Co-Gasification of Treated Solid Recovered Fuel Residue by Using Minerals Bed and Biomass Waste Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Properties of Feedstocks
2.3. Experimental Method
2.4. Tar and Gas Pollutants Sampling Methods
3. Results and Discussion
3.1. TSRFR Gasification
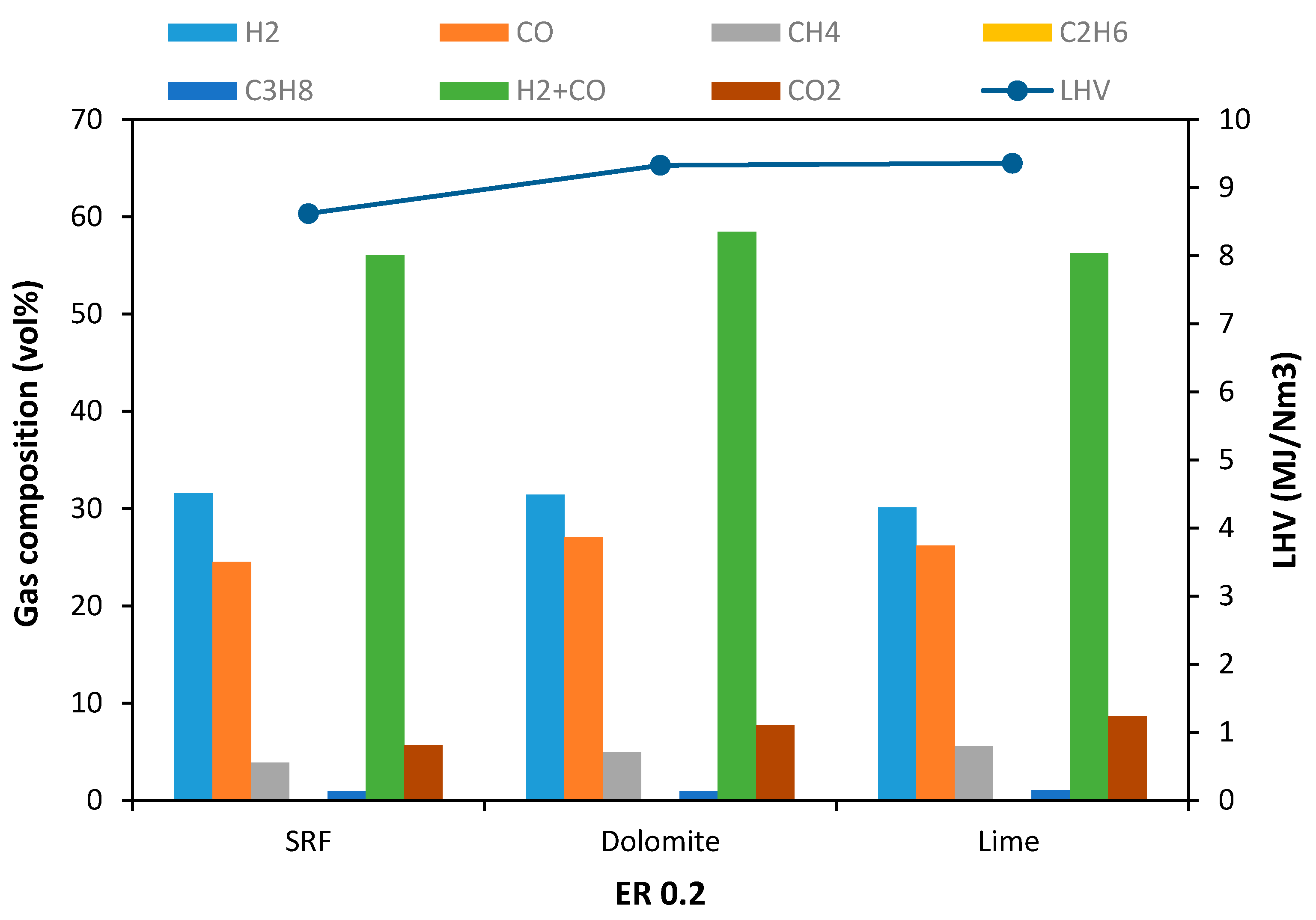
3.2. TSRFR Gasification Using Low-Cost Minerals as Bed Material
