1. Introduction
The efficient use of energy is of utmost importance for process sustainability and emission reduction [
1]. This is an area of increasing research and practical interest that has persisted to this day [
2]. All major economic sectors are under investigation, including industry [
3], transportation [
4] and agriculture [
5].
The use of all types of resources and the impacts of processes on the surroundings can be related to the use of the energy necessary to complete the tasks. The evaluation of industrial systems is most frequently performed by using mathematical models for the consistent estimation of their thermodynamic properties and related energy use. Commercial simulators provide this functionality [
6].
When comparing alternative processes, the energy demand is not always a suitable metric because it may not take into account the quality of the energy used. In this context, exergy is the property that can be used as a combined indicator of energy quality and quantity [
7]. This property of exergy allows the optimisation of the process design and operation based on realistic estimates of how much energy can be sourced, converted, supplied or reused. Extended Exergy Analysis also takes into account the economic aspects of a resource; e.g., a wind turbine in a more windy place has a higher exergy efficiency because it produces more energy with similar investment and operation costs [
8]. This concept can be applied to an isolated unit (e.g., a wind turbine), to an industrial process (e.g., concrete industry [
9]) or a farming system (e.g., canola [
10]).
Process systems can no longer be considered in isolation [
11], dealing only with the maximisation of their standalone efficiency. While process efficiency is important for obtaining profit, its environmental impact spans beyond the boundaries of the current system. This conflict between the usefulness of the streams and resources of a process and their effect on natural storage is solved by the concept of circularity [
12], in which the overall life cycle is considered.
The exergy concept has been shown to be key to addressing sustainability issues [
13]. The use of renewable resources is beneficial, as this takes advantage of natural energy flows across the Earth, without depleting accumulated terrestrial energy reserves, such as fossil fuels [
14]. Therefore, the share of renewable resources used in the economy should be increased, although the exergy obtained in some of the harvesting paths may be small. Another confirmation of the usefulness of exergy for sustainability modelling comes from the domain of water management and water treatment plants [
15]. However, despite being proven to be potentially useful, the use of the exergy concept is frequently limited only to the estimation of the exergy efficiency of various process contexts [
16], such as the exergy efficiency of a process or the share of exergy from the renewables provided to a process.
There are examples of exergy assessment in the literature. Changes in the concentration of a solvent give rise to a massive exergy loss, indicating the importance of solvent selection [
17]. An exergy analysis to evaluate the performance of a continuous Directional Solvent Extraction (DSE) desalination process using octanoic acid was presented in [
18]. Extractive solvent regeneration is a potential method to substitute stripping and reduce the exergy demands of CO
2 capture systems [
19].
To compare process alternatives using exergy, the selection of system boundaries and reference points (e.g., ambient conditions) must provide comparable output streams. The same issue is also typical for the implementations of the Life Cycle Assessment (LCA) framework [
20], where the choice of the system boundary and selection of life cycle stages is crucial to obtaining credible results. This similarity is useful for the potential integration of exergy-based criteria within the framework.
There have been many attempts to define a universal reference state [
21]. The restricted dead state is defined as the physical thermodynamic equilibrium with the reference state. However, a dead state which takes the chemical equilibrium into account is required for environmental assessments. A widely used variant is based on an Earth similarity condition [
22], where a reference substance is chosen for every element [
23]. Substance exergies are determined to start from those of the reference substances, considering balanced chemical reactions. Regretfully, some chemical exergies are negative, and the reference is not entirely consistent [
24].
The initial reference state has been updated according to new and more accurate geochemical and geological information. Thanatia [
25] is a thermodynamically dead planet in which all materials have reacted, dispersed and mixed; i.e., it represents a complete dispersed state of minerals and the complete combustion of fossil fuels. Thanatia is not a reference state but a baseline used to calculate concentration exergies, therefore providing the exergy replacement costs. To assess the exergy degradation of the natural capital, the reference environment has evolved to a Thermo-Ecological Cost (TEC) methodology which in combination with the concept of Exergy Replacement Costs (ERC) results in the TERC (Termal-Exergy Replacement Cost) methodology, which is used to assess the degradation of fossil and mineral capital [
25].
