Comparison of Bimetallic Fe-Cu and Fe-Ca Oxygen Carriers for Biomass Gasification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe-Cu and Fe-Ca Bimetallic Oxygen Carriers
2.3. Characterization of the Oxygen Carriers
2.4. TGA Analysis of Thermal Degradation Characteristics of Biomass with Oxygen Carriers
2.5. Biomass Gasification with the Oxygen Carriers in a Bench-Scale Fixed Bed Reactor
3. Results and Discussion
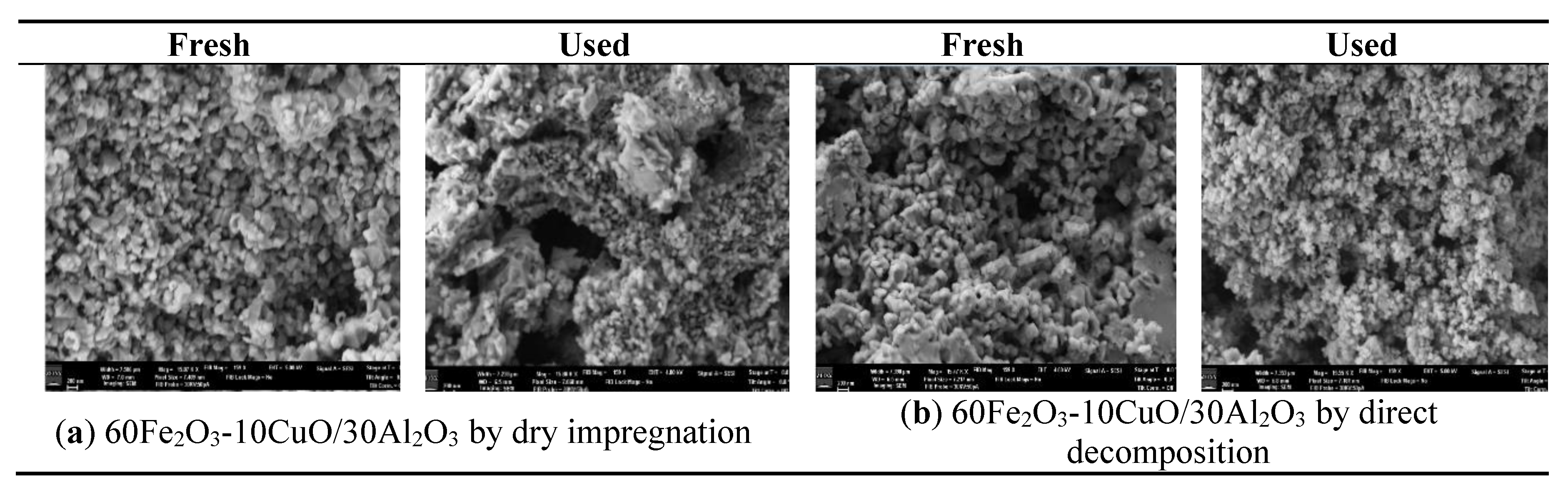
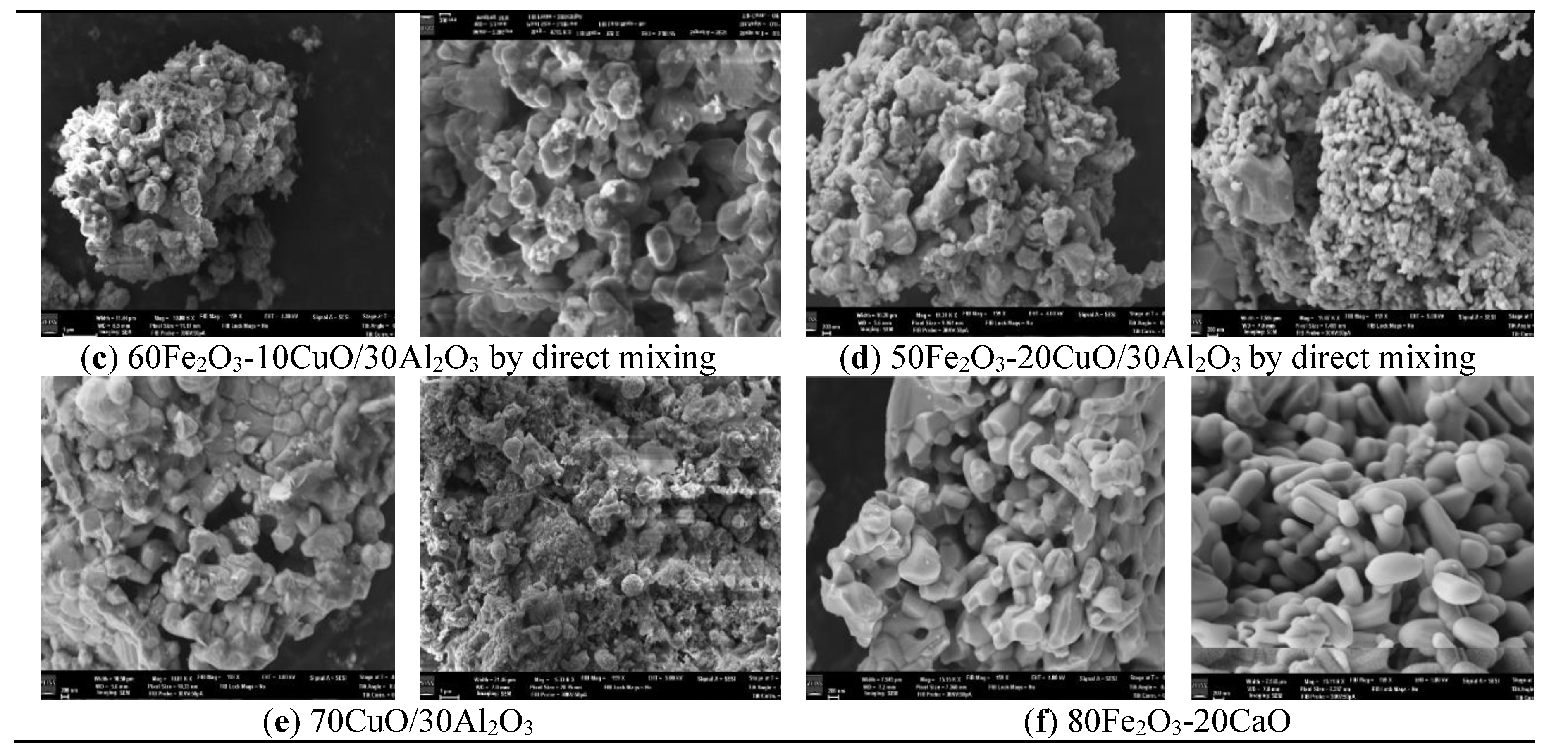
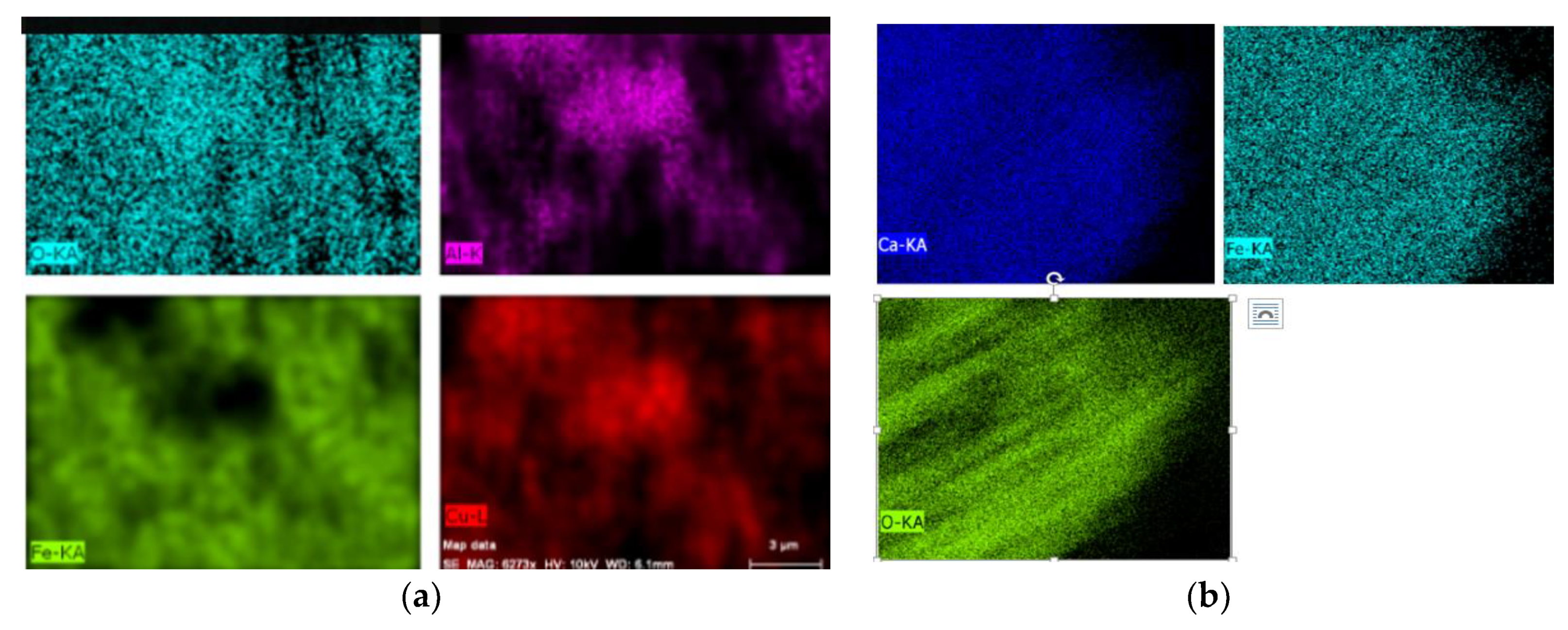
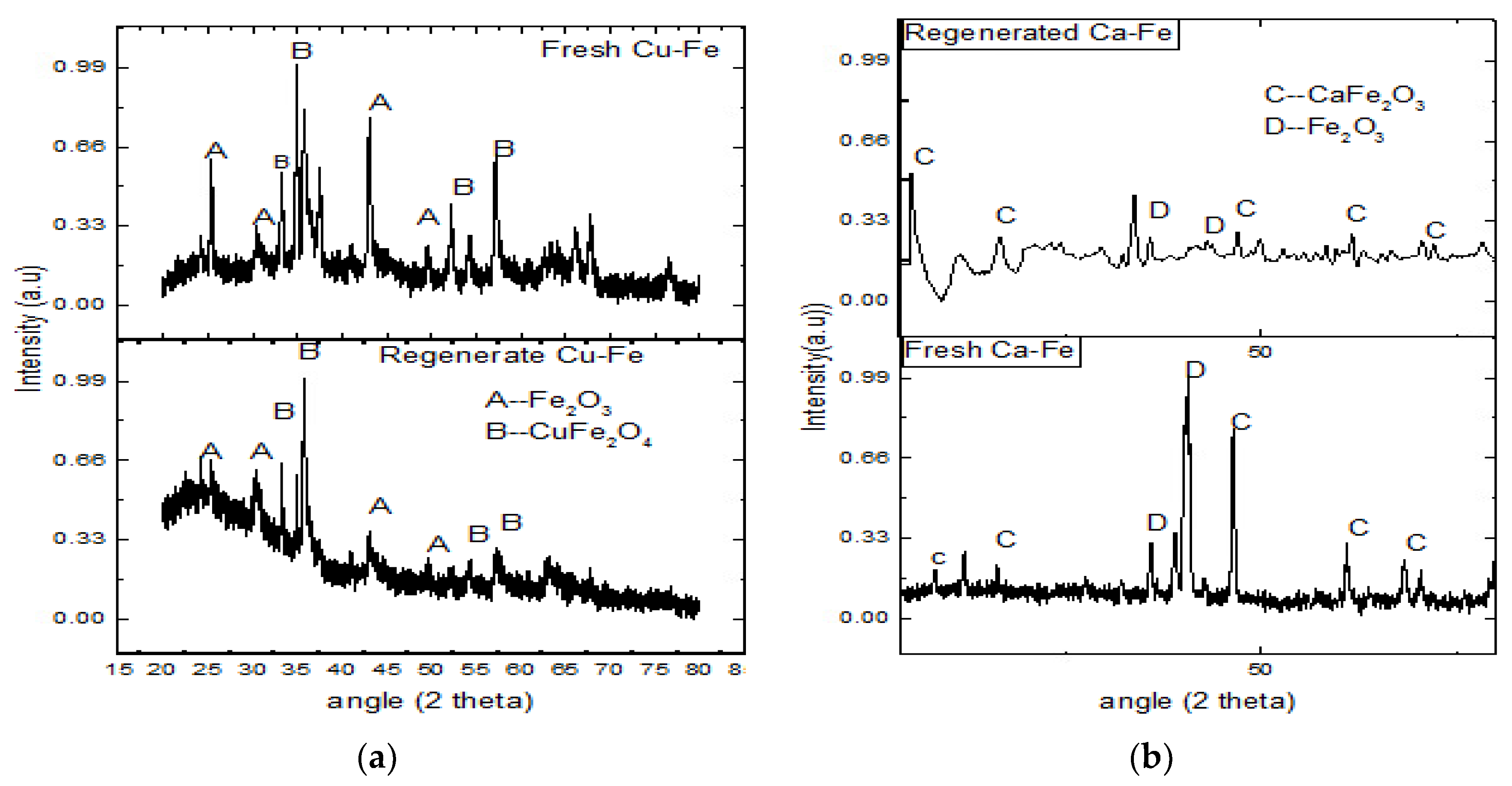
