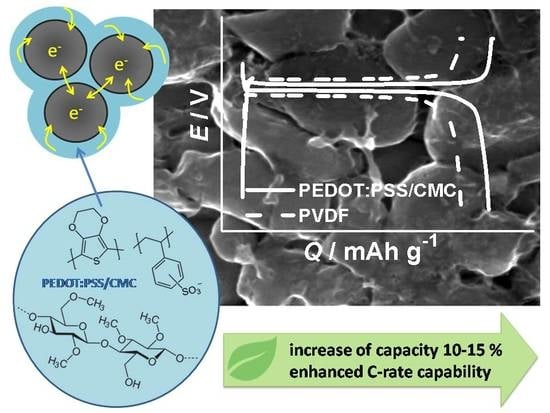
Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results
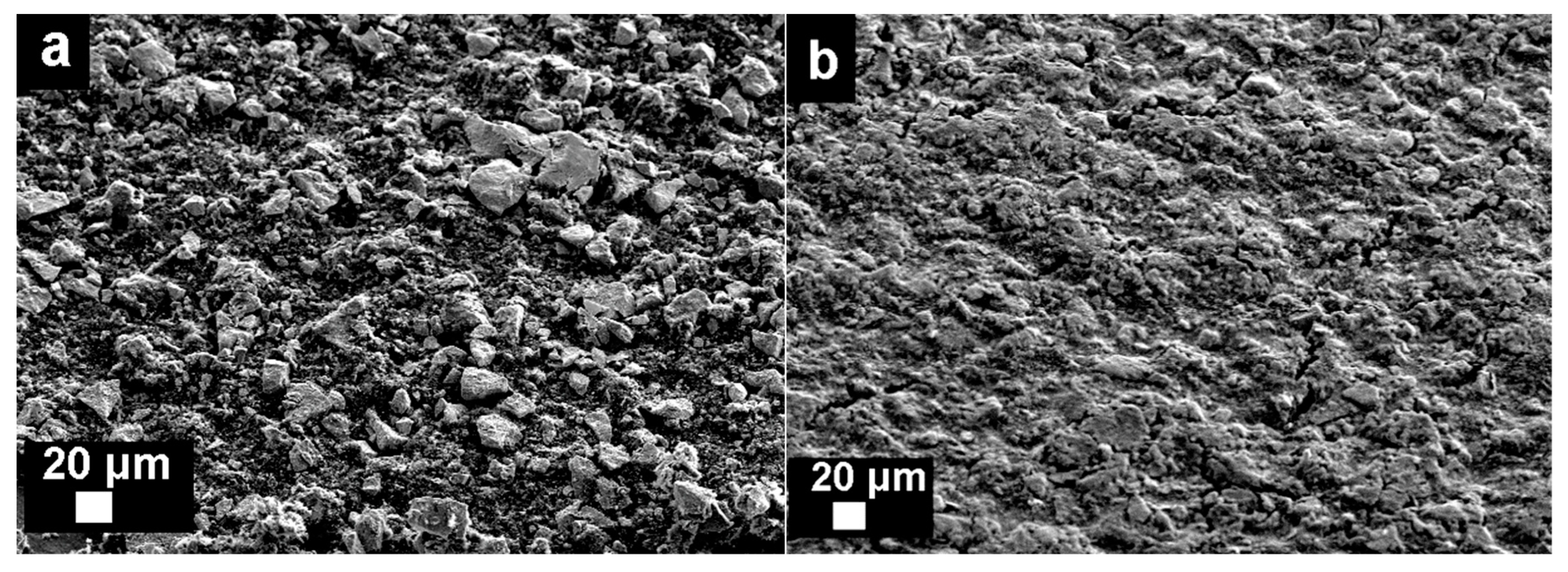
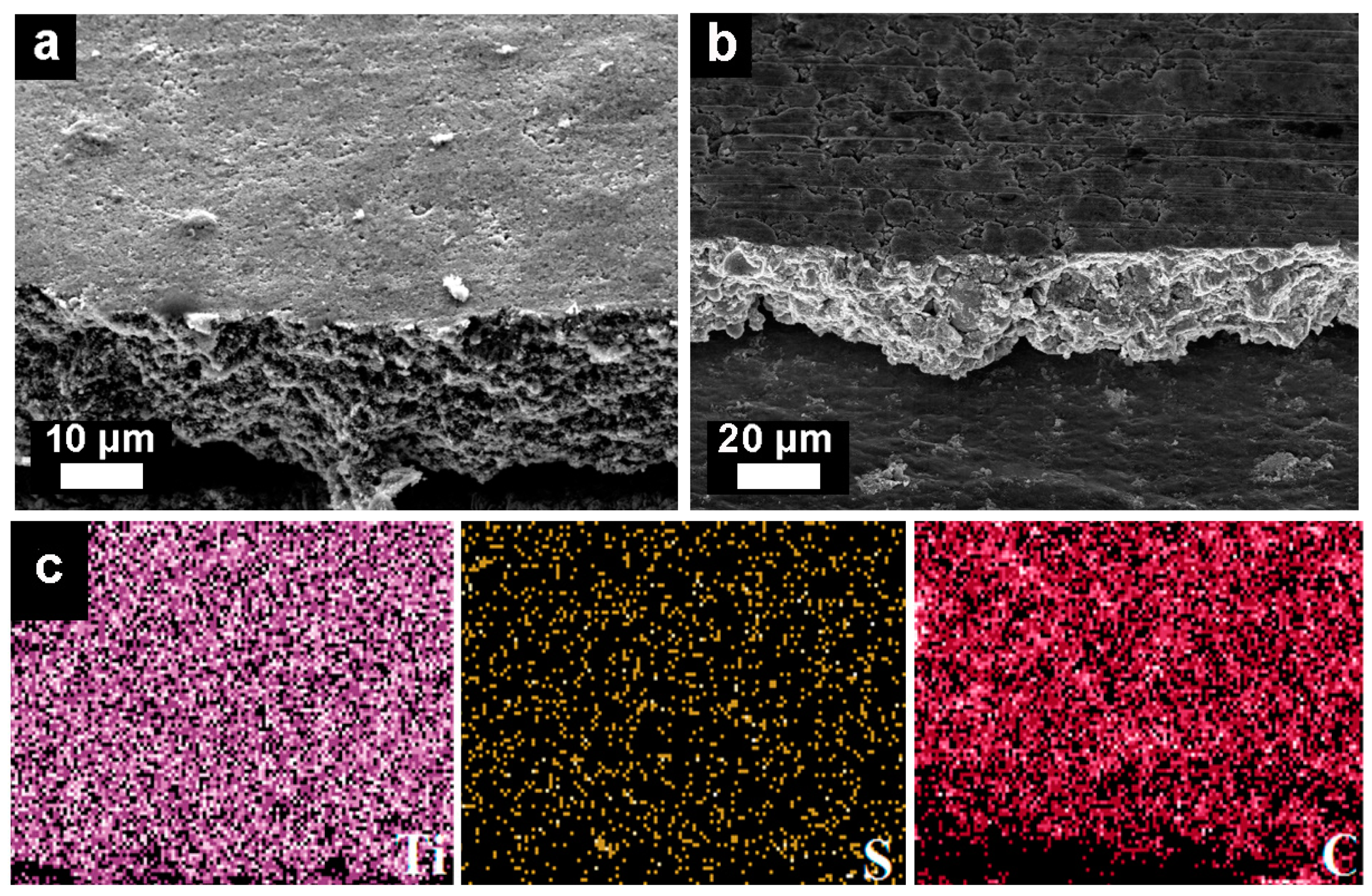
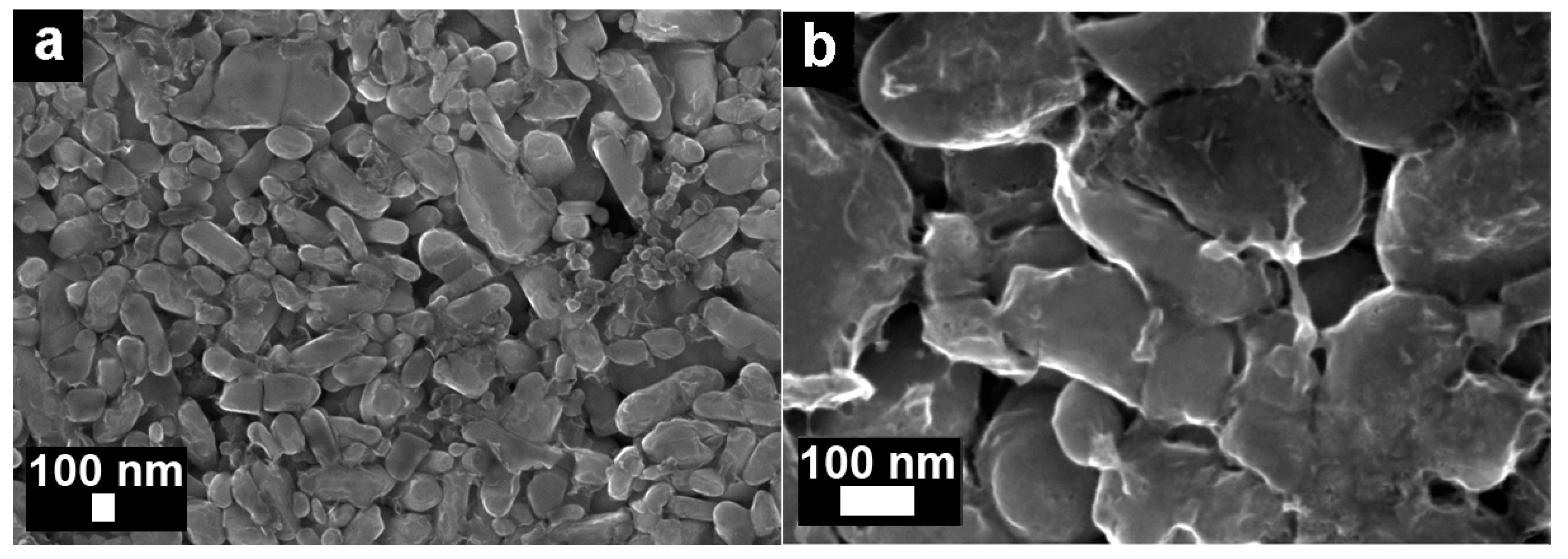