The choice of the reference conditions can also have a significant effect on the evaluation of the chemical exergy of particular substances such as fuels [
26]. This is even more important for the evaluation of the exergy efficiency of large-scale systems, such as the Turkish industrial sector. A study of the trends in this area [
27] revealed an increase from 25% to 29% when the ambient reference temperature decreased from 298 to 273 K.
From the perspectives of ecological modelling and the life cycle, it is possible to use the concept of embodied exergy: the cumulative amount of exergy inputs necessary to deliver a product or a service [
28]. The cited work has applied the concept to exergy costing and accounting for energy sector applications, linking exergy spending to monetary costs.
Although exergy is very useful for assessing the loss of resource quality, its use has not been widespread in environmental impact evaluation. LCA is one of the well-established techniques with which exergy has been combined to conduct the exergy analysis of a complete product life cycle [
29].
One method to quantify the environmental impact of a process based on exergy is the use of the environmental compatibility indicator, which takes into account the input exergy to the process and the exergy requirements for the abatement of process emissions and waste [
30]. In an ideal case (no impact considered), the discussed process emits only heat.
Furthermore, the highest exergy efficiency does not correspond to minimum costs [
31] or minimum environmental impact [
32]. Exergy efficiency, in that sense, is a local evaluation criterion and is only appropriate to specific energy conversion or use schemes.
Circular economic flow is based on the separation of technology and the economy as the main condition [
33]. This concept considers the inputs and outputs of operations during industrial production and focuses on cause-effect relationships. The author considers the circularity concept in terms of temporally repeating cycles of economic activity and presents the realisation that the economy cannot be considered separately from the environment.
Different industrial approaches to the improvement of the sustainability of human society and the environment have been attempted. The simple approaches to the substitution of materials and the end-of-pipe reduction of harmful emissions have been superseded by LCA-based methods for ecological design and economics [
34]. The understanding of the interconnections, inputs and outputs for the entire supply leads to the goals of the circular economy [
35]. In this context, close attention has to be paid to the full life cycle, including the facility construction and decommissioning, as has been shown in an analysis of the reuse of materials from wind turbines after their end of service [
36].
The utilisation and reuse of different types of waste may be analysed by systematic approaches: e.g., P-Graph offers a solution for closed-loop processing and the analysis of its impact [
37]. Process Integration also has great potential for analysing circular flows, especially in improving the sustainability of energy systems [
38].
For the effective application of targeting and optimisation models in the design, operation and retrofitting of industrial processes for the circular economy, it is necessary to have flexible and scalable modelling concepts and tools. Conventional logic treats process streams as either inputs or outputs, where the outputs are either products or waste streams [
39]. The waste streams were traditionally thought of as needing to be treated and disposed of. The circular economy paradigm for process design [
40] requires non-product outlet streams to be treated as sources of potential resources as well.
Besides research, regulatory action has also been taken; for example, the EU action plan for the circular economy [
41]. Some ideas related to circularity have been developed previous to the popularisation of the circularity concept; e.g., reuse, remanufacturing or recycling [
42]. Sustainable Consumption and Production (SCP) tools have been identified as a booster of circularity [
43]. The implementation of circularity has resulted in innovation opportunities [
44]. This is the case with the redesign of pharmaceutical supply chains to prevent the waste of medical supplies [
45].
A clear example of circularity is the mass flow in nature [
46]: a mixture of dead biomass is decomposed by microorganisms and fungi to simple molecules that are captured by plant roots to generate complex molecules again using solar energy. This nutrient flow takes place in natural environments but not in agriculture, where the products are transported away to consumers without returning back to fields [
46], breaking the natural cycle.