3.1. The Phase and Morphologic Properties of the Oxygen Carriers
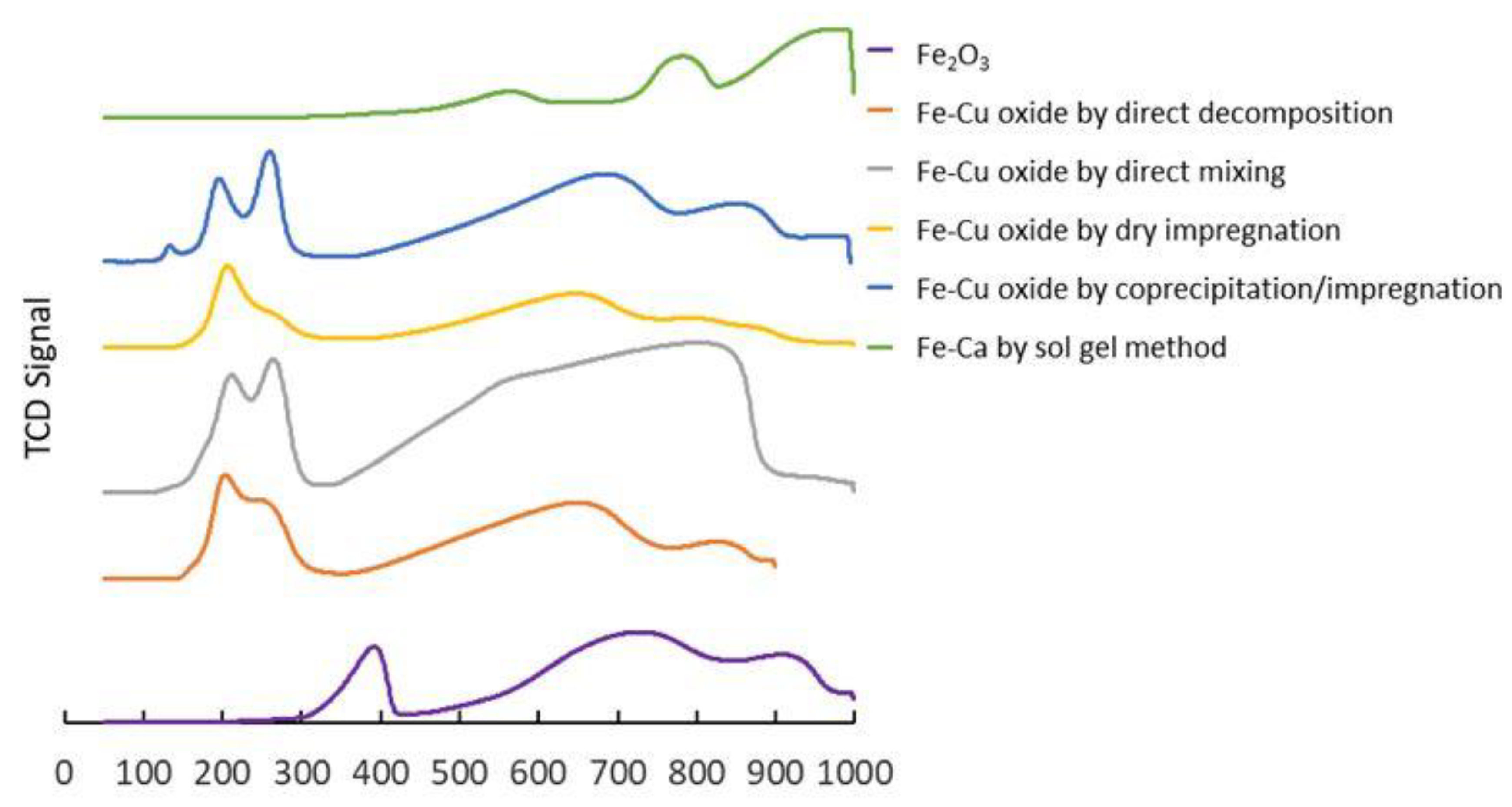
3.2. Reduction Characteristics of Oxygen Carriers
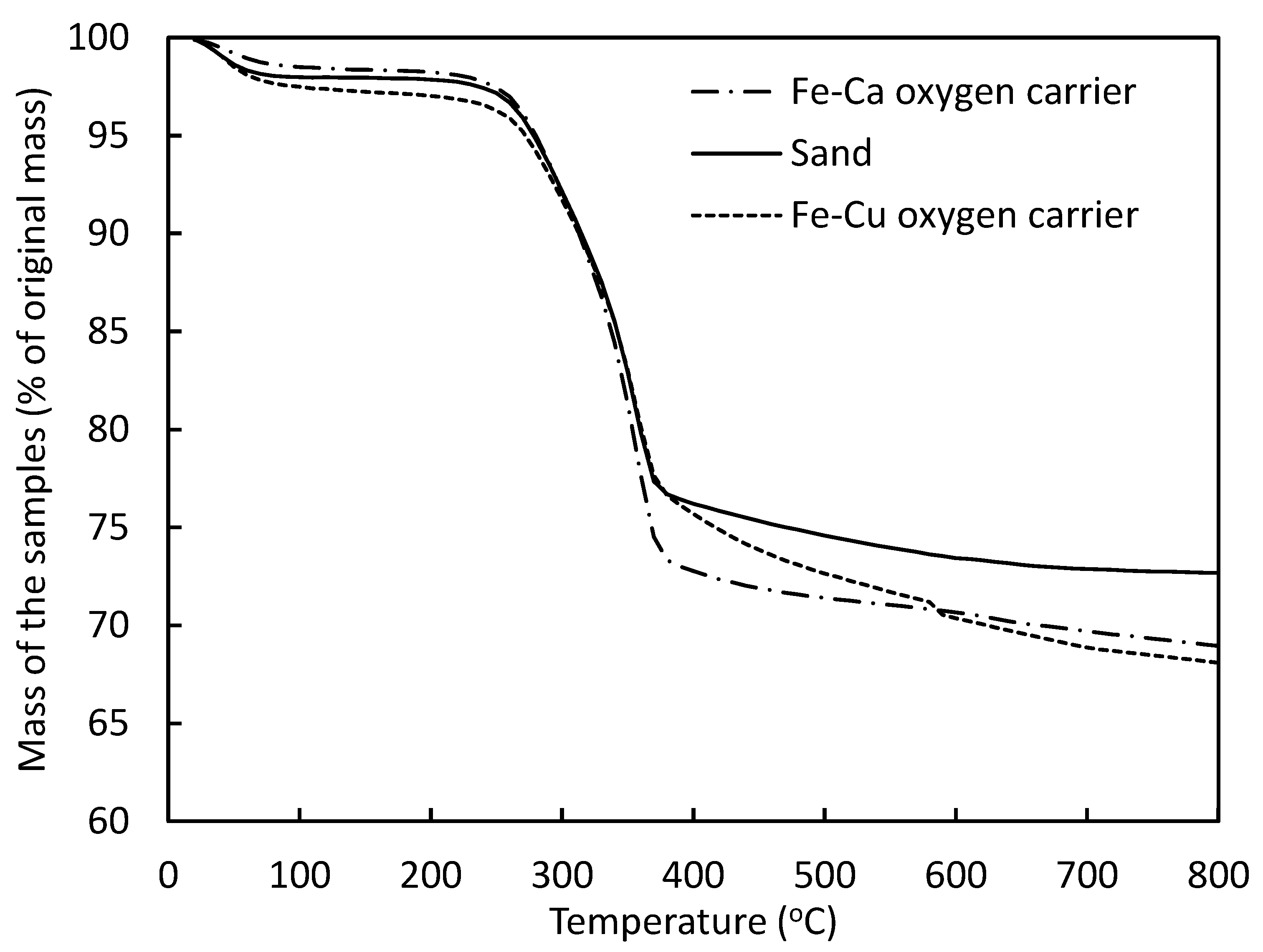
3.3. Thermal Degradation Characteristics of Biomass with Various Oxygen Carriers in a TGA
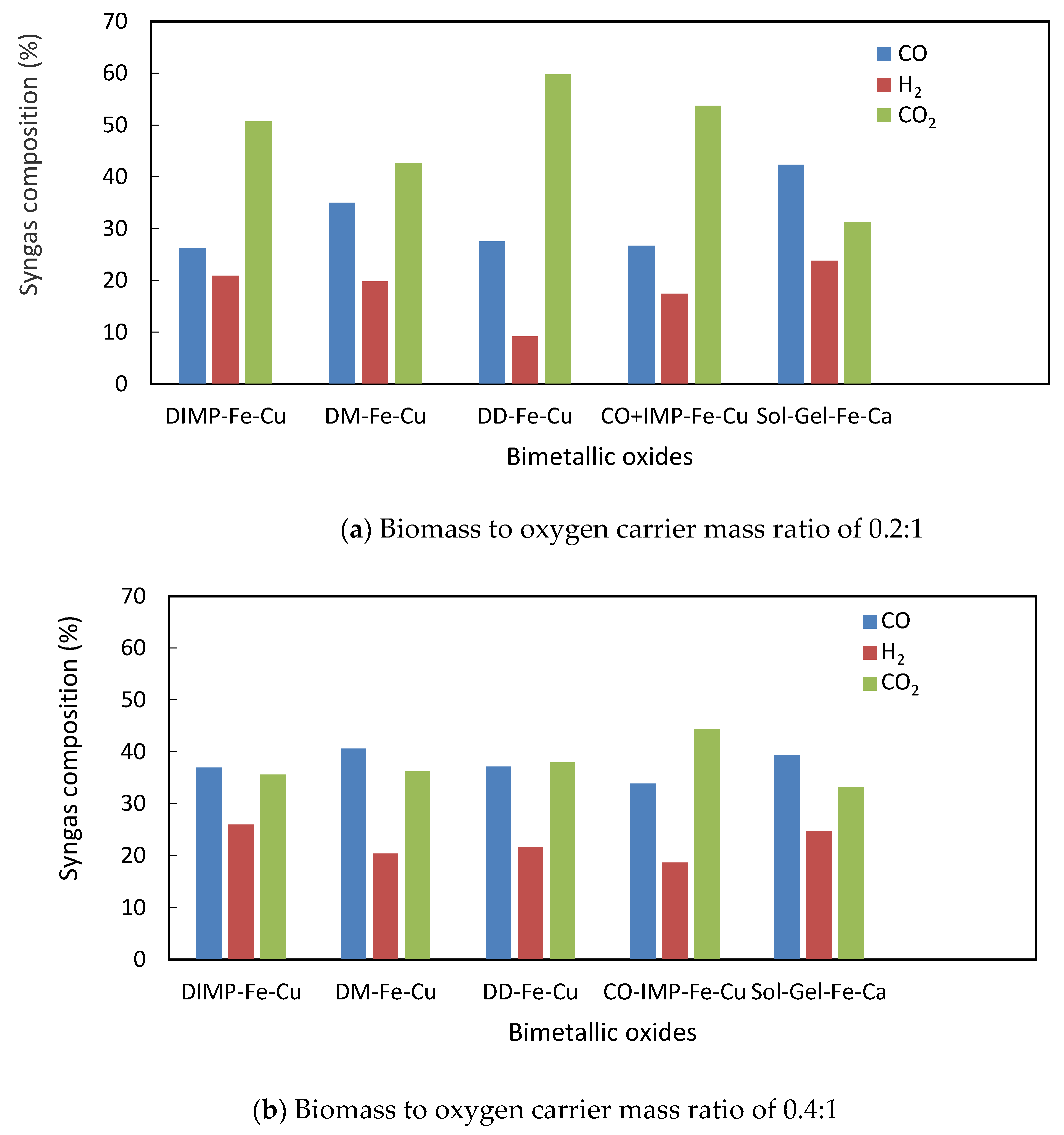
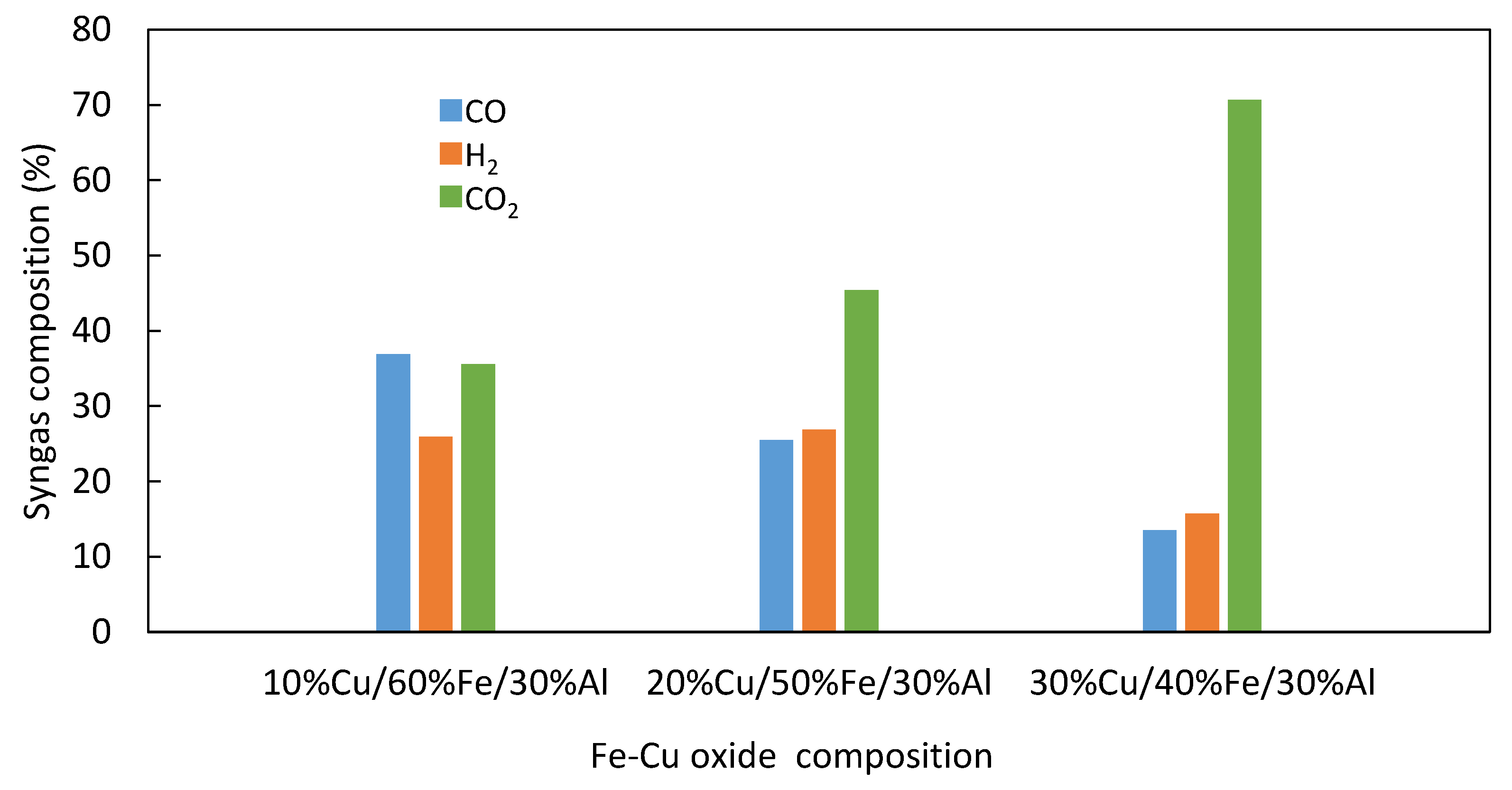
3.4. Biomass Gasification with the Oxygen Carriers in a Tubular Fixed Bed Reactor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Wang, L.J.; Weller, C.L.; Hanna, M.A.; Jones, D.D. Contemporary issues in thermal gasification of biomass and application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 1–9. