [Google Scholar] [CrossRef] [Green Version]
- KODEKX. Procedure for Measuring the Ultimate Strength of Cement Compounds Incorporating Radioactive Waste Using a Testing Cybertronic Testing Machine. 2013. Available online: http://docs.cntd.ru/document/437125221 (accessed on 10 March 2020).
- Federal Norms and Rules in the Field of Atomic Energy Use. “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements” (NP-019-15); Rostekhnadzor: Moscow, Russia, 2015; pp. 1–22. [Google Scholar]
- GOST R 52126-2003. Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms; Gosstandart 305: Moscow, Russian, 2003; pp. 1–8. [Google Scholar]
- ASTM C1285-14. Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT); ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 20 March 2020). [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- de Groot, G.J.; van der Sloot, H.A. Determination of leaching characteristics of waste materials leading to environmental product certification. In Stabilization and Solidification of Hazardous, Radioactive and Mixed Wastes; Gilliam, T.M., Wiles, G., Eds.; ASTMSTP 1123; American Society for Testing and Materials: Philadelphia, PA, USA, 1992; Volume 2, pp. 149–170. [Google Scholar] [CrossRef]
- Torras, J.; Buj, I.; Rovira, M.; de Pablo, J. Semi-dynamic leaching tests of nickel containing wastes stabilized/solidified with magnesium potassium phosphate cements. J. Hazard. Mater. 2011, 186, 1954–1960. [Google Scholar] [CrossRef]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
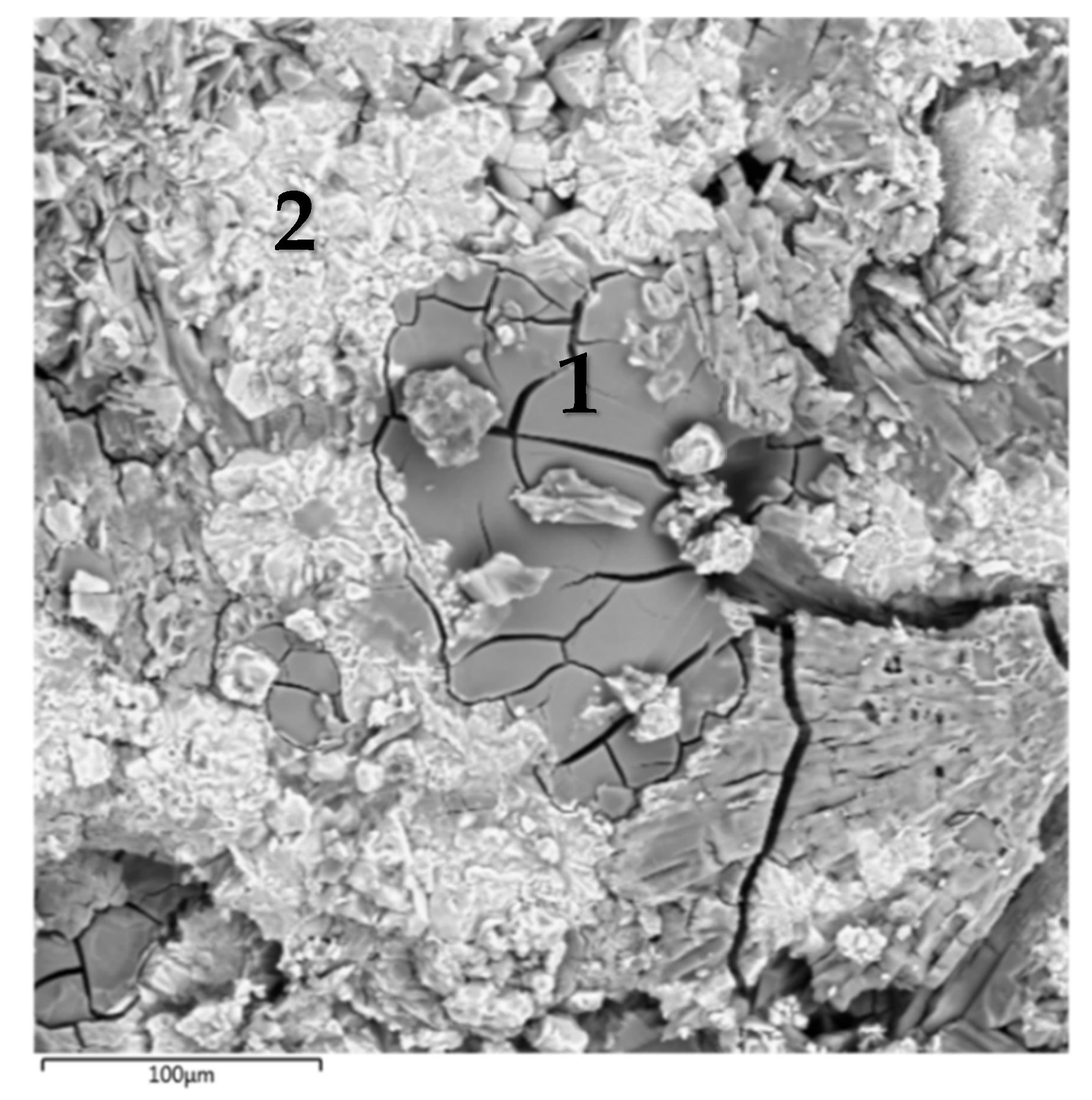


| No. | Content of Elements, at.% | |||||
|---|---|---|---|---|---|---|