There is intensive research available in the literature about circularity in the industry, such as in metals processing [
47], including copper [
48] and steel [
49]. Other fields have also been researched, such as construction [
50] or forest wood harvesting and utilisation [
51]. However, the global economy is not circular because large amounts of materials are used only once to provide energy or commercial value and are thus not available for recycling [
52].
Examples of circularity in the chemical industry are related to plastics recycling as a consequence of the strategy of the European Commission [
53]. The practices include plastic sorting [
54], product design [
55], or the design of chemical bonds suitable for biodegradation [
56].
Many authors have defined circularity and its advantages and provided tools to quantify it. Examples include Corona et al. in 2019 [
57], who focused on the circularity metrics, and Sassanelli et al. [
58], who dealt with the assessment methods and the identification of the systematic taxonomy of the indicators used for circular economy evaluation by Saidani et al. [
59].
The provided state-of-the-art review has shown that various tools and practices are available for process network optimisation, allowing the identification of the potential reuse paths for material components. However, accounting for the reuse of multiple resources within complex networks, containing multiple loops, creates a multi-dimensional optimisation problem if only approached directly. This observation reveals the need for an accounting framework and concepts that would measure the degree of sustainability and favorability of process networks adequately, taking into consideration the heterogeneous nature of the networks both in terms of their activities and the multitude of resources tracked.
The current work presents a system of analytical concepts, a framework and tools for evaluating the impacts of process systems based on thermodynamics. The framework is based on the concept of exergy as the unifying performance metric. It defines the tools of exergy assets and liabilities that enable the assessment of the sustainability of the considered systems. The trade-offs between the different feedstock and product flows and environmental impacts are modelled using the exergy assets and liabilities, leading to the calculation of the exergy footprint. The remaining content of the article presents the model and framework (
Section 2), followed by illustrative case studies (
Section 3) and a concluding discussion in
Section 4.
2. Model and Framework
Process systems and supply chains consist of various process units and sub-systems, each of them having input and output interfaces and internal relationships. The heterogeneity of processes and their characteristics are complemented by the system scalability: the ability of various process units and systems to be integrated as parts of larger systems, forming nested hierarchies. This section starts with the development of the modelling concepts and framework, including the material flow cycles and the energy cascading principle; that is followed by the formulation of the accounting framework and the modelling equations.
2.1. General Trends and Issues
To derive a unifying criterion for the assessment of heterogeneous process systems of varying sizes, it is necessary to formulate a suitable framework. This should be based on a common process representation and allow the scalability of the evaluation scope. An essential property of the desired framework is that it be based on indicators that quantify the resource supply, demand, availability and deficit in a seamless way. The quantitative criteria also have to reflect the need to attain a sustainable development path of the considered system. These requirements form the basis for selecting reference conditions for the desired system designs.
At the process level, there can be multiple inputs and outputs. An example can be taken from the domain of agriculture [
60]. As illustrated in
Figure 1, there are various input streams as well as output streams, which are of different natures and have different environmental impacts and economic significance. While input-output analysis is helpful in quantifying the net resource and footprint impacts, it is difficult to use in revealing possible reuse and recycling patterns because of the different natures and compositions of the inlet and outlet streams.
They are good examples of recycling from chemical processes involving reactors at the process level, where the unreacted feed is separated from the reaction products and recycled. Such an arrangement can be found in the ethanol production process by the hydration of ethylene [
57]. The usual pattern is a reactor (or a reactor network) followed by separators.
Figure 2 shows a summary of the process arrangement of the example given in [
61]. The key reactants are ethylene and water. First, the ethylene is separated by flashing and washing, and then the resulting water–ethanol mixture is separated in a series of distillation columns. The system features two loops: one for the ethylene recycling and another for the water recycling.
Several types of nexuses have been discussed in the research literature. Of these, the best-known is the energy–water nexus [
62], but the correlations among other resources and product flows have also been investigated; for example the energy–water–food nexus [
63], the joint consideration of water, land and food [
64], and even the consideration of terrain–emission interactions [
65]. All these nexuses can be represented as having two major parts: material and energy flows. An analysis of these two parts is presented below.