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Mattisson, T.; Chen, D.; Holmen, A.; Bjørgum, E. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1− xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4. Int. J. Greenh. Gas Control 2008, 2, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Shen, L. Review of reactor for chemical looping combustion of solid fuels. Int. J. Greenh. Gas Control 2018, 76, 92–110. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Using chemical-looping with oxygen uncoupling (CLOU) for combustion of six different solid fuels. Energy Procedia 2009, 1, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of chemical looping combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Mendiara, T.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Lee, D.H.; Zheng, Y.; Zhao, H.; Zheng, C. Characterization and evaluation of Fe2O3/Al2O3 oxygen carrier prepared by sol–gel combustion synthesis. J. Anal. Appl. Pyrolysis 2011, 91, 105–113. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Fan, J.A.; Fan, L.S. New insight into the development of oxygen carrier materials for chemical looping systems. Engineering 2018, 4, 343–351. [Google Scholar] [CrossRef]
- Forero, C.R.; Gayán, P.; García-Labiano, F.; De Diego, L.F.; Abad, A.; Adánez, J. Effect of gas composition in chemical-looping combustion with copper-based oxygen carriers: Fate of sulphur. Int. J. Greenh. Gas Control 2010, 4, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Luis, F.; Garcı, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kWth chemical-looping combustor during 200 h with a CuO–Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar]
- Siriwardane, R.; Tian, H.; Simonyi, T.; Poston, J. Synergetic effects of mixed copper–iron oxides oxygen carriers in chemical looping combustion. Fuel 2013, 108, 319–333. [Google Scholar] [CrossRef]