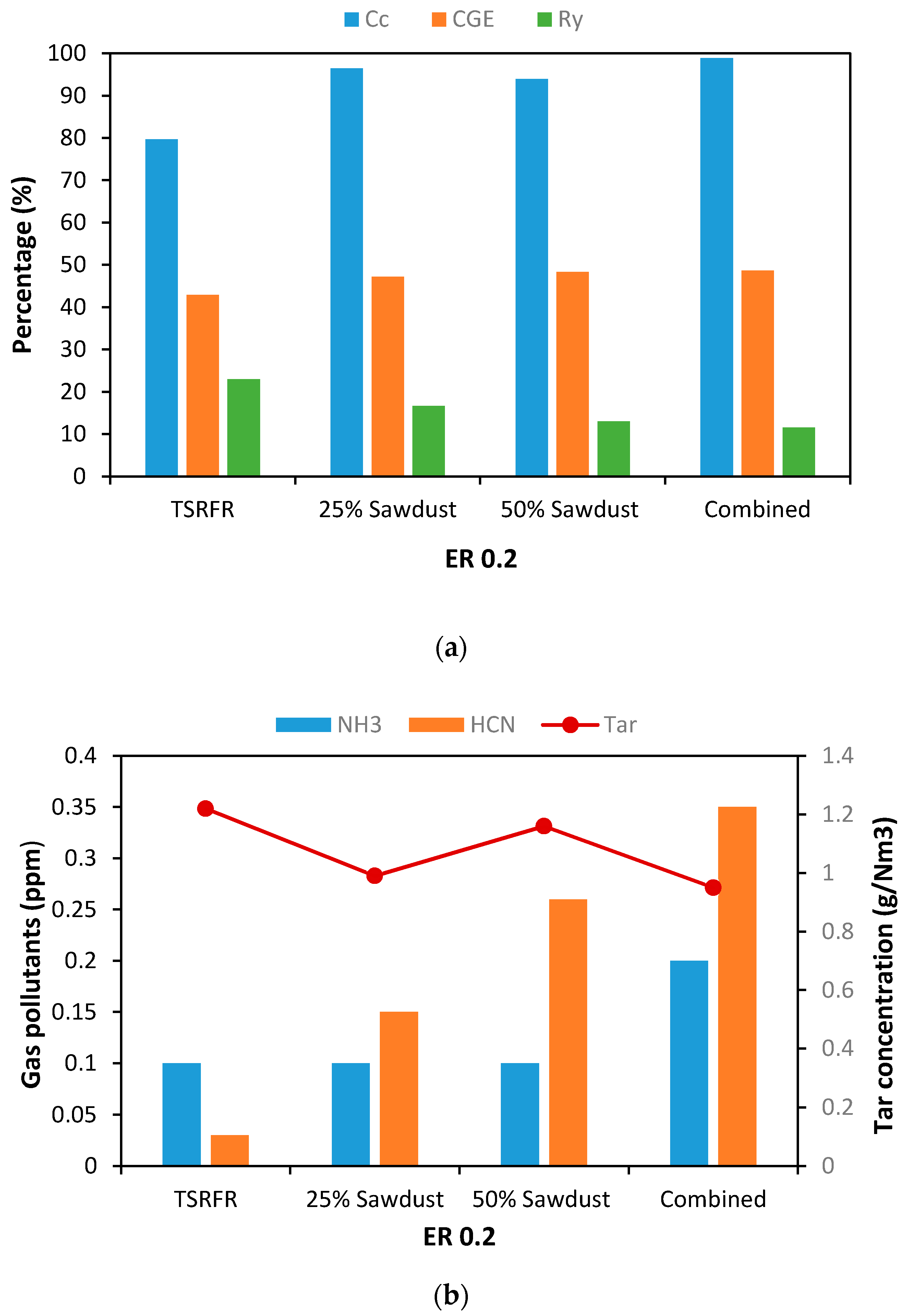
3.3. Co-Gasification of TSRFR and Biomass Waste Blends
4. Conclusions
- (i)
- For TSRFR gasification, ER 0.2 was found as the most suitable condition to gasify. At ER 0.2, the maximum syngas yield with the highest LHV was obtained. Also, the highest CGE and very high Cc was found at ER 0.2, and the concentration of tar and gas pollutants were found to be very low in this condition.