2.1.1. Material Flows and Their Cycles
At the regional level, placing industrial sites within the environmental context reveals that the significant material flows feature two types of cycles (
Figure 3), which can be extrapolated to a global (planetary) level. One type of material cycle is the traditional one: extracting resources (primary raw materials) from environmental storage sites, processing them into intermediate materials and further to products, the delivery and use of those products, the generation of waste streams and the disposal of the waste into the environment. The second cycle travels a shorter path, consisting of diverting part of the material flow of waste to the generation of secondary raw materials, which are used to substitute primary raw materials. Of these cycle types, the traditional route is more straightforward and is perceived as economically more favourable. While this may have been the case at the beginning of the industrial age, the increasing waste generation makes the recycling–reuse pattern desirable and viable for key materials such as paper [
66], metals [
67], and even electronic waste [
68].
One obvious essential feature is that both material flow patterns form closed cycles. In this sense, the major degree of freedom within the material flows network is the split between the recycled and non-recycled fractions of the generated waste.
2.1.2. Energy Flows—Cascading
Energy can be sourced either from renewable sources (mainly of solar origin) or from terrestrial deposits (
Figure 4). Energy flows follow the Laws of Thermodynamics, cascading from higher to lower quality [
69]. Harvested energy flows can be used to power various processes, resulting in the movement of the material flows within a system; i.e., an enterprise, a municipality, or a region. At the system level, at various scales, energy can be sourced, converted and used and ultimately is left to dissipate in the environment. The share of the losses to dissipation reaches two-thirds [
70]. This pattern reveals that there are two types of global system interface flows: energy inlets (renewable) and energy outlets (dissipation). Any non-renewable energy sources are internal to the system. This allows the classification of renewable energy sources as long-term degrees of freedom and the non-renewable as short term ones.
Energy cascading is thus used to power the closed material cycles for industrial and other activities in the global economy. Establishing this principle allows us to set up a framework for system state accounting which can be used to evaluate and optimise the system design and operation for various objective functions linked to the energy supply.
The analysis in this section clearly points to energy harvesting and use as the dominating factor, representing a key degree of freedom in driving the economy and societal activities. Moreover, energy is stored in various forms for conversion, transport and use. This view of energy transformations allows the consideration of industrial and business processes as networks of states and transitions, where the states are related to the energy content of materials and process streams, while the transitions are either intentional process operations or spontaneous transitions, transforming process streams from one state to another at the expense of exergy conversion and destruction.
There have been many proposed circular economy indicators; e.g., a recent review [
59] analysed 55 sets of circularity indicators. The choice and most beneficial use of indicators depends on the considered context. Within the context of a given supply chain or an industrial process, the degree of recycling of key materials is the most widely used indicator; in this context, the Circular Material Use rate (CMU) has been adopted by Eurostat [
71] to determine the degree of circularity of systems at various scales. In the case of Eurostat, this is applied to measure the circularity of the economies of EU member states. CMU is defined, within the context of a specific material, as the fraction of the recycled material (U) within the overall material intake by the system (M):
While CMU is a crucial indicator, it alone is not sufficient to characterise the sustainability of the considered systems. Additional indicators are therefore needed to provide sufficient characterisation. The model proposed here uses energy as the main indicator, in the form of exergy, with all remaining system properties used as specifications to ensure the sustainable conditions of all parts of the environment–economy–society macro-system.
2.1.3. Exergy as the Unifying Performance Metric
The identified need for an energy-based indicator needs to be put in the correct context. The process systems are evaluated based on certain requirements, which are intended to minimise or eliminate any adverse environmental impacts of the system.
Referring to
Figure 3 and
Figure 4, the material outputs of each process system cannot simply be released to the ambient environment. Before release, they have to be brought to a certain desired state at the point of release to the environment characterised by composition (or an equivalent specification) and temperature. Naturally, suitable pressure also has to be selected and specified.
