Microwave-Assisted Noncatalytic Esterification of Fatty Acid for Biodiesel Production: A Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Effects of Different Heating Processes
2.3. Analysis
2.4. Kinetics of Noncatalytic Esterification Using Microwave Irradiation
3. Results and Discussion
3.1. Effect of Various Heating Processes
3.2. Influence of Reactant Molar Ratio
3.3. Temperature Effect on Esterification
3.4. Effect of Microwave Power on Reaction
3.5. Kinetic Model Development
3.5.1. Determination of Reaction Rate Constant
3.5.2. Determination of Pre-Exponential Factor and Activation Energy
3.5.3. Relation of Microwave Power to Pre-Exponential Factor
3.6. Comparison with Other Works
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, H.C.; Liang, S.-H.; Chen, S.-S.; Su, C.-H.; Lin, J.-H.; Chien, C.-C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Santos, S.; Nobre, L.; Gomes, J.; Puna, J.; Quinta-Ferreira, R.; Bordado, J. Soybean oil transesterification for biodiesel production with micro-structured calcium oxide (CaO) from natural waste materials as a heterogeneous catalyst. Energies 2019, 12, 4670. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.; Park, S.; Roh, H.G.; Lee, C.S. Combustion and emission reduction characteristics of GTL-biodiesel fuel in a single-cylinder diesel engine. Energies 2019, 12, 2201. [Google Scholar] [CrossRef] [Green Version]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Selvaraj, R.; Praveenkumar, R.; Moorthy, I.G. A comprehensive review of biodiesel production methods from various feedstocks. Biofuels 2019, 10, 325–333. [Google Scholar] [CrossRef]
- Almasi, S.; Ghobadian, B.; Najafi, G.H.; Yusaf, T.; Dehghani Soufi, M.; Hoseini, S.S. Optimization of an ultrasonic-assisted biodiesel production process from one genotype of rapeseed (TERI (OE) R-983) as a novel feedstock using response surface methodology. Energies 2019, 12, 2656. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.C.; Liang, S.-H.; Doan, T.T.; Su, C.-H.; Yang, P.-C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Khan, I.U.; Yan, Z.; Chen, J. Optimization, transesterification and analytical study of Rhus typhina non-edible seed oil as biodiesel production. Energies 2019, 12, 4290. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, M.-C.; Chang, L.-W.; Hou, S.-S. Study of solid calcium diglyceroxide for biodiesel production from waste cooking oil using a high speed homogenizer. Energies 2019, 12, 3205. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H.; Nguyen, H.C.; Pham, U.K.; Nguyen, M.L.; Juan, H.-Y. Biodiesel production from a novel nonedible feedstock, soursop (Annona muricata L.) seed oil. Energies 2018, 11, 2562. [Google Scholar] [CrossRef] [Green Version]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Indra Mahlia, T.M.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Lucena, I.L.; Saboya, R.M.; Oliveira, J.F.; Rodrigues, M.L.; Torres, A.E.; Cavalcante, C.L., Jr.; Parente, E.J., Jr.; Silva, G.F.; Fernandes, F.A. Oleic acid esterification with ethanol under continuous water removal conditions. Fuel 2011, 90, 902–904. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Lee, H. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Melero, J.A.; Bautista, L.F.; Morales, G.; Iglesias, J.; Sánchez-Vázquez, R. Biodiesel production from crude palm oil using sulfonic acid-modified mesostructured catalysts. Chem. Eng. J. 2010, 161, 323–331. [Google Scholar] [CrossRef]
- Costa, A.A.; Braga, P.R.; de Macedo, J.L.; Dias, J.A.; Dias, S.C. Structural effects of WO3 incorporation on USY zeolite and application to free fatty acids esterification. Micropor. Mesopor. Mat. 2012, 147, 142–148. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Santos, N.A.; Saczk, A.A.; Hori, C.E.; Arroyo, P.A. Biodiesel production by free fatty acid esterification using lanthanum (La3+) and HZSM-5 based catalysts. Bioresour. Technol. 2013, 133, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Mateos, P.; García-Martín, J.; Guerrero-Vacas, F.; Naranjo-Calderón, C.; Barrios-Sánchez, C.; Pérez Camino, M.D.C. Valorization of a high-acidity residual oil generated in the waste cooking oils recycling industries. Grasas y Aceites 2019, 70, e335. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Si, Z.; Sun, W.; Zhang, P. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Selvakumar, P.; Sivashanmugam, P. Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production. Energy Convers. Manag. 2019, 179, 141–151. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.-Y.; Su, C.-H.; Chien, C.-C. Liquid lipase-catalyzed esterification of oleic acid with methanol for biodiesel production in the presence of superabsorbent polymer: Optimization by using response surface methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef] [Green Version]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Sundaram, S.; Tišma, M.; Hessel, V. Cost analysis of enzymatic biodiesel production in small-scaled packed-bed reactors. Appl. Energy 2018, 210, 268–278. [Google Scholar] [CrossRef]
- Rani, K.N.P.; Neeharika, T.S.V.R.; Kumar, T.P.; Satyavathi, B.; Sailu, C. Kinetics of non-catalytic esterification of free fatty acids present in Jatropha oil. J. Oleo Sci. 2016, 65, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Alenezi, R.; Leeke, G.; Winterbottom, J.; Santos, R.; Khan, A. Esterification kinetics of free fatty acids with supercritical methanol for biodiesel production. Energy Convers. Manag. 2010, 51, 1055–1059. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Lin, H.-M.; Lee, M.-J. Biodiesel production with continuous supercritical process: Non-catalytic transesterification and esterification with or without carbon dioxide. Bioresour. Technol. 2013, 145, 362–369. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, S.H.; Hong, S.W.; Yeo, Y.-K. A single step non-catalytic esterification of palm fatty acid distillate (PFAD) for biodiesel production. Fuel 2012, 93, 373–380. [Google Scholar] [CrossRef]