- (ii)
- The use of dolomite and lime minerals as bed materials successfully increased the syngas yield, LHV of product gas, CGE, and Cc. Uses of dolomite and lime also successfully decreased the amount of Ry and tar concentration. In addition, a low amount of gas pollutants was obtained in the product gas. Overall, dolomite showed comparatively better performance than lime.
- (iii)
- In the case of co-gasification of TSRFR with biomass waste blends, both blends (25 wt% sawdust, 50 wt% sawdust) showed excellent performance. After using biomass blends, Gy, syngas yield, LHV of product gas, CGE, Cc increased, and Ry decreased. Concentrations of tar and gas pollutants were deficient in both cases. Overall, the 25 wt% sawdust blends showed a better performance compared with 50 wt% sawdust.
- (iv)
- In an experiment conducted using 25 wt% sawdust blends with TSRFR as a feedstock and dolomite as the bed material, the highest syngas yield with the highest LHV of product gas was obtained. Moreover, CGE, Cc, and residue yield showed the highest performance, and tar and gas pollutants concentration were found very low in the product gas. Considering all the facts, it can be concluded that TSRFR blended with 25 wt% pine sawdust and accompanied by dolomite as bed material was found as the most viable condition for TSRFR gasification.
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.-W.; Lee, J.-S.; Yang, W.-S.; Alam, M.T.; Seo, Y.-C. A Comparative Study of the Gasification of Solid Refuse Fuel in Downdraft Fixed Bed and Bubbling Fluidized Bed Reactors. Waste Biomass Valorization 2020, 11, 2345–2356. [Google Scholar] [CrossRef]
- Alam, M.T.; Lee, J.-S.; Lee, S.-Y.; Bhatta, D.; Yoshikawa, K.; Seo, Y.-C. Low Chlorine Fuel Pellets Production from the Mixture of Hydrothermally Treated Hospital Solid Waste, Pyrolytic Plastic Waste Residue and Biomass. Energies 2019, 12, 4390. [Google Scholar] [CrossRef] [Green Version]
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-S.; Yang, W.-S.; Alam, M.T.; Seo, Y.-C.; Lee, S.-Y. Gasification characteristics of biomass for tar removal by secondary oxidant injection. J. Mater. Cycles Waste Manag. 2018, 20, 823–831. [Google Scholar] [CrossRef]
- Bourtsalas, A.T.; Seo, Y.; Alam, M.T.; Seo, Y.-C. The status of waste management and waste to energy for district heating in South Korea. Waste Manag. 2019, 85, 304–316. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012; Volume 15. [Google Scholar]
- Yang, W.-S.; Park, J.-K.; Park, S.-W.; Seo, Y.-C. Past, present and future of waste management in Korea. J. Mater. Cycles Waste Manag. 2015, 17, 207–217. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Gasification of a solid recovered fuel in a pilot scale fluidized bed reactor. Fuel 2014, 117, 528–536. [Google Scholar] [CrossRef]
- Seo, Y.-C.; Alam, M.T.; Yang, W.-S. Gasification of Municipal Solid Waste. In Gasification for Low-Grade Feedstock; IntechOpen: London, UK, 2018; pp. 115–141. [Google Scholar]
- Tchapda, A.H.; Pisupati, S.V. A review of thermal co-conversion of coal and biomass/waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef] [Green Version]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. From coal to biomass gasification: Comparison of thermodynamic efficiency. Energy 2007, 32, 1248–1259. [Google Scholar] [CrossRef]
- Guan, Y.; Luo, S.; Liu, S.; Xiao, B.; Cai, L. Steam catalytic gasification of municipal solid waste for producing tar-free fuel gas. Int. J. Hydrog. Energy 2009, 34, 9341–9346. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.; Yang, W.; Kang, J.; Sung, J.; Alam, M.T.; Seo, Y.; Rao, C.S.; Saravanakumar, A.; Kumar, K.V. For Waste to Energy, Assessment of Fluff Type Solid Refuse Fuel by Thermal Characteristics Analyses. Procedia Environ. Sci. 2016, 35, 498–505. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Yang, W.; Kang, J.; Alam, M.; Seo, Y.; Oh, J.; Gu, J.; Chennamaneni, S.; Kandasamy, V. Emission Characteristics of Gaseous Pollutants from Pilot-Scale SRF Gasification Process. In Waste Management and Resource Efficiency; Springer: Berlin/Heidelberg, Germany, 2019; pp. 51–58. [Google Scholar]
- Dunnu, G.; Panopoulos, K.; Karellas, S.; Maier, J.; Touliou, S.; Koufodimos, G.; Boukis, I.; Kakaras, E. The solid recovered fuel Stabilat®: Characteristics and fluidised bed gasification tests. Fuel 2012, 93, 273–283. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, J.S.; Park, S.W.; Kang, J.J.; Alam, M.T.; Seo, Y.C. Studies on combustion characteristics of low calorific waste generated in SRF manufactory by mechanical biological treatment (MBT). In Proceedings of the 3rd 3R International Scientific Conference on Material Cycles and Waste Management, Hanoi, Vietnam, 9–11 March 2016. [Google Scholar]
- Energy Recovery Policy. Available online: http://eng.me.go.kr/eng/web/index.do?menuId=144 (accessed on 15 November 2019).
