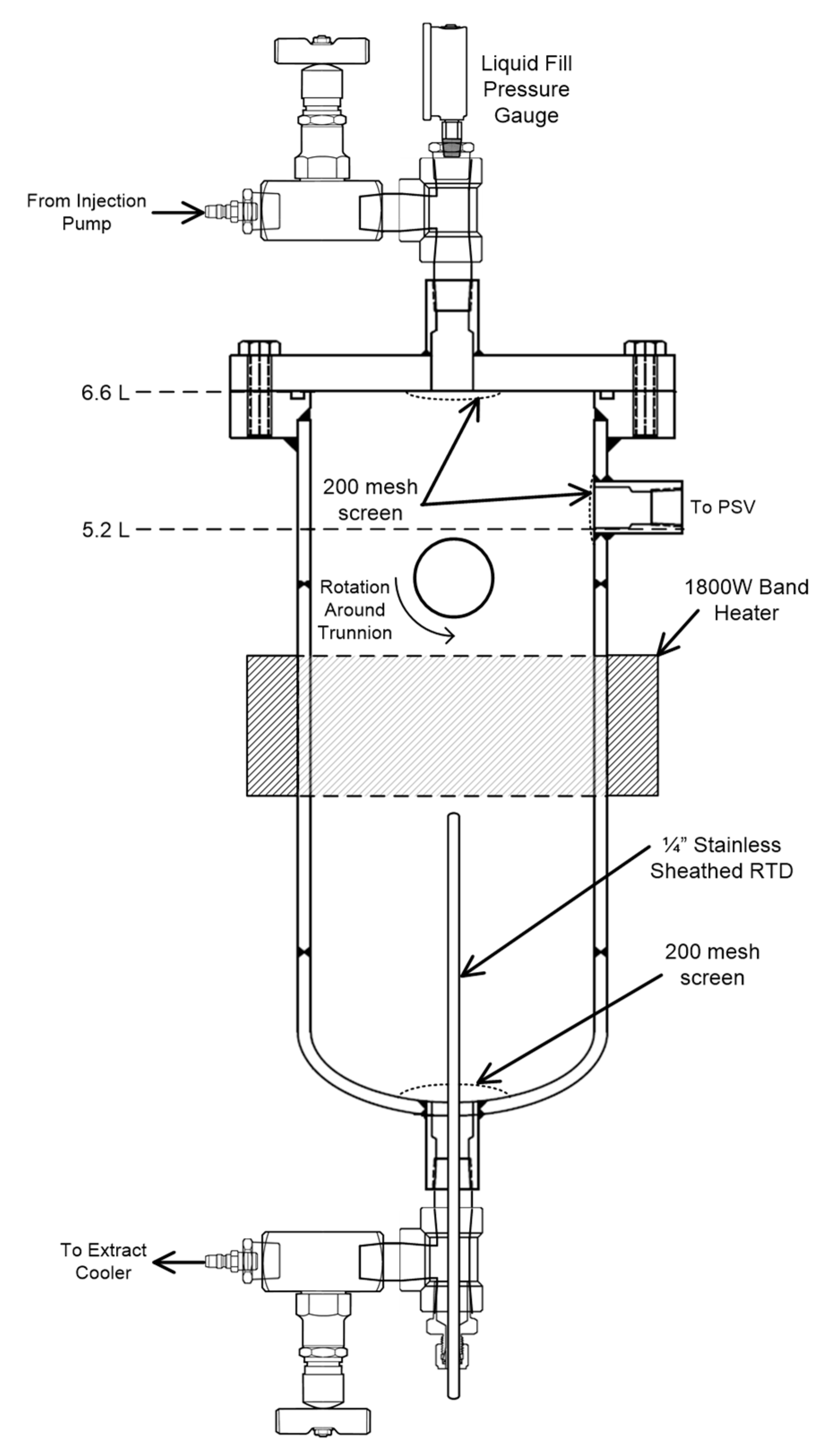
3.1. Study of Time/Temperature Effects
To develop an understanding of how WBC responds to hot water extraction, and enable comparison to other biomass types, extractions were conducted at a range of times and temperatures given in
Table 1. Higher temperatures (155 °C, 165 °C, 175 °C) were used compared to some other work [
9], as WBC has, in our experience, shown a less strong response to extraction. One of the clearest indicators of the extraction effect is the mass removal or yield. Plotted as mass removal versus time for each temperature, a set of parallel curvilinear trends are exhibited (
Figure 2a).These trends are quite similar in shape to those seen in the literature for other biomass types (e.g., sugar maple [
16]), but show a noticeably lower mass removal than seen for maple in previous studies (20.6% mass removal versus 22.3% for maple at 160 °C/120 min), or for mixed hardwoods in this study (22.4%, Table 4). It should be noted that lower mass removal for WBC compared to other hardwoods is most likely related to the lower average hemicellulose content (contributed to primarily by xylan), as indicated in
Table 3.