Such a state is usually defined by the environmental regulations concerning the corresponding natural storages. For instance, for wastewater discharge to environmental basins in the European Union, it is required that they contain a maximum of 25 mg/L BOD5 (Biological Oxygen Demand) at 20 °C [
72], which can be used to estimate the content of the main contaminants.
Similarly, there are regulatory limits on effluent discharge temperature. For instance, King County, Seattle, US [
73], allows a maximum of 40 °C at the entry of wastewater treatment plants. The Environmental Protection Agency of Taiwan [
74] imposes limits from 35 to 42 °C for the points of discharge at sea, with the addition of a requirement that the water stream does not deviate from the surrounding surface water by more than 4 °C. The significance of this stipulation is that it relates the target stream temperature to that of the ambient conditions.
From the above reasoning, it becomes clear that all energy flows and storage contents that relate to the considered process systems are limited only to the energy that can be extracted as a difference from the conditions of the surrounding environment. This is equivalent to the definition of exergy, also known as availability [
69].
In this case, the referenced environmental conditions are not necessarily the currently existing conditions but those mandated in the environmental regulations and standards. This provides a reference point for estimating the exergy balance (deficit or excess) to achieve zero deviation from the desired environmental conditions and minimise the potential environmental impacts.
The observations below aid in establishing the basis of the evaluation model:
- (1)
For any process system, only the interface streams—inputs and outputs—can be considered as producing environmental impacts. Internal streams have no direct impact on the environment.
- (2)
The inputs represent the demands of the system which are passed to upstream providers of resources, products and services. Similarly, the outputs represent the interface with their downstream counterparts: users/consumers, utilities, artificial (landfills, tailing ponds) and natural storage systems (the atmosphere, rivers, lakes, oceans, the ground).
The next section defines the necessary elements for using exergy as the metric to determine the quality of a process stream by defining exergy components associated with the stream, divided into assets and liabilities. The follow-up sections build on this by formulating the overall framework for exergy accounting and computing the exergy profit or loss associated with a process system.
2.2. Exergy Accounting Framework
For the evaluation of a process system’s performance regarding its environmental impacts and its sustainability, it is necessary to capture the interfaces—i.e., the inlet and outlet streams (
Figure 2)—as only they have the potential for impact. The internal constraints and internal flows are resolved by the system calculation model; i.e., simulation or optimisation. EXA and EXL denote the exergy assets and the exergy liabilities of a stream, respectively.
Consider again
Figure 2, in which the input and output streams are highlighted. The process inputs are the streams labelled as ethylene feed, water feed, and water (wash water). The outputs are the streams labelled as “purge”, wastewater, and ethanol product.
Inputs and outputs can be distinguished from the interface streams. An output stream is either a product or waste. In the case of product output, liabilities are not assigned because a product stream only carries useful value but does not involve the exergy penalty. Exergy assets can be assigned to a product stream only if the stream content implies or has the goal of retrieving exergy capable of driving economic activities such as chemical processes or transport operations.
For waste streams, the determination of exergy assets or liabilities employs a notional (potential) workflow (
Figure 5). The workflow involves attempted operations for exergy extraction/recovery first, followed by the end of pipe treatment of the residual stream and finally discharge. Any potential for exergy extraction and utilisation is defined as an asset, and the need to add exergy to the remaining potential workflow is added to the liabilities.
The input streams to the processing system have to be considered. For this, it is necessary to realise that the input to any human-operated process system is a product output of an upstream system. This includes, besides intermediate products, the resource streams extracted from nature (since the extraction itself is already an operation). Following the principles defined for products, the direct exergy liabilities are not assigned to the input streams, while exergy assets are assigned only in the case of an energy conversion system as the main object of evaluation.