- André, R.N.; Pinto, F.; Franco, C.; Dias, M.; Gulyurtlu, I.; Matos, M.; Cabrita, I. Fluidised bed co-gasification of coal and olive oil industry wastes. Fuel 2005, 84, 1635–1644. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Pazo, A.; López, J. Gasification and co-gasification of biomass wastes: Effect of the biomass origin and the gasifier operating conditions. Fuel Process. Technol. 2008, 89, 828–837. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Perdikaris, N.; Boukis, I. Gasification improvement of a poor quality solid recovered fuel (SRF). Effect of using natural minerals and biomass wastes blends. Fuel 2014, 117, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.; van Paasen, S.V.; Bergman, P.C.; Kiel, J.H. Catalytic decomposition of biomass tars: Use of dolomite and untreated olivine. Renew. Energy 2005, 30, 565–587. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Horio, M.; Tangsathitkulchai, C. Effects of gasifying conditions and bed materials on fluidized bed steam gasification of wood biomass. Bioresour. Technol. 2009, 100, 1419–1427. [Google Scholar] [CrossRef]
- Jo, M.-H.; Lee, B.-J.; Lee, J.-Y. Effect of modified mechanical treatment facilities on SRF yield in Korea. Sci. J. Riga Tech. Univ. Environ. Clim. Technol. 2013, 12, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S.; Choi, H.S. A study on torrefaction characteristics of waste sawdust in an auger type pyrolyzer. J. Mater. Cycles Waste Manag. 2016, 18, 460–468. [Google Scholar] [CrossRef]
- Chen, G.; Leung, D. Experimental investigation of biomass waste, (rice straw, cotton stalk, and pine sawdust), pyrolysis characteristics. Energy Sources 2003, 25, 331–337. [Google Scholar] [CrossRef]
- Park, H.J.; Park, Y.-K.; Kim, J.S. Influence of reaction conditions and the char separation system on the production of bio-oil from radiata pine sawdust by fast pyrolysis. Fuel Process. Technol. 2008, 89, 797–802. [Google Scholar] [CrossRef]
- Kathirvale, S.; Yunus, M.N.M.; Sopian, K.; Samsuddin, A.H. Energy potential from municipal solid waste in Malaysia. Renew. Energy 2004, 29, 559–567. [Google Scholar] [CrossRef]
- Cozzani, V.; Nicolella, C.; Petarca, L.; Rovatti, M.; Tognotti, L. A fundamental study on conventional pyrolysis of a refuse-derived fuel. Ind. Eng. Chem. Res. 1995, 34, 2006–2020. [Google Scholar] [CrossRef]