The mass removal data generated for WBC shows more cook to cook variability than was expected. This is likely due to the higher variability seen in the WBC, as compared to clean, debarked, and screened maple chips. As found with prior work and through analyzing data from Mittal [
14], the time/temperature data consolidates cleanly into a single curve with P-factor allowing for comparison across all conditions simultaneously (
Figure 2b), and thus all further data will be normalized to P-factor rather than to time and temperature. As HWE is generally accepted to be autocatalytic, a logistic regression (Equation (2)), as suggested by Chaffee [
9] and Cramer [
29], and is appropriate for mass removal, as well as methanol, acetic acid, and formic acid yields. These three compounds are believed to be stable under HWE conditions, and thus only formation reactions need to be considered. Loss reactions, such as those that effect furfural, would make the logistics fit inappropriate. This model has fitting parameters of X
inf and X
0 with units of the dependent variable (e.g., g/100 g wood for acetic acid), and r with units of inverse hours (inverse P-factor). The logistic fit for mass removal is shown in
Figure 2b and does appear appropriate with an R
2 of 95.5%. Additionally, residual plots (not shown) support this fit as appropriate.
The solid content of the extract is shown in
Figure 3. Some components (acetic and formic acids, furfural, methanol) are not accounted for in this measurement as they evaporate during drying. Acid salts (e.g., sodium formate), however, do remain in the extract.
Figure 3a exhibits increasing solids content to around 575 to 800 P-factor. This indicates an increase in extracted sugar and lignin in solution. At higher P-factors, the solids content begins to fall. This suggests the rate of losses to components not recovered here outpaces the rate of extraction. The losses have historically been assumed to be to low molecular weight components, particularly furans and related low molecular weight degradation products that are likely to evaporate (e.g., methylglyoxal, glycolaldehyde, etc. as per Antal [
30]). At very high P-factors, it was noted that some material coalesced and dropped out of solution as the extract cooled. While it was not possible to quantify this completely, on the cook conducted at 1500 P-factor, 1.2 g/100 g wood of this material was recovered. This indicates that losses to non-soluble precipitation—which is assumed to be due to polymerization of furans and lignin—were significant, as this is over half the total losses of solids content when comparing the 1500 P-factor and 575 P-factor data points.
Similar overall trends were present for the post-centrifugation solids concentration (
Figure 3a). This is believed to represent the solids content with most high molecular weight lignin and lignin-carbohydrate complexes removed. The post-centrifugation measurement should leave mostly sugars, ash, and organic acid salts, along with some soluble low molecular weight lignin. The difference (shown in
Figure 3b) provides an idea of the colloidal, and/or partially soluble lignin and lignin-carbohydrate complexes. The data in
Figure 3b shows significant apparent scatter. Some of this is due to the amplifying effects of the different scale, while some is likely due to the compounding of the scatter in the data for the solids content and post-centrifugation solids content. A very rough but similar trend to the solids contents can be seen regardless of this noise. The amount of centrifugable solids rises to the 500–1000 P-factor range, before falling at higher P-factors. As this material is believed to be lignin related, polymerization and loss of stability in solution is a more likely loss pathway than degradation to volatiles. This may indicate that these centrifugable components are a large source of the material noted above that dropped out of solution before solids content could be measured, and was thus not captured in the solids content.