3.1. Morphology of Electrodes
3.2. Cyclic Voltammetry
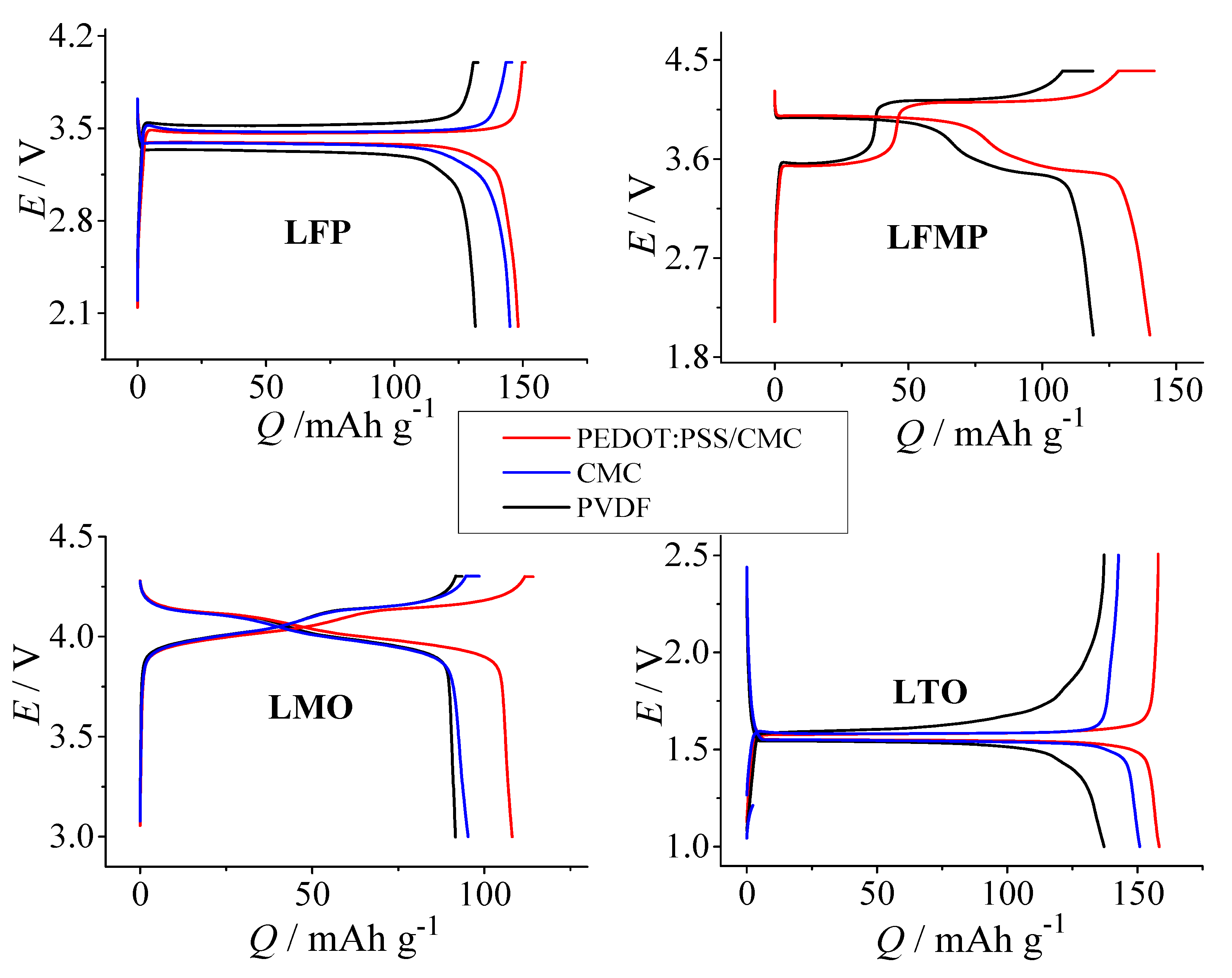
3.3. Galvanostatic Charge–Discharge
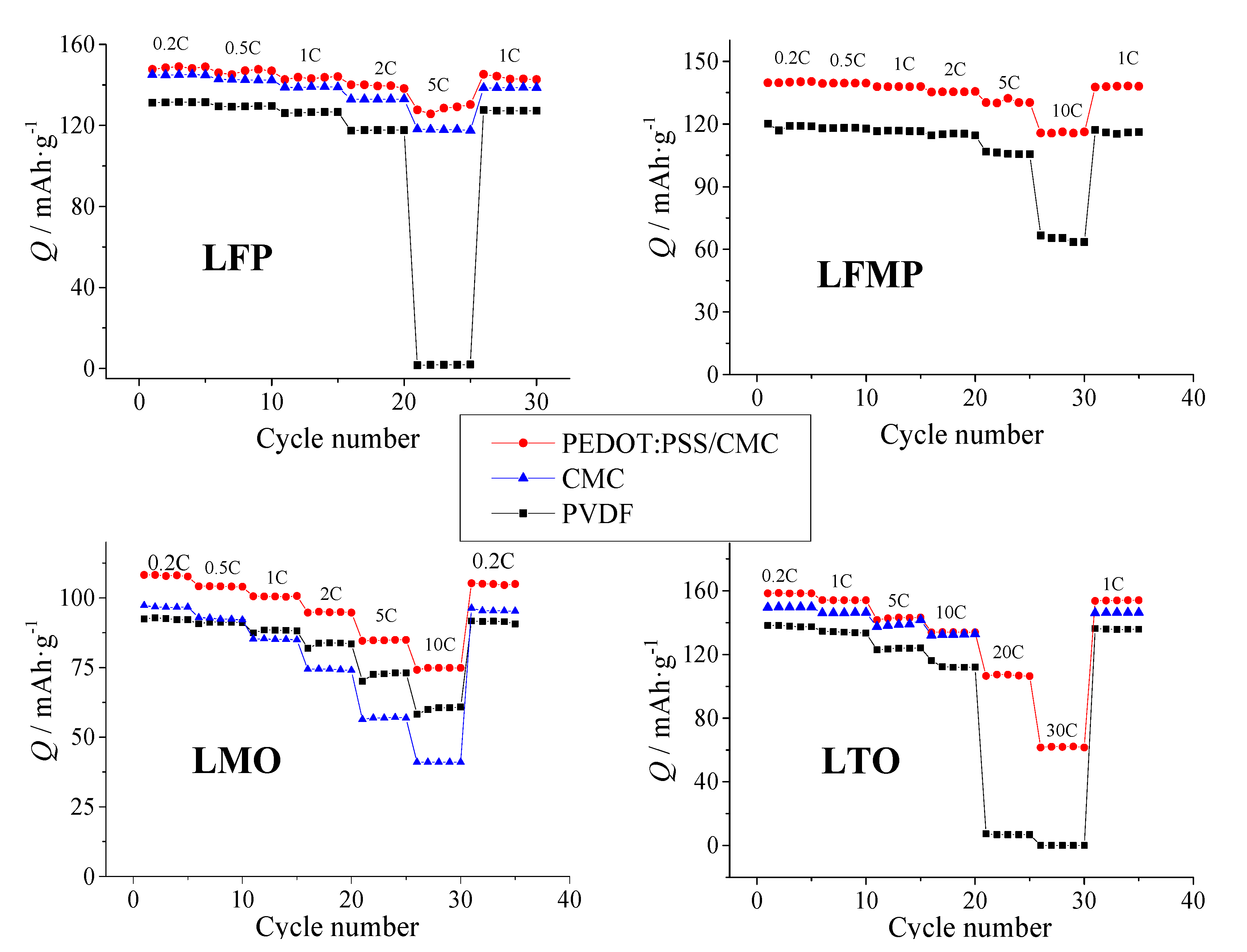
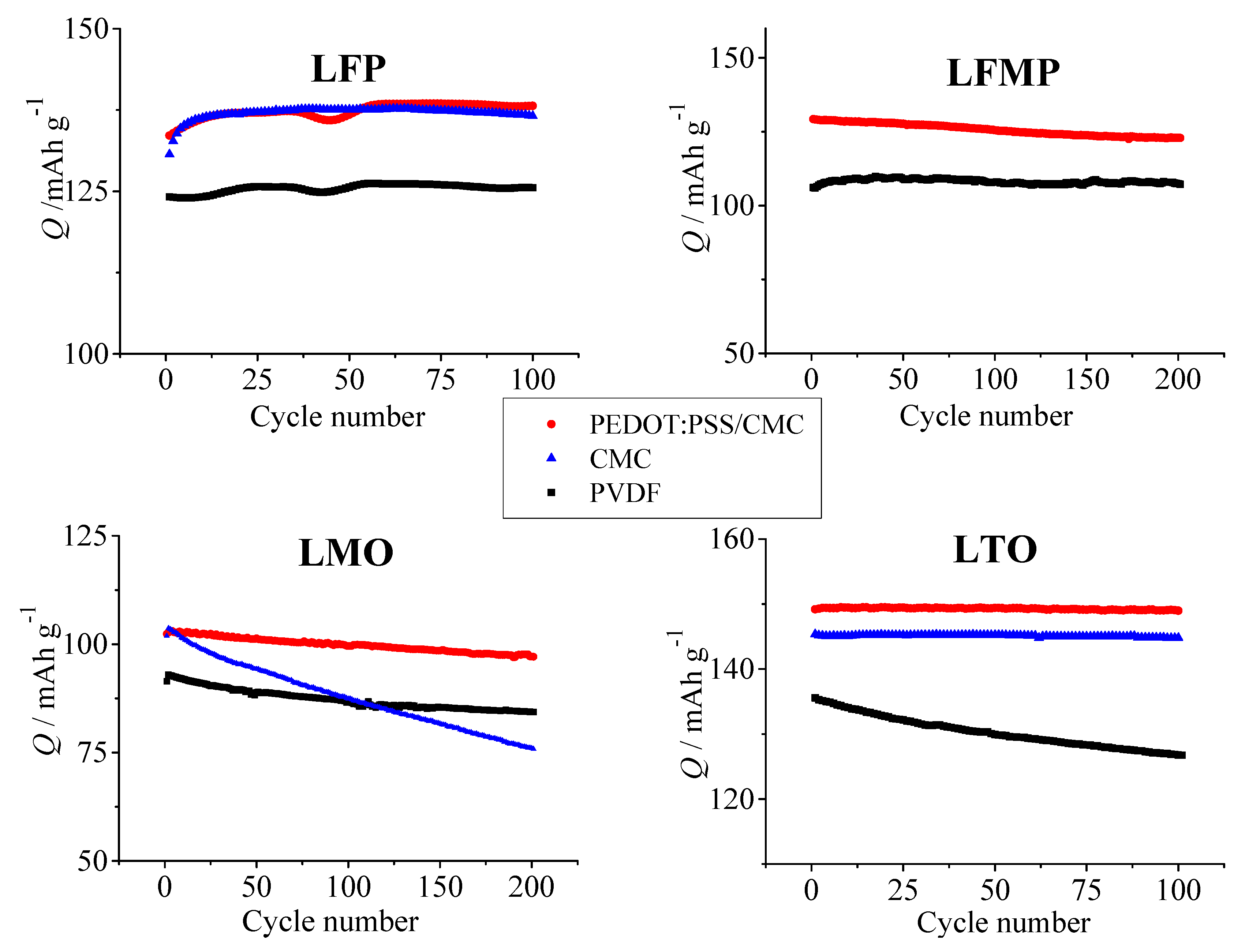