- Yang, W.-S.; Lee, J.-S.; Park, S.-W.; Kang, J.-J.; Alam, T.; Seo, Y.-C. Gasification applicability study of polyurethane solid refuse fuel fabricated from electric waste by measuring syngas and nitrogenous pollutant gases. J. Mater. Cycles Waste Manag. 2016, 18, 509–516. [Google Scholar] [CrossRef]
- Son, Y.-I.; Sato, M.; Namioka, T.; YOSIKAWA, K. A study on measurement of light tar content in the fuel gas produced in small-scale gasification and power generation systems for solid wastes. J. Environ. Eng. 2009, 4, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Rezaiyan, J.; Cheremisinoff, N.P. Gasification Technologies: A Primer for Engineers and Scientists; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Guo, X.; Xiao, B.; Liu, S.; Hu, Z.; Luo, S.; He, M. An experimental study on air gasification of biomass micron fuel (BMF) in a cyclone gasifier. Int. J. Hydrogen Energy 2009, 34, 1265–1269. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.D.; Hanna, M.A. Steam–air fluidized bed gasification of distillers grains: Effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef]
- Cho, S.-J.; Jung, H.-Y.; Seo, Y.-C.; Kim, W.-H. Studies on gasification and melting characteristics of automobile shredder residue. Environ. Eng. Sci. 2010, 27, 577–586. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Brage, C.; Yu, Q.; Chen, G.; Sjöström, K. Tar evolution profiles obtained from gasification of biomass and coal. Biomass Bioenergy 2000, 18, 87–91. [Google Scholar] [CrossRef]
- Kinoshita, C.; Wang, Y.; Zhou, J. Tar formation under different biomass gasification conditions. J. Anal. Appl. Pyrolysis 1994, 29, 169–181. [Google Scholar] [CrossRef]
- Narvaez, I.; Orio, A.; Aznar, M.P.; Corella, J. Biomass gasification with air in an atmospheric bubbling fluidized bed. Effect of six operational variables on the quality of the produced raw gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Kwak, T.-H.; Lee, S.; Park, J.-W.; Maken, S.; Yoo, Y.D.; Lee, S.-H. Gasification of municipal solid waste in a pilot plant and its impact on environment. Korean J. Chem. Eng. 2006, 23, 954–960. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Wu, Z. Nitrogen release during fixed-bed gasification of several coals with CO2: Factors controlling formation of N2. Fuel 1999, 78, 521–527. [Google Scholar] [CrossRef]