The bulk of the solids in solution (61–83%, see Figure 5b) are in the form of sugars, which were measured using HPLC. As shown in
Figure 4a, the total sugar yield (including monomers and estimated polymer content) rises to a peak between 575–800 P-factor and then decreases, likely as the loss reaction rate increases compared to the rate of sugar release. The rate of sugar release would be expected to drop as the potentially available sugars from the hemicellulose fraction become depleted. Monomer yield peaks closer to 1000 P-factor, as shown in
Figure 4b. Monomeric sugar yield does not drop significantly at higher P-factors, suggesting hydrolysis of polymers to monomers and losses to furans potentially balance out to the highest P-factor used in this study (1935).
Breaking the total monomer plot from
Figure 4b into individual sugars provides some further information, as shown in
Figure 5a. It is clear that xylose rapidly becomes the dominant monomer, as would be expected, as the WBC hemicelluloses are composed primarily of xylan (
Table 3). Arabinose yields initially rise quickly compared to xylose then drop off, suggesting the arabinose in the willow is more readily hydrolysable from the hemicelluloses than the xylan, and that the reservoir of hydrolysable arabinose units in the polymers is rapidly depleted. The glucose yields start close to their peak (about 0.4 g/100 wood) and plateau at 0.55 g/100 g wood beginning at around the 600 P-factor. HMF generation (shown in
Figure 6b) suggests that there is some degradation of glucose and mannose. The relatively small amount of glucose (even including that converted to HMF) indicates that cellulose degradation is minimal with glucose likely being derived from glucomannan, as seen by Mittal [
14] in work on maple. It is also instructive to compare the total sugar (
Figure 4a) and solids contents (
Figure 3a) data trends. This comparison, displayed as total sugar as a percentage of total solids, is shown in
Figure 5b. The resulting plot shows that, despite similar curve shapes, there is a significant swing in what portion of the solids in solution is sugars. As sugars are a preferred product,
Figure 5b indicates a preferred P-factor range of 575–900, with some indication that lower temperatures (155 °C and 160 °C) may provide a small but significant advantage.
In HWE, furan generation is a factor needing consideration, as past work has shown a positive correlation of furan generation with HWE severity [
14], and furan production affects process value. Though they have considerable commercial value, furans are not an ideal product to generate during HWE, as the conditions prevalent in HWE are likely to result in selectivity under 50% (high losses) [
31]. As shown in
Figure 6, both furfural and HMF generation show a positive and linear response to P-Factor (R
2 of 98.9% for furfural, and 97.6% for HMF). As furfural is likely to be fairly stable once formed under these conditions [
32], the furfural yield likely represents primarily formation, with losses after formation being less than 20%, even at 1900 P-Factor. The HMF generation represents more of a balance of loss and generation reactions. The linearity in HMF yield is surprising, considering that it comes from hexose sugars (with relatively flat concentrations, as per
Figure 5a) and that it degrades much more rapidly than furfural.
Three other compounds of note are generated during HWE: acetic acid, formic acid, and methanol. Organic acids were measured both by HPLC (formic and acetic acids, specifically) and by direct titration (all free acids totaled). As shown in
Figure 7, both formic and acetic acid yields rise before plateauing, with a logistic (autocatalytic) model explaining the patterns effectively (R
2 of 98.2% for acetic acid and 92.5% for formic acid). Acetic acid is known to come from acetate groups on the hemicelluloses, and is thus a limited reservoir of acetate is available for release, with the logistics fit suggesting a value of 3.8 g/100 g wood being the total reservoir. This would be on the high side of average for a range of hardwoods (3.6 g/100 g, [
18]), but is reasonable and compares well with the slightly higher acetate content for WBC, as shown in
Table 3.