3.4. Cycling Performance
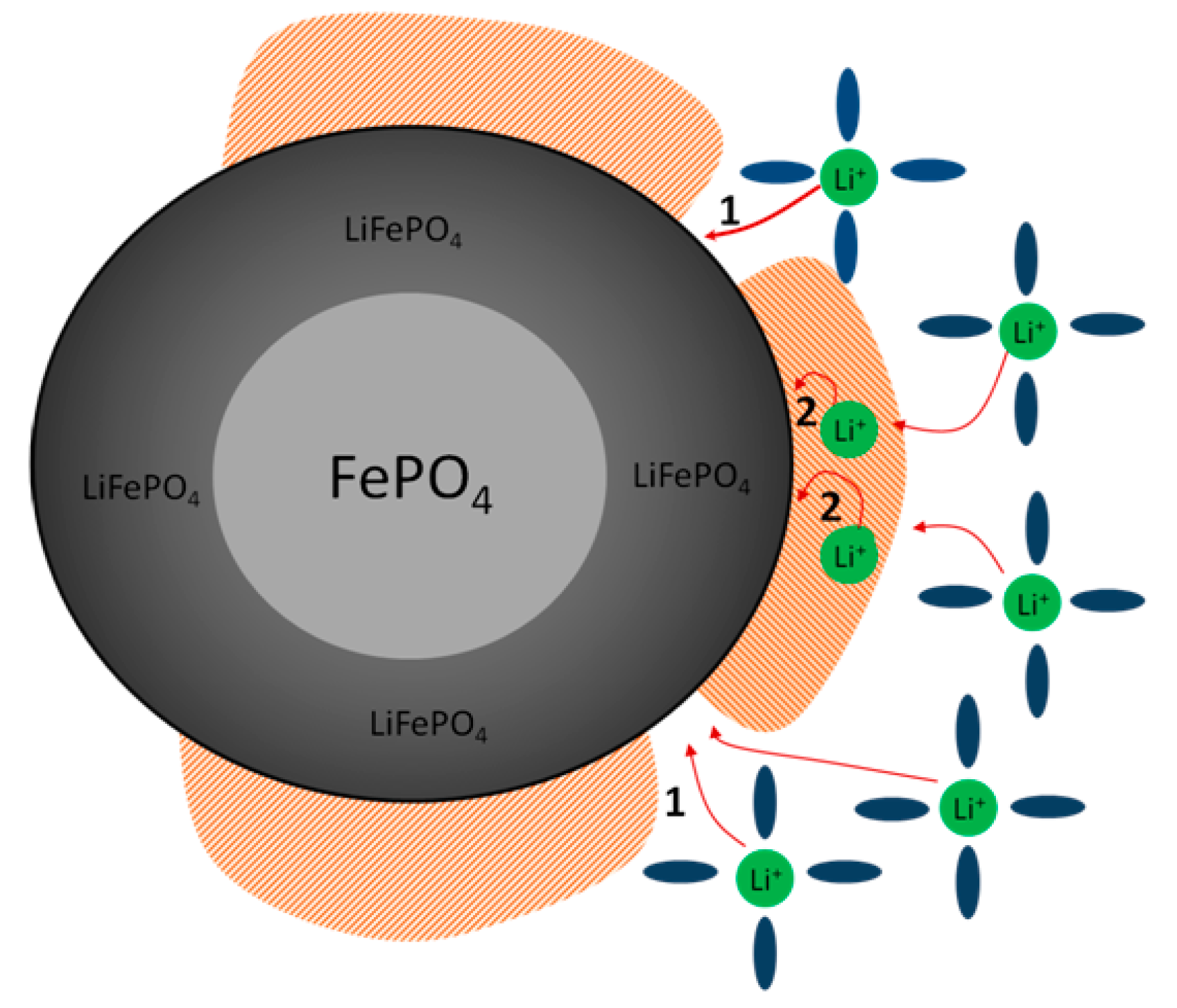
4. Discussion
- (1)
- PEDOT:PSS acts as an electronic/ionic conductive component, providing more conductive media between active grains with tight electrical contacts.