The above discussion only reflects the perspective of the local to downstream impacts of a process system. To enable accounting for complete supply chains as well as the overall LCA [
75], it is important also to include the upstream environmental impacts, leading to the need to account for the embodied exergy [
28]. In this case, instead of the potential downstream exergy flows, the account includes the upstream exergy inputs (liabilities/credit) and the exergy content of the evaluated streams, assigned as assets.
Having estimated the exergy assets and liabilities for each of the interface streams for a process system, they are summed up, producing the total exergy assets (Equation (2)) and the total exergy liabilities (Equation (3)) of the system.
Equations (2) and (3) can be applied to various contours, including specific process systems, supply chains or complete life cycles. They can be used to evaluate downstream and/or upstream impacts.
2.3. Exergy Content of a Single Process Stream
Referring to the observations formulated at the end of
Section 2.1.3, the values of EXA and EXL can be estimated for any stream in the considered system. Each process stream is modelled as having two exergy sets: EXA is assigned positive values, and EXL is assigned negative values. Summing the assets and the liabilities for the stream produces the net balance, resulting in the potential exergy profit (positive balance) or loss (negative balance).
The model development starts with the identification of the potential components of the exergy content in a stream. According to the theory presented in [
7], the following components can be distinguished in the exergy content of a thermodynamic system, including a process stream [
7]:
- (1)
Thermo-mechanical/physical exergy: This is based on the thermal and pressure conditions of the system and can be expressed as in Equation (4) when no pressurised gas is present:
where Ex
phy (MW) is the thermo-mechanical exergy flow rate, H and H
0 are the enthalpy flow rates of the stream (MW) at the current conditions and at the reference conditions, respectively, T
0 (°C) is the temperature at the reference conditions, and S and S
0 are the entropy flow rates (kW/°C). The typical reference conditions are 25 °C and 1 atm. It has to be noted that the temperature-related quantities are given in °C. While the definitions of the thermodynamic properties are based on the Kelvin scale, the usual temperature specifications are in °C, which is the much more commonly used scale in engineering calculations.
- (2)
Chemical exergy: This is the retrievable exergy from the system by applying potential chemical and physical conversions or the exergy input required for cleaning/separation. This component can be expressed in different ways, depending on the particular processes (chemical and/or biochemical). For chemical reactions, the chemical exergy can be evaluated as
where Ex
chem (kW) is the chemical exergy flow rate, μ
i and μ
i,0 (kJ/kmol) are the chemical potentials at current, and reference conditions, respectively, and N
i (kmol/s) is the molar flow rate of the flow. In this work, the reference state of the materials is evaluated based on the Szargut method [
76]. The detailed calculation steps of the chemical exergy are shown in [
6]. For simplicity, an open-source online tool [
77] is used to estimate the chemical exergy of materials in this paper.
- (3)
Gravitational exergy: This expresses the potential energy (directly convertible to exergy; see [
7]) resulting from the elevation of the system above a certain base point:
where Ex
G (kW) is the gravitational (potential) exergy, m (kg/s) is the mass flow rate, g (m/s
2) is the acceleration due to gravity, and Δh (m) is the elevation difference between the current location of the stream and the location of the environmental reservoir selected for the reference point.
- (4)
Kinetic exergy: This expresses the kinetic energy (directly convertible to exergy).
where Ex
k (kW) is the kinetic exergy, m (kg/s) is the mass flow rate, and v (m/s) is the velocity of the stream.
- (5)
Electromagnetic exergy: The component (Ex
EM) can also be defined for electrochemical systems and problems, expressing the potential of the system within an electromagnetic field. This can be calculated as equivalent to the energy delivered by the electric current [
7].
For each modelling context, the significance and the relevance of each of the components have to be evaluated, and only the significant ones should be retained in the model. In the current work, only the thermo-mechanical and the chemical exergy components are evaluated. The other components are relevant to specific applications: the gravitational component is applicable to accounting for process layout, and the electromagnetic component is relevant to the electrochemistry and electromagnetism domains.