Formic acid yields show a similar trend to acetic acid (
Figure 7b), though unlike acetic acid, its source is not well understood. Some is certainly the result of the losses associated with degradation of hexoses to HMF and subsequent degradation of HMF [
33]. Other sources are currently unknown. The shape of the formic acid plot and reasonable fit with a logistics model suggests that some reservoir or source of formic acid exists that is being depleted, which does not clearly fit with the source of this material being hexose sugars and related degradation. Some un-identified secondary source of formic acid seems likely. An illuminating point becomes apparent when comparing the total of the acetic and formic acids with apparent quantity of acid indicated by titration (converted to mol/100 g wood,
Figure 7c). There appears to be a consistent gap between the titrated and HPLC acid concentrations, which is in fact the case, as shown in the “difference” series in
Figure 7c. This difference averages 0.017 mol/100 g wood, and has a coefficient of variation of only 9%. As the HPLC method would convert any acid salts (e.g., potassium acetate) to free acid (potassium sulfate and acetic acid), it seems that this difference is likely due to soluble acetate and formate salts of ash removed from the wood (e.g., sodium, potassium, calcium, magnesium, etc.). This constant quantity of organic acid salts suggests that effectively all ash removal (a known effect of HWE, though not one measured here) occurs before 230 P-factor. Finally, methanol is a known product of HWE, and is generally assumed to be lignin or glucuronic acid derived. Methanol generation in HWE also appears to fit a logistic model, as shown in
Figure 7d. The plateau at higher P-factors, along with the logistic fit, seems to indicate a reservoir of methanol source material that is depleted by the extraction, with a maximum available quantity of 0.83 g/100 g wood.
As the primary purpose of HWE is to extract valuable products and upgrade and reduce the variability of the quality of the biomass, the preferred conditions for HWE are those that recover the most value across the processed biomass and the recoverable products. Sugars, lignin, acetic acid, formic acid, furfural, and methanol are all intended products of HWE biorefining. To maximize sugar recovery, a P-factor in the 575–800 range appears to be preferred. At the 575 P-factor, 85% of the potential methanol, 77% of the potential formic acid, and 37% of the available acetic acid are already in the solution. The maximum quantity of sugar (or very close to it) and overall solids are in solution, and losses of sugars to furans and other compounds is low. Further mass removal beyond 575 P-factor likely does not result in increased sugar yield and will overall reduce the mass available in the biomass stream for other products, suggesting the lower end of the 575–800 P-factor range is preferable. Reduced P-factor also has the advantage of reducing extractor vessel size or lowering reaction temperature, either of which has value in industrial practice. The 575 P-factor condition appears to be a reasonable maxima for overall value, and aligns with the 160 °C/120 min at temperature condition used as a preferred condition in other work [
9]. It should also be noted that, though the acetic acid recovery is low at this condition, work by Mittal [
14] suggests that a considerable portion of the available acetate may in fact be in bound form still attached to polymeric hemicellulose sugars in solution. The 575 P-Factor (160 °C, 120 min) condition is used for the experiments in
Section 3.2 and
Section 3.3 as being representative of likely industrial conditions.
3.2. Short Rotation Willow and Mixed Hardwood Combined Extraction
As it is generally expected that biorefineries and other biomass consuming facilities would use a mix of WBC and conventional wood chips from forestry operations, the effect of mixing these raw materials is potentially quite important. To study this, extractions were conducted on three mixes of WBC and mixed hardwoods, as well as pure samples of WBC and pure MHW. The 575 P-Factor (160 °C, 120 min) condition was used, as noted above. These extractions were compared to determine if there were unexpected advantages or disadvantages as a result of the mixed feedstock. It is instructive to begin with a comparison of the pure WBC and pure MHW samples, as shown in
Table 4. Most values, including mass removal, solids content, and sugar yield, were noticeably lower for WBC than for MHW, as would be expected from the estimated compositions given in
Table 3. Organic acids, methanol, and HMF yields all were higher for WBC. The higher acetic acid yield is consistent with the higher average acetate content of WBC compared to the MHW feedstocks, as shown in
Table 3. Formic acid yield rises similarly, though to a larger extent than for acetic acid. The source of this is unclear, but parallels an increase in HMF yield. This may indicate that, counter to the indications discussed earlier, the main source of formic acid may in fact be hexose sugar degradation. The higher pH combined with the higher acid content suggests more buffering, likely from dissolved ash in the extract. Overall, this suggests somewhat less value could be derived (in sugars and lignin) from willow than for mixed hardwoods, though more processed biomass is available for other applications (e.g., pellets), along with more value from acetic acid, formic acid, and methanol.