- (2)
- CMC additive acts as a thickening agent with good ionic conductivity, improving material by adjustable porosity and wettability by electrolyte, which facilitate Li+ ion movement.
- (3)
- In addition, the presence of ionogenic groups in the binder components (such as CMC and PEDOT:PSS) can create an increased concentration of lithium ions around the active material particles, which can also contribute to more efficient mass transfer during the discharge process.
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chou, S.L.; Pan, Y.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Sengodu, P.; Deshmukh, A.D. Conducting polymers and their inorganic composites for advanced Li-ion batteries: A review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem.–Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Uchaker, E.; Yuan, C.; Cao, G. Li4Ti5O12 nanoparticles embedded in a mesoporous carbon matrix as a superior anode material for high rate lithium ion batteries. Adv. Energy Mater. 2012, 2, 691–698. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Bewlay, S.L.; Chen, Y.; Liu, H.K.; Ahn, J.H. Electrochemical properties of carbon coated LiFePO4 cathode materials. J. Power Sources 2005, 146, 521–524. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Q.; Peng, D.; Liu, H.; Chen, Y. Fast precipitation-induced LiFe0.5Mn0.5PO4/C nanorods with a fine size and large exposure of the (010) faces for high-performance lithium-ion batteries. J. Alloys Compd. 2019, 794, 178–185. [Google Scholar] [CrossRef]
- Guan, Y.; Shen, J.; Wei, X.; Zhu, Q.; Zheng, X.; Zhou, S.; Xu, B. LiFePO4 activated carbon/graphene composite with capacitive-battery characteristics for superior high-rate lithium-ion storage. Electrochim. Acta 2019, 294, 148–155. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Xu, M.; Feng, M.; Gu, J.; Liu, Y.; Wang, L. Improved electrochemical performances of carbon-coated LiFePO4 microspheres for Li-ion battery cathode. Mater. Res. Express 2019, 6, 115520. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Qiu, Y.; Liu, Z.; He, H.; Li, B.; Cao, H. Synthesis and superior cathode performance of sandwiched LiMn2O4@rGO nanocomposites for lithium-ion batteries. Mater. Today Adv. 2019, 1, 100001. [Google Scholar] [CrossRef]
- Li, L.; Sui, J.; Qin, W. Superior capacity, rate, long cycle life and high temperature performance of multilayered porous ultralong LiMn2O4 nanorods for lithium ion batteries. J. Electroanal. Chem. 2019, 833, 304–312. [Google Scholar] [CrossRef]
- Yi, T.; Li, Y.; Fang, Z.; Cui, P.; Luo, S.; Xie, Y. Improving the cycling stability and rate capability of LiMn0.5Fe0.5PO4/C nanorod as cathode materials by LiAlO2 modification. J. Mater. 2020, 6, 33–44. [Google Scholar] [CrossRef]
- Choi, D.; Wang, D.; Viswanathan, V.V.; Bae, I.T.; Wang, W.; Nie, Z.; Zhang, J.G.; Graff, G.L.; Liu, J.; Yang, Z.; et al. Li-ion batteries from LiFePO4 cathode and anatase/graphene composite anode for stationary energy storage. Electrochem. Commun. 2010, 12, 378–381. [Google Scholar] [CrossRef]
- Shin, H.C.; Cho, W., II; Jang, H. Electrochemical properties of carbon-coated LiFePO4 cathode using graphite, carbon black, and acetylene black. Electrochim. Acta 2006, 52, 1472–1476. [Google Scholar] [CrossRef]
- Yue, H.J.; Huang, X.K.; Lv, D.P.; Yang, Y. Hydrothermal synthesis of LiMn2O4/C composite as a cathode for rechargeable lithium-ion battery with excellent rate capability. Electrochim. Acta 2009, 54, 5363–5367. [Google Scholar] [CrossRef]
- Jo, M.; Yoo, H.; Jung, Y.S.; Cho, J. Carbon-coated nanoclustered LiMn0.71Fe0.29PO4 cathode for lithium-ion batteries. J. Power Sources 2012, 216, 162–168. [Google Scholar] [CrossRef]
- Mi, Y.; Gao, P.; Liu, W.; Zhang, W.; Zhou, H. Carbon nanotube-loaded mesoporousLiFe0.6Mn0.4PO4/C microspheres as high performance cathodes for lithium-ion batteries. J. Power Sources 2014, 267, 459–468. [Google Scholar] [CrossRef]
- Lin, B.; Yin, Q.; Hu, H.; Lu, F.; Xia, H. LiMn2O4 nanoparticles anchored on graphene nanosheets as high-performance cathode material for lithium-ion batteries. J. Solid State Chem. 2014, 209, 23–28. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Yu, S.; Xiao, Q.; Lei, G.; Ding, Y. Carbon-encapsulated LiMn2O4 spheres prepared using a polymer microgel reactor for high-power lithium-ion batteries. J. Power Sources 2016, 301, 376–385. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Li, Y.; Chen, Y.; Luo, T. Electrochemical performance of Li4Ti5O12/carbon nanotubes/graphene composite as an anode material in lithium-ion batteries. Int. J. Hydrogen Energy 2017, 42, 7195–7201. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Jin, J.; Wei, L.; Chen, W.; Lin, Y.; Huang, Z. Graphene-wrapped Li4Ti5O12 hollow spheres consisting of nanosheets as novel anode material for lithium-ion batteries. Electrochim. Acta 2017, 254, 287–298. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Chen, Y.; Wang, J.Z.; Bewlay, S.; Liu, H.K. An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim. Acta 2005, 50, 4649–4654. [Google Scholar] [CrossRef]
- Huang, Y.H.; Goodenough, J.B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem. Mater. 2008, 20, 7237–7241. [Google Scholar] [CrossRef]
- Boyano, I.; Blazquez, J.A.; de Meatza, I.; Bengoechea, M.; Miguel, O.; Grande, H.; Huang, Y.; Goodenough, J.B. Preparation of C-LiFePO4/polypyrrole lithium rechargeable cathode by consecutive potential steps electrodeposition. J. Power Sources 2010, 195, 5351–5359. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Zhu, D.; Huang, L.; Chen, Y. Improvement of the overall performances of LiMn2O4 via surface-modification by polypyrrole. Mater. Res. Bull. 2015, 71, 91–97. [Google Scholar] [CrossRef]
- Fedorková, A.; Oriňáková, R.; Oriňák, A.; Talian, I.; Heile, A.; Wiemhöfer, H.D.; Kaniansky, D.; Arlinghaus, H.F. PPy doped PEG conducting polymer films synthesized on LiFePO4 particles. J. Power Sources 2010, 195, 3907–3912. [Google Scholar] [CrossRef]
- Gong, C.; Deng, F.; Tsui, C.P.; Xue, Z.; Ye, Y.S.; Tang, C.Y.; Zhou, X.; Xie, X. PANI-PEG copolymer modified LiFePO4 as a cathode material for high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 19315–19323. [Google Scholar] [CrossRef]
- Lian, J.; Wang, X.; Zhang, W.; Huang, Y.; Xia, T.; Lian, Y. A ternary polyaniline/active carbon/lithium iron phosphate composite as cathode material for lithium ion battery. J. Nanosci. Nanotechnol. 2016, 16, 6494–6497. [Google Scholar] [CrossRef]