| Mg | K | P | O | Cs | Cl | |
| 1 | 9.00 ± 1.10 | 9.35 ± 1.50 | 9.50 ± 1.00 | 71.55 ± 8.00 | 0.45 ± 0.15 | 0.15 ± 0.05 |
| 2 | 1.50 ± 0.40 | 2.60 ± 0.65 | 12.60 ± 1.20 | 48.00 ± 5.50 | 35.00 ± 3.00 | 0.30 ± 0.05 |
| Sample | Specific Surface Area SBET, m2/g | Pores Volume, cm3/g/Average Diameter, nm |
|---|---|---|
| MPPZ | 15.9 | 0.053/5.48 |
| MPPZ_180 °C | 9.5 | 0.052/5.68 |
| MPPZ_450 °C | 6.4 | 0.031/4.89 |
| Compound | LR of Compound Components, g/(cm2∙day) | |||||
|---|---|---|---|---|---|---|
| Mg | P | K | Cs | Li | ||
| Blank MPP matrix | 1.8 × 10−10 | 7.9 × 10−7 | 1.5 × 10−6 | - | - | |
| 10 wt% LiCl-KCl-CsCl | MPPZ | 6.7 × 10−10 | 4.9 × 10−7 | 1.0 × 10−6 | 9.2 × 10−7 | 2.0 × 10−7 |
| MPPZ_180 °C | 1.6 × 10−9 | 7.8 × 10−7 | 1.7 × 10−6 | 1.4 × 10−6 | 2.1 × 10−7 | |
| MPPZ_450 °C | 1.2 × 10−9 | 8.3 × 10−7 | 2.2 × 10−6 | 4.2 × 10−7 | 1.2 × 10−7 | |
| 20 wt% LiCl-KCl-CsCl | MPPZ | 7.6 × 10−10 | 1.6 × 10−7 | 1.6 × 10−6 | 1.9 × 10−6 | 2.6 × 10−8 |
| MPPZ_180 °C | 1.3 × 10−9 | 2.6 × 10−7 | 2.6 × 10−6 | 2.9 × 10−6 | 3.3 × 10−7 | |
| MPPZ_450 °C | 2.8 × 10−9 | 4.1 × 10−7 | 3.7 × 10−6 | 2.1 × 10−6 | 4.0 × 10−7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. https://doi.org/10.3390/en13081963
Kulikova SA, Belova KY, Tyupina EA, Vinokurov SE. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies. 2020; 13(8):1963. https://doi.org/10.3390/en13081963
Chicago/Turabian StyleKulikova, Svetlana A., Kseniya Yu. Belova, Ekaterina A. Tyupina, and Sergey E. Vinokurov. 2020. "Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound" Energies 13, no. 8: 1963. https://doi.org/10.3390/en13081963