While HWE of feedstocks containing increasing quantities of WBC yields lower quantities of sugar and lignin, the potentially lower value of the co-product suite needs to be viewed in its overall context. A potential advantage of willow biomass crops is their high yields, very short rotations, and coppice management, where the plants resprout and grow after each harvest. Yields can be an order of magnitude greater than natural forests in the region and can use a wide range of open land. This creates opportunities for feedstock for a given size of biorefinery to be generated on a much smaller land base, and in much closer proximity to the facility. Both of these could contribute to lower feedstock costs. In addition, the biomass produced from willow crops can have a negative carbon footprint [
34], which would impact the overall carbon footprint of this system. Ultimately, the economic and environmental impact of different proportions of willow in the feedstock should be assessed using tools like life cycle assessment and techno-economic analysis across the entire system.
Extractions of mixes of MHW and WBC generally showed the expected linear relationship between the WBC fraction and each outcome, as shown in the plots in
Figure 8. While noisy, mass removal (
Figure 8a) is roughly linear with willow percentage. Most other outcomes have less variation, with the linearity of the extract solids and sugar contents suggesting the roughness in the mass removal is probably noise. While linearity seems to be lacking in the methanol plot (
Figure 8e), methanol was generally the noisiest of the HPLC measurements, due to a combination of low concentrations and non-zero baseline. As seen in the P-factor studies in
Figure 3b, centrifugable solids (solids content minus post-centrifugation solids content) data had considerable scatter, but for the WBC/MHW extractions shows a slight downward trend in centrifugable solids with increasing WBC. The average lignin content of the WBC is slightly lower than that of the MHW feedstock (
Table 3), indicating the likely source of this difference.
3.3. Water-To-Wood Ratio and Other Variables
HWE processing has historically been assumed to be unaffected by the water-to-wood ratio, in terms of mass removal and yield of recovered products [
23]. However, unpublished research on extraction of non-woody materials suggests that the water-to-wood ratio may affect multiple extraction outcomes. Additionally, developing a better understanding of the effects of presteaming and counter-current extraction are of significant interest for industrial applications. As laboratory analyses of these techniques result in water-to-wood ratios that are not consistently 4:1, these tests will be compared with the water-to-wood ratio trends, rather than the base 4:1 data points. To enable these comparisons, logarithmic curve fits have been used to approximate the diffusion effects of higher water-to-wood ratios, and 95% confidence intervals have been plotted around them (
Figure 9). This is intended to give an approximate indication of the significance of any differences between the base water-to-wood ratio data set and the presteamed extractions and the counter-current simulation extractions.
As in the P-factor studies presented above, mass removal and solids content are the first indicators of the effect of water-to-wood ratio.
Figure 9a demonstrates that changes are clear, with mass removal rising considerably as water-to-wood ratio is increased, from an average of 19.1% at 3:1, through 20.6% at 4:1, to 23.3% at 10:1. This is a considerable range (equivalent to decreasing P-factor by 100 points, and increasing it by 240 points respectively) around the 4:1 base value and indicates that there are important changes resulting from differing water-to-wood ratios. This indication is supported by a similar rise in extract solids yield across this range (
Figure 9b), from an average of 12.7 g/100 g wood at 3:1 to 16.2 g/100 g wood at 10:1. The increase in solids yield is more rapid than the increase in mass removal, with the recoverable solids (solids content/mass removed) increasing from 66% to 70%. Total sugar yields (
Figure 9c) also rise with increasing water-to-wood ratio, as do monomer yields (
Figure 9d), with monomers increasing as a proportion of total sugars. This increased percentage may be due to lower furan generation, as shown in
Figure 9e. This shows a 33% reduction in furfural generation, compared to the 24% increase in monomer yield. In absolute terms, the furfural not generated accounts for approximately 50% of the increase in monomer yield. Under the conditions of HWE, 50% selectivity (molar yield) would not be surprising, indicating that reduced losses may be the primary source of the extra monomer.