2.4. Exergy Profit and Exergy Footprint
The exergy assets and liabilities for a stream are both calculated using the equations in
Section 2.3. They assign exergy extraction and utilisation potentials to the assets, and the exergy demands to the liabilities. Establishing the balance of the total exergy assets (EX
asset) (Equation (2)) and the total exergy liabilities (EX
liability) (Equation (3)) produces the exergy profit (EX
profit) of the process system:
The opposite difference (the negation) of the exergy profit is termed the exergy footprint (EX
footprint):
In this way, a positive value for the footprint means an adverse impact on the environment by imposing the equivalent demand to be supplied from outside sources. With this criterion, the sustainability contribution of the evaluated process system can be clearly measured. A higher exergy profit, meaning a lower exergy footprint, also translates to a better sustainability contribution of the system.
All exergy components can be used in the general case. However, in the current study, only the thermo-mechanical and the chemical components are evaluated, since they are the most typical for chemical and waste processing.
4. Conclusions
This article reveals that the fundamental trade-offs between the various resource flows and environmental impacts—such as water–energy and water–energy–food nexuses—converge to the issues of material flow circularity and energy flow cascading. Based on this understanding, the concepts of exergy assets, exergy liabilities, and exergy profit/footprint are formulated, supplemented with a comprehensive evaluation framework.
Two case studies from completely different industrial domains are provided which illustrate the applicability of the framework for the seamless assessment of the energy/exergy needs of the process systems. These include the processes of acetic acid production and MSW treatment areas.
The results from the first case study show that the separation and reuse of the acetic-acid-containing purge stream are exergy-prohibitive and that it is not probable that such a solution would be sustainable. The follow-up analysis of the acetic acid production shows that the process requires a substantial external exergy input. Determining the degree of sustainability of such a process needs further analysis of the possible sources of providing such exergy.
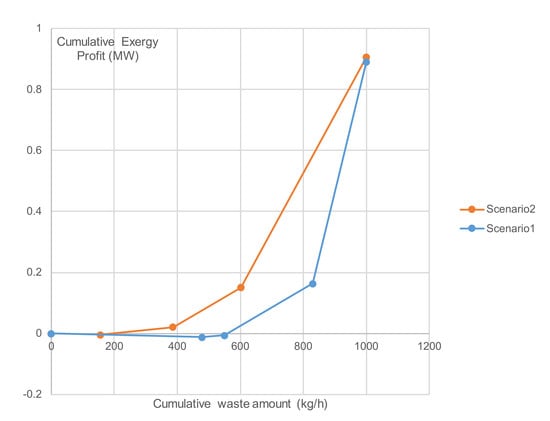
The second case study clearly shows the sustainability potential of the MSW treatment for obtaining either useful energy directly or first extracting useful chemicals before the waste-to-energy process. The developed cumulative Exergy Composite Curves show a marginal advantage (less than 10%) of the chemical extraction route over the direct waste-to-energy route. The developed curves demonstrate that the proposed framework represents a useful toolset for evaluating process systems and alternative solutions.
The proposed concept can be further developed to create a complete framework which is capable of accounting for the thermodynamic irreversibility of processes. This will help us to reach a deeper understanding of the exergy flows, storages and losses and their relation to process sustainability.
Building on this, future work should incorporate economic metrics into the evaluation, leading to a complete toolset accounting for both the technical and economic performance of the considered process systems. This will make the tools suitable for decision-making in real engineering projects and for use by process managers and potential investors.
The correct selection of the system boundaries for the analysis of exergy footprints is key to the practical applicability of the concept. Full Life Cycle Assessment requires the collection of a large amount of information, which sometimes depends on subjective considerations. In many cases, not all stages of the life cycle are really significant with respect to the chosen criteria. In this context, further work should also be directed towards embedding this accounting framework within the Life Cycle Assessment framework, allowing for the scalability of the concepts and their adaptation to the modelling contexts.