- Hui, Y.; Cao, L.; Xu, Z.; Huang, J.; Ouyang, H.; Li, J.; Hu, H. In situ synthesis of core-shell Li4Ti5O12 @ polyaniline composites with enhanced rate performance for lithium-ion battery anodes. J. Mater. Sci. Technol. 2017, 33, 231–238. [Google Scholar] [CrossRef]
- Xu, D.; Wang, P.; Yang, R. Enhanced electrochemical performance of core-shell Li4Ti5O12/PTh as advanced anode for rechargeable lithium-ion batteries. Ceram. Int. 2017, 43, 7600–7606. [Google Scholar] [CrossRef]
- Arbizzani, C.; Balducci, A.; Mastragostino, M.; Rossi, M.; Soavi, F. Li1.01Mn1.97O4 surface modification by poly(3,4-ethylenedioxythiophene). J. Power Sources 2003, 119–121, 695–700. [Google Scholar] [CrossRef]
- Wang, X.; Shen, L.; Li, H.; Wang, J.; Dou, H.; Zhang, X. PEDOT coated Li4Ti5O12 nanorods: Soft chemistry approach synthesis and their lithium storage properties. Electrochim. Acta 2014, 129, 283–289. [Google Scholar] [CrossRef]
- Cíntora-Juárez, D.; Pérez-Vicente, C.; Ahmad, S.; Tirado, J.L. Improving the cycling performance of LiFePO4 cathode material by poly(3,4-ethylenedioxythiopene) coating. RSC Adv. 2014, 4, 26108–26114. [Google Scholar] [CrossRef]
- Vicente, N.; Haro, M.; Cíntora-Juárez, D.; Pérez-Vicente, C.; Tirado, J.L.; Ahmad, S.; Garcia-Belmonte, G. LiFePO4 particle conductive composite strategies for improving cathode rate capability. Electrochim. Acta 2015, 163, 323–329. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, D.; Zhong, H.; Zhang, Q.; Yang, J.; Zhang, L. Facile synthesis of nanostructured Li4Ti5O12/PEDOT:PSS composite as anode material for lithium-ion batteries. RSC Adv. 2016, 6, 95512–95517. [Google Scholar] [CrossRef]
- Maleki, H.; Deng, G.; Kerzhner-Haller, I.; Anani, A.; Howard, J.N. Thermal stability studies of binder materials in anodes for lithium-ion batteries. J. Electrochem. Soc. 2000, 147, 4470–4475. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Simens, A.S.; Minor, A.M.; Song, X.; Battaglia, V.S. Optimization of acetylene black conductive additive and PVDF composition for high-power rechargeable lithium-ion cells. J. Electrochem. Soc. 2007, 154, 1129–1134. [Google Scholar] [CrossRef]
- Zaghib, K.; Striebel, K.; Guerfi, A.; Shim, J.; Armand, M.; Gauthier, M. LiFePO4/polymer/natural graphite: Low cost Li-ion batteries. Electrochim. Acta 2004, 50, 263–270. [Google Scholar] [CrossRef]
- Li, J.; Christensen, L.; Obrovac, M.N.; Hewitt, K.C.; Dahn, J.R. Effect of heat treatment on Si electrodes using polyvinylidene fluoride binder. J. Electrochem. Soc. 2008, 155, A234–A238. [Google Scholar] [CrossRef] [Green Version]
- Gören, A.; Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. State of the art and open questions on cathode preparation based on carbon coated lithium iron phosphate. Compos. Part B Eng. 2015, 83, 333–345. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Huang, L.; Sun, S.-G. Water soluble binder, an electrochemical performance booster for electrode materials with high energy density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Lee, J.H.; Paik, U.; Hackley, V.A.; Choi, Y.M. Effect of carboxymethyl cellulose on aqueous processing of natural graphite negative electrodes and their electrochemical performance for lithium batteries. J. Electrochem. Soc. 2005, 152, 1763–1769. [Google Scholar] [CrossRef]
- Park, H.; Lee, D.; Song, T. Synthesis of carboxymethyl cellulose lithium by weak acid treatment and its application in high energy-density graphite anode for Li-Ion batteries. Ind. Eng. Chem. Res. 2018, 57, 8895–8901. [Google Scholar] [CrossRef]
- Liu, G.; Shen, X.; Ui, K.; Wang, L.; Kumagai, N. Influence of the binder types on the electrochemical characteristics of tin nanoparticle negative electrode for lithium secondary batteries. J. Power Sources 2012, 217, 108–113. [Google Scholar] [CrossRef]
- Bo, D.; Xuanning, H.; Zhenfei, C.; Yangzhou, M.; Guangsheng, S.; Weidong, Y.; Cuie, W. Effects of binders on electrochemical properties of high capacity silicon composite anodes. Inorg. Chem. Commun. 2020, 113, 107771. [Google Scholar] [CrossRef]
- Huang, S.; Ren, J.; Liu, R.; Yue, M.; Huang, Y.; Yuan, G. The progress of novel binder as a non-ignorable part to improve the performance of Si-based anodes for Li-ion batteries. Int. J. Energy Res. 2018, 42, 919–935. [Google Scholar] [CrossRef]
- Ryou, M.H.; Hong, S.; Winter, M.; Lee, H.; Choi, J.W. Improved cycle lives of LiMn2O4 cathodes in lithium ion batteries by an alginate biopolymer from seaweed. J. Mater. Chem. A 2013, 1, 15224–15229. [Google Scholar] [CrossRef]
- De Giorgio, F.; La Monaca, A.; Dinter, A.; Frankenberger, M.; Pettinger, K.H.; Arbizzani, C. Water-processable Li4Ti5O12 electrodes featuring eco-friendly sodium alginate binder. Electrochim. Acta 2018, 289, 112–119. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, L.; Qu, Q.; Zhang, L.; Shen, M.; Zheng, H. Chitosan, a new and environmental benign electrode binder for use with graphite anode in lithium-ion batteries. Electrochim. Acta 2013, 105, 378–383. [Google Scholar]
- He, J.; Wang, J.; Zhong, H.; Ding, J.; Zhang, L. Cyanoethylated carboxymethyl chitosan as water soluble binder with enhanced adhesion capability and electrochemical performances for LiFePO4 cathode. Electrochim. Acta 2015, 182, 900–907. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, H.S.; Park, J.H.; Kim, T.H.; Song, H.K.; Lee, S.Y. Conducting polymer-skinned electroactive materials of lithium-ion batteries: Ready for monocomponent electrodes without additional binders and conductive agents. ACS Appl. Mater. Interfaces 2014, 6, 12789–12797. [Google Scholar] [CrossRef] [PubMed]
- Levin, O.V.; Eliseeva, S.N.; Alekseeva, E.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Composite LiFePO4/poly-3,4-ethylenedioxythiophene cathode for lithium-ion batteries with low content of non-electroactive components. Int. J. Electrochem. Sci. 2015, 10, 8175–8189. [Google Scholar]
- Das, P.R.; Komsiyska, L.; Osters, O.; Wittstock, G. PEDOT:PSS as a functional binder for cathodes in lithium ion batteries. J. Electrochem. Soc. 2015, 162, A674–A678. [Google Scholar] [CrossRef]
- Shkreba, E.V.; Eliseeva, S.N.; Apraksin, R.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Kondratiev, V.V. Electrochemical performance of lithium titanate anode fabricated using a water-based binder. Mendeleev Commun. 2019, 29, 105–107. [Google Scholar] [CrossRef]
- Chiu, H.C.; Lu, X.; Zhou, J.; Gu, L.; Reid, J.; Gauvin, R.; Zaghib, K.; Demopoulos, G.P. Capacity fade mechanism of Li4Ti5O12 nanosheet anode. Adv. Energy Mater. 2016, 7, 1601825. [Google Scholar] [CrossRef]