HMF yields showed the opposite trend (
Figure 9f), rising with increasing water-to-wood ratio. While this would seem directly counter to the discussion above, this may suggest that HMF concentrations are more controlled by loss reactions than by formation reactions. Loss reactions would be slowed significantly by the reduced acid concentrations and reduced HMF concentration resulting from the dilution effects of increased water-to-wood ratio. This appears to have a larger effect than the lower formation rates that would be expected from the lower absolute (g/L) hexose sugar concentrations. Acetic acid recoveries responded unexpectedly as well, staying effectively flat across the range of water-to-wood ratios tested. This indicates that diffusion pressures are rather un-important in the reactions generating acetic acid, also suggesting that acid concentration is less important than acetate available for hydrolysis. Formic acid recoveries follow the same trend as monomeric sugar yield, and rise by an almost identical proportion (23.3% for formic acid, 24% for monomer sugars). This may indicate that the main source of formic acid is in fact hexose sugar degradation. Methanol yield did decrease ~27% across this range, but with so much noise that it is difficult to clearly interpret.
The other modified extractions tested all resulted in varying effective water-to-wood ratios. As the water-to-wood ratios seen in these extractions did not align directly with the water-to-wood ratios used in the water-to-wood ratio study, comparisons are to the trend lines identified (and their 95% confidence interval) to indicate if differences are significant. Presteaming is an important tool in industrial pulping practice to remove air from chips, pre-heat them, and improve penetration of pulping chemicals. As shown in
Figure 9 (the orange + symbols) presteaming seems to have neither significant advantages nor disadvantages for HWE. This is also true with methanol (not shown), for which no change is apparent. The presteaming utilized here (15 min at 15 PSI) is quite aggressive, with 1–5 min at 15 psi being a common range in this team’s experience. This indicates that for industrial applications presteaming can be tailored to operating needs (e.g., air removal and preheating) without concern for changing downstream process chemistry.
Continuous counter-current extraction appears to be a highly attractive technique for commercial applications: providing washing and potentially yield improvements if advantageous diffusion pressures can be harnessed. While laboratory scale testing cannot accurately duplicate the chemistry in a continuous extractor, by breaking a cook into stages, we can to some extent simulate the stronger diffusion effects (lower product concentrations) that would be seen in this format, as compared to batch cooking. The 2 and 3 stage cooks were intended to simulate this effect to different extents, giving a two-tier indication of the scale of this effect. As demonstrated in
Figure 9a, the mass removal is not significantly impacted by multi-stage cooking (beyond the simple dilution effect), while solids (
Figure 9b) and sugar yields (
Figure 9c) rise significantly. The rise in sugars was primarily due to an increase in polymeric sugars, with monomeric sugar yield (
Figure 9d) not changing significantly. Furan generation drops significantly (
Figure 9e,f), likely due to the lesser maximum time (½ or ⅓ that of a batch cook to the same P-factor), and an average quantity of monomeric sugar was held at temperature. Acetic acid yields (
Figure 9g) may have dropped slightly, though not enough to be clearly differentiated from background noise. A drop in free acetic acid (as compared to bound acetate, which was not measured in this study) would make sense, paralleling the effect seen with monomer above. Formic acid yield did not change at all with the multi-stage cook, and as with presteaming, no change in methanol yield could be clearly separated from noise. The changes in solids, sugars, and furans all suggest significant potential benefits to counter-current extraction.