- Zhou, J.; Masutani, S.M.; Ishimura, D.M.; Turn, S.Q.; Kinoshita, C.M. Release of fuel-bound nitrogen during biomass gasification. Ind. Eng. Chem. Res. 2000, 39, 626–634. [Google Scholar] [CrossRef]
- Tian, F.-J.; Yu, J.; McKenzie, L.J.; Hayashi, J.-I.; Li, C.-Z. Conversion of fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: A comparative study of coal and biomass. Energy Fuels 2007, 21, 517–521. [Google Scholar] [CrossRef]
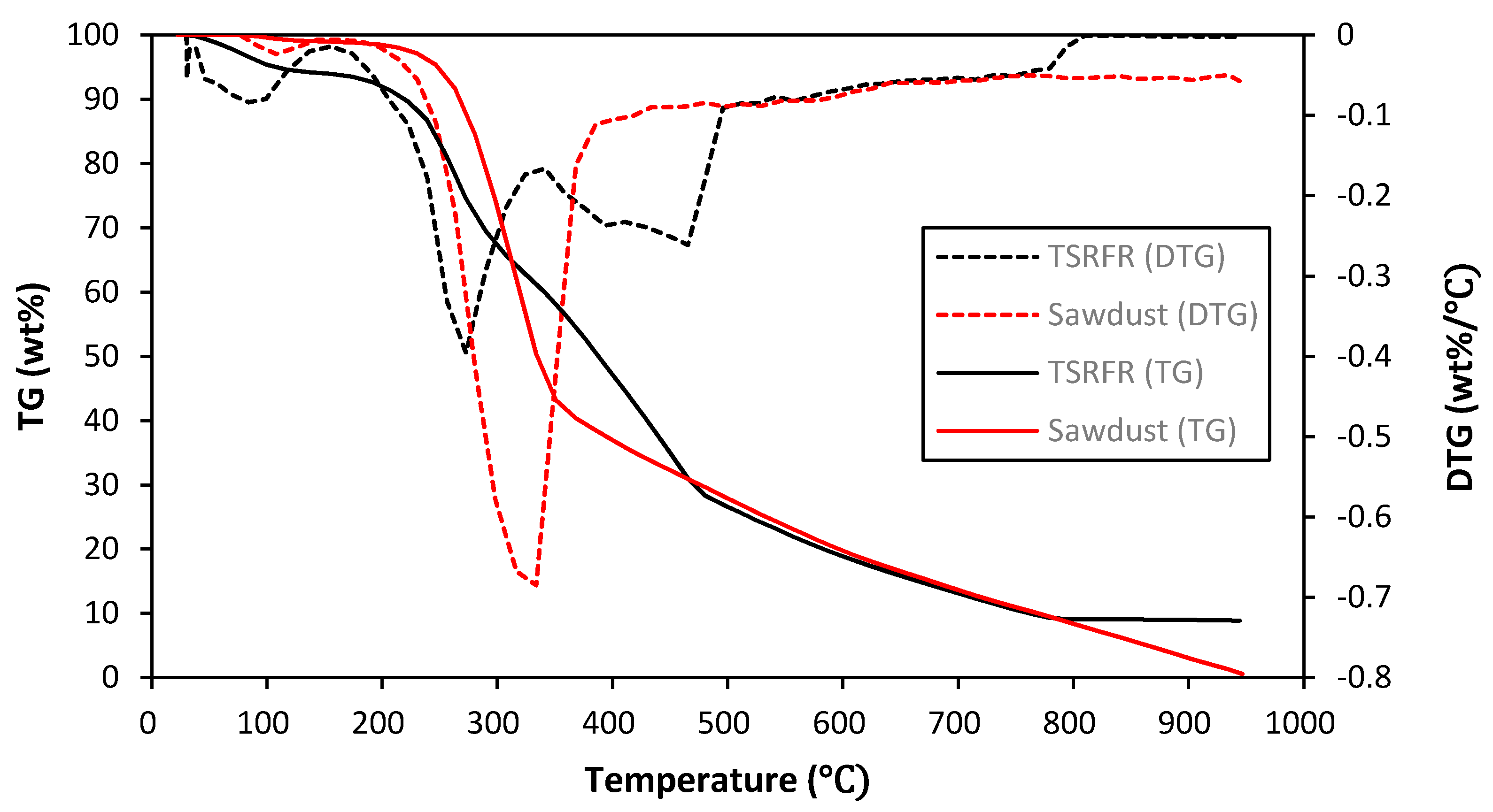





| Feedstock | Elemental Analysis (wt%) | Proximate Analysis (wt%) | HHV (MJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Cl | MC | VM | FC | AC | ||
| TSRFR | 43.24 | 6.03 | 41.89 | 0.44 | ND | ND | 5.04 | 78.09 | 7.52 | 9.35 | 21.54 |
| Pine sawdust | 45.66 | 5.81 | 45.32 | 0.11 | ND | ND | 1.40 | 81.52 | 16.65 | 0.43 | 19.38 |
| Parameter | Condition 1 | Condition 2 | Condition 3 | Condition 4 |
|---|---|---|---|---|
| Feedstock | TSRFR | TSRFR | TSRFR + Pine sawdust (75 wt%+25 wt%) TSRFR + Pine sawdust (50 wt%+50 wt%) | Optimum one from Condition 3 |
| Temperature (°C) | 950 | 950 | 950 | 950 |
| Equivalent ratio | 0.2, 0.3, 0.4 | Optimum result from Condition 1 | Optimum result from Condition 1 | Optimum result from Condition 1 |
| Feeding rate (g/min) | 5 | 5 | 5 | 5 |
| Particle size (mm) | 15–45 | 15–45 | 15–45 (TSRFR); 1–2 (Pine sawdust) | 15–45 (TSRFR); 1–2 (Pine sawdust) |
| Bed material | Silica sand | Silica sand + dolomite (67 wt% + 33 wt%) Silica sand + lime (67 wt% + 33 wt%) | Silica sand | Optimum result from Condition 2 |
| Gasification agent | Air | Air | Air | Air |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.T.; Park, S.-W.; Lee, S.-Y.; Jeong, Y.-O.; Girolamo, A.D.; Seo, Y.-C.; Choi, H.S. Co-Gasification of Treated Solid Recovered Fuel Residue by Using Minerals Bed and Biomass Waste Blends. Energies 2020, 13, 2081. https://doi.org/10.3390/en13082081
Alam MT, Park S-W, Lee S-Y, Jeong Y-O, Girolamo AD, Seo Y-C, Choi HS. Co-Gasification of Treated Solid Recovered Fuel Residue by Using Minerals Bed and Biomass Waste Blends. Energies. 2020; 13(8):2081. https://doi.org/10.3390/en13082081
Chicago/Turabian StyleAlam, Md Tanvir, Se-Won Park, Sang-Yeop Lee, Yean-Ouk Jeong, Anthony De Girolamo, Yong-Chil Seo, and Hang Seok Choi. 2020. "Co-Gasification of Treated Solid Recovered Fuel Residue by Using Minerals Bed and Biomass Waste Blends" Energies 13, no. 8: 2081. https://doi.org/10.3390/en13082081