- Memm, M.; Hoffmann, A.; Wohlfahrt-Mehrens, M. Water-based LiNi1/3Mn1/3Co1/3O2-cathodes with good electrochemical performance by use of additives. Electrochim. Acta 2018, 260, 664–673. [Google Scholar] [CrossRef]
- Cetinel, F.A.; Bauer, W. Processing of water-based LiNi1/3Mn1/3Co1/3O2 pastes for manufacturing lithium ion battery cathodes. Bull. Mater. Sci. 2014, 37, 1685–1690. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Shao, Z.; Wang, W.; Wang, F.; Wang, D.; Zhou, Z.; Xiang, P.; Xu, C. Novel functional carboxymethyl cellulose lithium (CMC-Li) for enhanced performance of lithium-ion batteries. RSC Adv. 2014, 4, 24859–24862. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, W.; Wang, F.; Wang, J. Enhanced electrochemical properties of LiFePO4 (LFP) cathode using the carboxymethyl cellulose lithium (CMC-Li) as novel binder in lithium-ion battery. Carbohydr. Polym. 2014, 111, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dupré, N.; Gaillot, A.C.; Lestriez, B.; Martin, J.F.; Daniel, L.; Patoux, S.; Guyomard, D. CMC as a binder in LiNi0.4Mn1.6O4 5 V cathodes and their electrochemical performance for Li-ion batteries. Electrochim. Acta 2012, 62, 77–83. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, G.T.; Chao, D.; Loeffler, N.; Copley, M.; Lin, J.; Shen, Z.; Passerini, S. Toward greener lithium-ion batteries: Aqueous binder-based LiNi0.4Co0.2Mn0.4O2 cathode material with superior electrochemical performance. J. Power Sources 2017, 372, 180–187. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Shkreba, E.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Holze, R.; Kondratiev, V.V. Effects of conductive binder on the electrochemical performance of lithium titanate anodes. Solid State Ionics 2019, 333, 18–29. [Google Scholar] [CrossRef]
- Apraksin, R.V.; Eliseeva, S.N.; Kamenskii, M.A.; Tolstopyatova, E.G.; Lang, G.G.; Kondrat’ev, V.V. Impedance of LiFe0.4Mn0.6PO4 electrodes with combined conducting polymer binder of PEDOT:PSS and carboxymethyl cellulose. Russ. J. Electrochem. 2019, 55, 1047–1057. [Google Scholar] [CrossRef]
- Vorobeva, K.A.; Eliseeva, S.N.; Apraksin, R.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Kondratiev, V.V. Improved electrochemical properties of cathode material LiMn2O4 with conducting polymer binder. J. Alloys Compd. 2018, 766, 33–44. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Apraksin, R.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Electrochemical impedance spectroscopy characterization of LiFePO4 cathode material with carboxymethylcellulose and poly-3,4-ethylendioxythiophene/polystyrene sulfonate. Electrochim. Acta 2017, 227, 357–366. [Google Scholar] [CrossRef]
- Apraksin, R.V.; Eliseeva, S.N.; Tolstopjatova, E.G.; Rumyantsev, A.M.; Zhdanov, V.V.; Kondratiev, V.V. High-rate performance of LiFe0.4Mn0.6PO4 cathode materials with poly(3,4-ethylenedioxythiopene): poly(styrene sulfonate)/carboxymethylcellulose. Mater. Lett. 2016, 176, 248–252. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Levin, O.V.; Tolstopjatova, E.G.; Alekseeva, E.V.; Apraksin, R.V.; Kondratiev, V.V. New functional conducting poly-3,4-ethylenedioxythiopene:polystyrene sulfonate/carboxymethylcellulose binder for improvement of capacity of LiFePO4-based cathode materials. Mater. Lett. 2015, 161, 117–119. [Google Scholar] [CrossRef]
- Shao, D.; Zhong, H.; Zhang, L. Water-soluble conductive composite binder containing PEDOT: PSS as conduction promoting agent for Si anode of lithium-ion batteries. ChemElectroChem 2014, 1, 1679–1687. [Google Scholar] [CrossRef]
- Lee, B.R.; Oh, E.S. Effect of molecular weight and degree of substitution of a sodium-carboxymethyl cellulose binder on Li4Ti5O12 anodic performance. J. Phys. Chem. C 2013, 117, 4404–4409. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, T.; Lai, Y.; Jia, M.; Li, J. A comparative study of different binders and their effects on electrochemical properties of LiMn2O4 cathode in lithium ion batteries. J. Power Sources 2014, 247, 1–8. [Google Scholar] [CrossRef]
- Du, G.; Xi, Y.; Tian, X.; Zhu, Y.; Zhou, Y.; Deng, C.; Zhu, H.; Natarajan, A. One-step hydrothermal synthesis of 3D porous microspherical LiFePO4/graphene aerogel composite for lithium-ion batteries. Ceram. Int. 2019, 45, 18247–18254. [Google Scholar] [CrossRef]
- Lepage, D.; Savignac, L.; Saulnier, M.; Gervais, S.; Schougaard, S.B. Modification of aluminum current collectors with a conductive polymer for application in lithium batteries. Electrochem. Commun. 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Lepage, D.; Michot, C.; Liang, G.; Gauthier, M.; Schougaard, S.B. A soft chemistry approach to coating of LiFePO4 with a conducting polymer. Angew. Chem.–Int. Ed. 2011, 50, 6884–6887. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhong, H.; Wang, J.; Zhang, L. Investigation on xanthan gum as novel water soluble binder for LiFePO4 cathode in lithium-ion batteries. J. Alloys Compd. 2017, 714, 409–418. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Su, S.; Han, G.; Liu, J. Hybrid humics/sodium carboxymethyl cellulose water-soluble binder for enhancing the electrochemical performance of a Li-ion battery cathode. Powder Technol. 2019, 351, 203–211. [Google Scholar] [CrossRef]
- Lux, S.F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low cost, environmentally benign binders for lithium-ion batteries. J. Electrochem. Soc. 2010, 157, A320. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, F.; Wang, W.; Wang, J. Novel polymer Li-ion binder carboxymethyl cellulose derivative enhanced electrochemical performance for Li-ion batteries. Carbohydr. Polym. 2014, 112, 532–538. [Google Scholar] [CrossRef]
- Kubarkov, A.V.; Drozhzhin, O.A.; Karpushkin, E.A.; Stevenson, K.J.; Antipov, E.V.; Sergeyev, V.G. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonic acid)–Polymer composites as functional cathode binders for high power LiFePO4 batteries. Colloid Polym. Sci. 2019, 297, 475–484. [Google Scholar] [CrossRef]
- Hong, J.; Wang, F.; Wang, X.; Graetz, J. LiFexMn1-xPO4: A cathode for lithium-ion batteries. J. Power Sources 2011, 196, 3659–3663. [Google Scholar] [CrossRef]
- Hu, L.; Qiu, B.; Xia, Y.; Qin, Z.; Qin, L.; Zhou, X.; Liu, Z. Solvothermal synthesis of Fe-doping LiMnPO4 nanomaterials for Li-ion batteries. J. Power Sources 2014, 248, 246–252. [Google Scholar] [CrossRef]