- Siriwardane, R.; Riley, J.; Tian, H.; Richards, G. Chemical looping coal gasification with calcium ferrite and barium ferrite via solid–solid reactions. Appl. Energy 2016, 165, 952–966. [Google Scholar] [CrossRef]
- Udomsirichakorn, J.; Basu, P.; Abdul Salam, P.; Acharya, B. CaO-based chemical looping gasification of biomass for hydrogen-enriched gas production with in situ CO2 capture and tar reduction. Fuel Process. Technol. 2014, 127, 7–12. [Google Scholar] [CrossRef]

| Oxygen Carrier | Type of Oxidation | Reaction Formula (Ignore N and S in Biomass) | Mass Ratio of Biomass to Oxide |
|---|---|---|---|
| Fe2O3 | Gasification | 2.19:1 | |
| Combustion | 0.26:1 | ||
| CuO | Gasification | 1.54:1 | |
| Combustion | 0.17:1 | ||
| CaO | Gasification | 2.19:1 | |
| Combustion | 0.25:1 |
| Oxygen Carrier | Composition (wt.%) | |||
|---|---|---|---|---|
| Fe2O3 | CuO | Al2O3 | CaO | |
| 70Fe2O3/30Al2O3 | 70 | 0 | 30 | - |
| 60Fe2O3-10CuO/30Al2O3 | 60 | 10 | 30 | - |
| 50Fe2O3-20CuO/30Al2O3 | 50 | 20 | 30 | - |
| 40Fe2O3-30CuO/30Al2O3 | 40 | 30 | 30 | - |
| 70CuO/30Al2O3 | 0 | 70 | 30 | - |
| 80Fe2O3-20CaO | 80 | - | - | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriungi, B.; Wang, L.; Shahbazi, A. Comparison of Bimetallic Fe-Cu and Fe-Ca Oxygen Carriers for Biomass Gasification. Energies 2020, 13, 2019. https://doi.org/10.3390/en13082019
Muriungi B, Wang L, Shahbazi A. Comparison of Bimetallic Fe-Cu and Fe-Ca Oxygen Carriers for Biomass Gasification. Energies. 2020; 13(8):2019. https://doi.org/10.3390/en13082019
Chicago/Turabian StyleMuriungi, Beatrice, Lijun Wang, and Abolghasem Shahbazi. 2020. "Comparison of Bimetallic Fe-Cu and Fe-Ca Oxygen Carriers for Biomass Gasification" Energies 13, no. 8: 2019. https://doi.org/10.3390/en13082019