- Yan, S.Y.; Wang, C.Y.; Gu, R.M.; Li, M.W. Enhanced kinetic behaviors of LiMn0.5Fe0.5PO4/C cathode material by Fe substitution and carbon coating. J. Solid State Electrochem. 2015, 19, 2943–2950. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yuan, L.X.; Zhang, W.X.; Huang, Y.H. LiFe0.8Mn0.2PO4/C cathode material with high energy density for lithium-ion batteries. J. Alloys Compd. 2012, 532, 25–30. [Google Scholar] [CrossRef]
- Ding, J.; Su, Z.; Tian, H. Synthesis of high rate performance LiFe1−xMnxPO4/C composites for lithium-ion batteries. Ceram. Int. 2016, 42, 12435–12440. [Google Scholar] [CrossRef]
- Martha, S.K.; Grinblat, J.; Haik, O.; Zinigrad, E.; Drezen, T.; Miners, J.H.; Exnar, I.; Kay, A.; Markovsky, B.; Aurbach, D.; et al. LiMn0.8Fe0.2PO4: An advanced cathode material for rechargeable lithium batteries. Angew. Chem.–Int. Ed. 2009, 48, 8559–8563. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Wang, Y.; Zhang, J. PVP-assisted solvothermal synthesis of LiMn0.8Fe0.2PO4/C nanorods as cathode material for lithium ion batteries. Ceram. Int. 2016, 42, 9018–9024. [Google Scholar] [CrossRef]
- Xiang, W.; Wu, Z.G.; Wang, E.H.; Chen, M.Z.; Song, Y.; Zhang, J.B.; Zhong, Y.J.; Chou, S.L.; Luo, J.H.; Guo, X.D.; et al. Confined synthesis of graphene wrapped LiMn0.5Fe0.5PO4 composite via two step solution phase method as high performance cathode for Li-ion batteries. J. Power Sources 2016, 329, 94–103. [Google Scholar] [CrossRef]
- Saravanan, K.; Ramar, V.; Balaya, P.; Vittal, J.J. Li(MnxFe1-x)PO4/C (x = 0.5, 0.75 and 1) nanoplates for lithium storage application. J. Mater. Chem. 2011, 21, 14925–14935. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, Y.; Deng, Y.; Qin, X.; Chen, G. The enhanced rate performance of LiFe0.5Mn0.5PO4/C cathode material via synergistic strategies of surfactant-assisted solid state method and carbon coating. J. Mater. Chem. A 2015, 3, 996–1004. [Google Scholar] [CrossRef]
- Li, J.; Xiang, M.; Wang, Y.; Wu, J.; Zhao, H.; Liu, H. Effects of adhesives on the electrochemical performance of monodisperse LiMn0.8Fe0.2PO4/C microspheres as cathode materials for high power lithium-ion batteries. J. Mater. Chem. A 2017, 5, 7952–7960. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Li, H.J.; Ozawa, K.; Wang, J.Z. Enhancing the high rate capability and cycling stability of LiMn2O4 by coating of solid-state electrolyte LiNbO3. ACS Appl. Mater. Interfaces 2014, 6, 22155–22165. [Google Scholar] [CrossRef] [PubMed]
- Han, C.G.; Zhu, C.; Saito, G.; Akiyama, T. Improved electrochemical performance of LiMn2O4 surface-modified by a Mn4+-rich phase for rechargeable lithium-ion batteries. Electrochim. Acta 2016, 209, 225–234. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, H.; Hu, Y.; Dai, Y.; Zhang, L.; Li, C. Synergistic enhancement effect of Al doping and highly active facets of LiMn2O4 cathode materials for lithium-ion batteries. Ind. Eng. Chem. Res. 2015, 54, 3800–3805. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Varadaraju, U.V.; Gopalan, R.; Prakash, R. Structural stability and superior electrochemical performance of Sc-doped LiMn2O4 spinel as cathode for lithium ion batteries. Electrochim. Acta 2019, 301, 342–351. [Google Scholar] [CrossRef]
- Maazi, S.; Navarchian, A.H.; Khosravi, M.; Chen, P. Effect of poly (vinylidene fluoride)/poly (vinyl acetate) blend composition as cathode binder on electrochemical performances of aqueous Li-ion battery. Solid State Ionics 2018, 320, 84–91. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.Y.; Lee, H.; Oh, E.S. Effects of polymeric binders on electrochemical performances of spinel lithium manganese oxide cathodes in lithium ion batteries. J. Power Sources 2014, 269, 418–423. [Google Scholar] [CrossRef]
- Hou, L.; Qin, X.; Gao, X.; Guo, T.; Li, X.; Li, J. Zr-doped Li4Ti5O12 anode materials with high specific capacity for lithium-ion batteries. J. Alloys Compd. 2019, 774, 38–45. [Google Scholar] [CrossRef]
- Hu, G.; Wu, J.; Du, K.; Peng, Z.; Jia, M.; Yang, H.; Cao, Y. Surface-fluorinated Li4Ti5O12 nanowires/reduced graphene oxide composite as a high-rate anode material for Lithium ion batteries. Appl. Surf. Sci. 2019, 479, 158–166. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, J.; Yu, Z.; Luo, L. Conductive PEDOT-decorated Li4Ti5O12 as next-generation anode material for electrochemical lithium storage. Solid State Ionics 2018, 325, 7–11. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, G.; Gao, L. Electrochemical characterization of Li4Ti5O12/C anode material prepared by starch-sol-assisted rheological phase method for Li-ion battery. J. Nanomater. 2012, 2012, 11–13. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Räsänen, S.; Jokinen, M.; Yliniemi, K.; Worsley, D.A.; Kuusivaara, J.; Juurikivi, J.; Ekqvist, R.; Kallio, T.; Karppinen, M.; et al. Water soluble binder for fabrication of Li4Ti5O12 electrodes. J. Power Sources 2013, 226, 134–139. [Google Scholar] [CrossRef]
- Birrozi, A.; Copley, M.; von Zamory, J.; Pasqualini, M.; Calcaterra, S.; Nobili, F.; Cicco, A.D.; Rajantie, H.; Briceno, M.; Bilbé, E.; et al. Scaling up “Nano” Li4Ti5O12 for high-power lithium-ion anodes using large scale flame spray pyrolysis. J. Electrochem. Soc. 2015, 162, A2331–A2338. [Google Scholar] [CrossRef]
- Chou, S.-L.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery. J. Phys. Chem. C 2011, 115, 16220–16227. [Google Scholar] [CrossRef]
- Lee, B.; Kim, S.; Oh, E. Bio-derivative galactomannan gum binders for Li4Ti5O12 negative electrodes in lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A2128–A2132. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Eliseeva, S.N.; Holze, R.; Kondratiev, V.V. Performance of negative lithium titanate electrodes containing minimized amounts of conducting polymer and modified guar gum as binder. J. Electrochem. Soc. 2019, 166, A3354–A3361. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chou, S.L.; Gu, Q.F.; Liu, H.K.; Dou, S.X. The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Guerfi, A.; Kaneko, M.; Petitclerc, M.; Mori, M.; Zaghib, K. LiFePO4 water-soluble binder electrode for Li-ion batteries. J. Power Sources 2007, 163, 1047–1052. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Casalegno, M.; Castiglione, F.; Passarello, M.; Mele, A.; Passerini, S.; Raos, G. Association and diffusion of Li+ in carboxymethylcellulose solutions for environmentally friendly Li-ion batteries. ChemSusChem 2016, 9, 1804–1813. [Google Scholar] [CrossRef] [Green Version]




| Electrodes | Active Material, wt. % | C, wt. % | PEDOT:PSS, wt. % | CMC, wt. % | PVDF, wt. % |
|---|---|---|---|---|---|
| LFPPEDOT:PSS/CMC | 92 | 4 | 2 | 2 | – |
| LFPCMC | 92 | 4 | – | 4 | – |
| LFPPVDF | 84 | 8 | – | – | 8 |
| LFMPPEDOT:PSS/CMC | 92 | 4 | 2 | 2 | – |
| LFMPPVDF | 80 | 10 | – | – | 10 |
| LMOPEDOT:PSS/CMC | 86 | 10 | 2 | 2 | – |
| LMOCMC | 92 | 4 | – | 4 | – |
| LMOPVDF | 80 | 10 | – | – | 10 |
| LTOPEDOT:PSS/CMC | 90 | 6 | 2 | 2 | – |
| LTOCMC | 90 | 6 | – | 4 | – |
| LTOPVDF | 80 | 10 | – | – | 10 |
| Electrode | Qelectrode, mAhg−1 | Qelectroactive material,mAhg−1 |
|---|---|---|
| LFPPEDOT:PSS/CMC | 149 | 162 |
| LFPCMC | 145 | 158 |
| LFPPVDF | 131 | 156 |
| LFMPPEDOT:PSS/CMC | 139 | 151 |
| LFMPPVDF | 119 | 142 |
| LMOPEDOT:PSS/CMC | 108 | 126 |
| LMOCMC | 95 | 103 |
| LMOPVDF | 92 | 115 |
| LTOPEDOT:PSS/CMC | 158 | 174 |
| LTOCMC | 151 | 168 |
| LTOPVDF | 137 | 171 |
| Cathode Material Based on LiFePO4 | QLFP, mAh·g−1 | Ref | ||
|---|---|---|---|---|
| 0.2 C | 1 C | 10 C | ||
| [LiFePO4/graphene aerogel-10%]/C/PVDF | 168 | 155 | 115 | [73] |
| [LiFePO4/activated carbon/graphene]/C/PVDF | – | 167 | 143 | [10] |
| [LiFePO4/graphene]/C/PVDF | – | 163 | 137 | [10] |
| [Carbon-coated LFP microspheres]/C/PVDF | 143 | 113 | – | [11] |
| C-LiFePO4/PVDF/C on Al substrate | – | 143 | 92 | [74] |
| PEDOT/LiFePO4/PVDF on C-coated Al substrate | 160 | 155 | 125 | [75] |
| C-LiFePO4/PANI/C/PVDF | 160 | 150 | 80 | [30] |
| [LiFePO4-PEDOT blend]/C/PVDF | 135 | 125 | – | [36] |
| LiFePO4/PANI/C/PTFE | 150 | 120 | 65 | [25] |
| LiFePO4/PPy/C/PTFE | 150 | 130 | 100 | [25] |
| LiFePO4-PEDOT/PVDF | 163 | 151 | 123 | [75] |
| LiFePO4/PEDOT (binder free) | 125 | 105 | – | [35] |
| C-LiFePO4/C/PVDF on PEDOT-coated Al substrate | – | 151 | 110 | [74] |
| LiFePO4/C/xanthan gum | 157 | 138 | 80 (5 C) | [76] |
| LiFePO4/C/cyanoethylated carboxymethyl chitosan | 158 | 139 | 90 (5 C) | [52] |
| LiFePO4/C/CMC | 155 | 128 | 78 (5 C) | [52] |
| LiFePO4/C/CMC | 130 | 115 | 70 | [77] |
| LiFePO4/C/CMC | – | 140 | – | [78] |
| LiFePO4/C/CMC | 176 | 140 | 90 (5 C) | [61] |
| LiFePO4/C/CMC | 150 | 137 | 80 (5 C) | [79] |
| LiFePO4/C/CMC | – | 115 | 75 (8 C) | [77] |
| LiFePO4/C/[humic acid-CMC] | – | 147 | 105 (8 C) | [77] |
| LiFePO4/C/PEDOT:PSS | 155 | 135 | 90 | [80] |
| C-LFP/PEDOT:PSS (8%) | - | 110 | – | [55] |
| C-LiFePO4/C/PEDOT:PSS/CMC | 161 | 155 | 139 (5 C) | [67] |
| Cathode Material Based on LiFeMnPO4 | QLFMP, mAh·g−1 | Ref | ||
| 0.2 C | 1 C | 10 C | ||
| LiFe0.2Mn0.8PO4/C/PVDF | 112 | 98 | 55 (5 C) | [81] |
| LiMn0.9Fe0.1PO4/C/PVDF | 135 | 75 | – | [82] |
| LiFe0.4Mn0.6PO4/C/PVDF | 146 | 140 | – | [82] |
| [LiMn0.5Fe0.5PO4/C]/C/PVDF | 142 | 130 | 105 | [83] |
| [LiFe0.6Mn0.4PO4/C composites with MWCNTs]/C/PVDF | – | 145 | 125 | [19] |
| Carbon-coated nanoclustered LiMn0.71Fe0.29PO4/C/PVDF | 156 | 142 | 85 (7 C) | [18] |
| [LiFe0.8Mn0.2PO4/C]/C/PTFE | 152 | 147 | 122 | [84] |
| [LiFe0.9Mn0.1PO4/C]/C/PTFE | – | 140 | 110 | [85] |
| C-LiMn0.8Fe0.2PO4/C/PVDF | 157 | 150 | 94-95 | [86] |
| [LiMn0.8Fe0.2PO4/C nanorods]/C/PVDF | 145 | 130 | – | [87] |
| [LiMn0.5Fe0.5PO4/C nanorods, LiAlO2 modification]/C/PVDF | 132 | 120 | 105 (5 C) | [14] |
| [LiFe0.5Mn0.5PO4/C nanorods]/C/PVDF | 157 | 143 | 102 | [9] |
| [Graphene wrapped LiMn0.5Fe0.5PO4 composite]/C/PVDF | 156 | 126 | 101 | [88] |
| [LiFe0.5Mn0.5PO4 /C nanoplates]/C/PVDF | – | 120 | 60 (9 C) | [89] |
| [LiFe0.5Mn0.5PO4 /C]/C/PVDF | – | 141 | 121 (5 C) | [90] |
| LiMn0.8Fe0.2PO4/C/LA133 | 155 | 145 | 130 | [91] |
| LiFe0.4Mn0.6PO4/C/PEDOT:PSS/CMC | 151 | 131 | 129 | [65] |
| Cathode Material Based on LiMn2O4 | QLMO, mAh g-1 | Ref | ||
| 0.2 C | 1 C | 10 C | ||
| Multilayered porous ultralong LiMn2O4 nanorods/C/PVDF | 134 | 119 | 102 | [13] |
| Sandwiched LiMn2O4@rGO nanocomposites/C/PVDF | 124 | 116 | 97 | [12] |
| [LiMn2O4 with LiNbO3 coating]/C/PVDF | – | 122 | 117 | [92] |
| [LiMn2O4 surface modified by a Mn4+-rich phase]/C/PVDF | – | 123 | 107 (7 C) | [93] |
| Al-doped LiMn2O4/C/PVDF/ | 116 | 110 | 102 | [94] |
| Sc-doped LiMn2O4/C/PVDF | – | 111 | 96 | [95] |
| LiMn2O4 coated by polypyrrole/C/PVDF | – | 117 | 92 | [27] |
| [Li1.01Mn1.97O4 surface modified by PEDOT]/PVDF | 119 | 111 | – | [33] |
| LiMn2O4/C/[PVDF-PVA] (25:75) | 110 | 105 | – | [96] |
| LiMn2O4/C/sodium alginate | – | 118 | – | [49] |
| LiMn2O4/C/PVDF | 122 | 115 | 92 (3 C) | [72] |
| LiMn2O4/C/CMC | 130 | 110 | 85 (3 C) | [72] |
| LiMn2O4/C/PAA (in NMP) | 128 | 110 | 82 (3 C) | [72] |
| LiMn2O4/C/polyacrylonitrile | 92 | 89 | – | [97] |
| LiMn2O4/C/PEDOT:PSS/CMC | 128 | 116 | 87 | [66] |
| Anode Material Based on Li4Ti5O12 | QLTO, mAh·g−1 | Ref | ||
| 0.2 C | 1 C | 10 C | ||
| [Mesoporous Li4Ti5O12/C nanocomposites]/C/PVDF | 162 | 145 | 121 | [7] |
| Zr-doped Li4Ti5O12/C/PVDF | – | 188 | 149 | [98] |
| [Surface-fluorinated Li4Ti5O12 nanowires/rGO composite]/C/PVDF | – | 167 | 133 | [99] |
| [Li4Ti5O12 coated by PANI]/C/PVDF | 175 | 155 | 100 | [31] |
| [Li4Ti5O12 nanorods coated by PEDOT]/C/PVDF | 172 | 169 | 135 | [34] |
| [Li4Ti5O12 coated by PEDOT]/C/PVDF | 172 | 160 | 140 | [100] |
| [Li4Ti5O12/PEDOT:PSS composites]/C/PVDF (0.1 C charge) | 174 | 170 | 161 | [37] |
| [Li4Ti5O12 coated by polythiophene]/C/PVDF | 170 | 167 | 140 | [32] |
| Li4Ti5O12/C/sodium alginate | – | 152 | 135 | [50] |
| Li4Ti5O12/C/[CMC-styrene butadiene rubber] | 160 | 125 | 55 | [101] |
| Li4Ti5O12/C/Acryl S020 | 180 | 170 | 120 | [102] |
| Li4Ti5O12/C/CMC | 165 | 150 | 60 | [71] |
| Li4Ti5O12/C/CMC | 148 | 134 | 118 | [103] |
| Li4Ti5O12/C/CMC | 160 | 145 | 78 | [104] |
| Li4Ti5O12/C/CMC | 165 | 150 | 110 | [105] |
| Li4Ti5O12/C/PEDOT:PSS/guar gum | 166 | 164 | 119 | [106] |
| Li4Ti5O12/C/PEDOT:PSS/CMC | 175 | 167 | 138 | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliseeva, S.N.; Kamenskii, M.A.; Tolstopyatova, E.G.; Kondratiev, V.V. Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries. Energies 2020, 13, 2163. https://doi.org/10.3390/en13092163
Eliseeva SN, Kamenskii MA, Tolstopyatova EG, Kondratiev VV. Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries. Energies. 2020; 13(9):2163. https://doi.org/10.3390/en13092163
Chicago/Turabian StyleEliseeva, Svetlana N., Mikhail A. Kamenskii, Elena G. Tolstopyatova, and Veniamin V. Kondratiev. 2020. "Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries" Energies 13, no. 9: 2163. https://doi.org/10.3390/en13092163
APA StyleEliseeva, S. N., Kamenskii, M. A., Tolstopyatova, E. G., & Kondratiev, V. V. (2020). Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries. Energies, 13(9), 2163. https://doi.org/10.3390/en13092163