- Thakkar, K.; Shah, K.; Kodgire, P.; Kachhwaha, S.S. In-situ reactive extraction of castor seeds for biodiesel production using the coordinated ultrasound–microwave irradiation: Process optimization and kinetic modeling. Ultrason. Sonochem. 2019, 50, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guerra, E.; Howlader, M.S.; Shields-Menard, S.; French, W.T.; Gude, V.G. Optimization of wet microalgal FAME production from Nannochloropsis sp. under the synergistic microwave and ultrasound effect. Int. J. Energy Res. 2018, 42, 1934–1949. [Google Scholar] [CrossRef]
- Encinar, J.; González, J.; Martínez, G.; Sánchez, N.; Pardal, A. Soybean oil transesterification by the use of a microwave flow system. Fuel 2012, 95, 386–393. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. Microwave-assisted transesterification of industrial grade crude glycerol for the production of glycerol carbonate. Chem. Eng. J. 2016, 284, 469–477. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.-H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, Y. Direct transesterification of wet Cryptococcus curvatus cells to biodiesel through use of microwave irradiation. Appl. Energy 2014, 119, 438–444. [Google Scholar] [CrossRef]
- Ye, W.; Gao, Y.; Ding, H.; Liu, M.; Liu, S.; Han, X.; Qi, J. Kinetics of transesterification of palm oil under conventional heating and microwave irradiation, using CaO as heterogeneous catalyst. Fuel 2016, 180, 574–579. [Google Scholar] [CrossRef]
- Teo, C.L.; Idris, A. Evaluation of direct transesterification of microalgae using microwave irradiation. Bioresour. Technol. 2014, 174, 281–286. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, H.; Yang, Y.; Huang, K.; Raghavan, G.V. Dynamic analysis of a continuous-flow microwave-assisted screw propeller system for biodiesel production. Chem. Eng. Sci. 2019, 202, 146–156. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lee, H.-Y.; Su, C.-H.; Shih, W.-J.; Chien, C.-C. Green process for fatty acid production from soybean oil through microwave-mediated autocatalytic synthesis. Chem. Eng. Process. 2020, 147, 107782. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huang, K.-C.; Su, C.-H. Green process for the preparation of phytosterol esters: Microwave-mediated noncatalytic synthesis. Chem. Eng. J. 2020, 382, 122796. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Ong, H.C.; Pham, T.T.T.; Dinh, T.K.K.; Su, C.H. Microwave-mediated noncatalytic synthesis of ethyl levulinate: A green process for fuel additive production. Int. J. Energy Res. 2019, 44, 1698–1708. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Proces. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.; Du, W.; Li, Y.; Liu, D. Ethanol as the acyl acceptor for biodiesel production. Renew. Sustain. Energy Rev. 2013, 25, 742–748. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Wu, J.; Zhang, Y.; Yuan, Z. Enhanced esterification of oleic acid and methanol by deep eutectic solvent assisted Amberlyst heterogeneous catalyst. Bioresour. Technol. 2016, 220, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H. Kinetic study of free fatty acid esterification reaction catalyzed by recoverable and reusable hydrochloric acid. Bioresour. Technol. 2013, 130, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Warrand, J.; Janssen, H.-G. Controlled production of oligosaccharides from amylose by acid-hydrolysis under microwave treatment: Comparison with conventional heating. Carbohydr. Polym. 2007, 69, 353–362. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of microwave and conduction-convection heating autohydrolysis pretreatment for bioethanol production. Bioresour. Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef]
- Loong, T.C.; Idris, A. Rapid alkali catalyzed transesterification of microalgae lipids to biodiesel using simultaneous cooling and microwave heating and its optimization. Bioresour. Technol. 2014, 174, 311–315. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of glycerolysis reaction of higher free fatty acid containing sustainable feedstock using microwave irradiation. Fuel Process. Technol. 2014, 118, 110–116. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Pisarello, M.; Querini, C.A. Butia Yatay coconut oil: Process development for biodiesel production and kinetics of esterification with ethanol. Energy Convers. Manag. 2014, 85, 407–416. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef] [Green Version]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, Y.C. Low cost guinea fowl bone derived recyclable heterogeneous catalyst for microwave assisted transesterification of Annona squamosa L. seed oil. Energy Convers. Manag. 2017, 138, 627–637. [Google Scholar] [CrossRef]
- Su, C.H.; Fu, C.C.; Gomes, J.; Chu, I.; Wu, W.T. A heterogeneous acid-catalyzed process for biodiesel production from enzyme hydrolyzed fatty acids. AIChE J. 2008, 54, 327–336. [Google Scholar] [CrossRef]
- Melo-Júnior, C.A.; Albuquerque, C.E.; Fortuny, M.; Dariva, C.; Egues, S.; Santos, A.F.; Ramos, A.L. Use of microwave irradiation in the noncatalytic esterification of C18 fatty acids. Energy Fuels 2009, 23, 580–585. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Lin, Z.-R.; Zeng, X.-A.; Yu, S.-J.; Sun, D.-W. Enhancement of ethanol–acetic acid esterification under room temperature and non-catalytic condition via pulsed electric field application. Food Bioprocess Technol. 2012, 5, 2637–2645. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Mutyala, S.; Fairbridge, C.; Paré, J.J.; Bélanger, J.M.; Ng, S.; Hawkins, R. Microwave applications to oil sands and petroleum: A review. Fuel Process. Technol. 2010, 91, 127–135. [Google Scholar] [CrossRef]



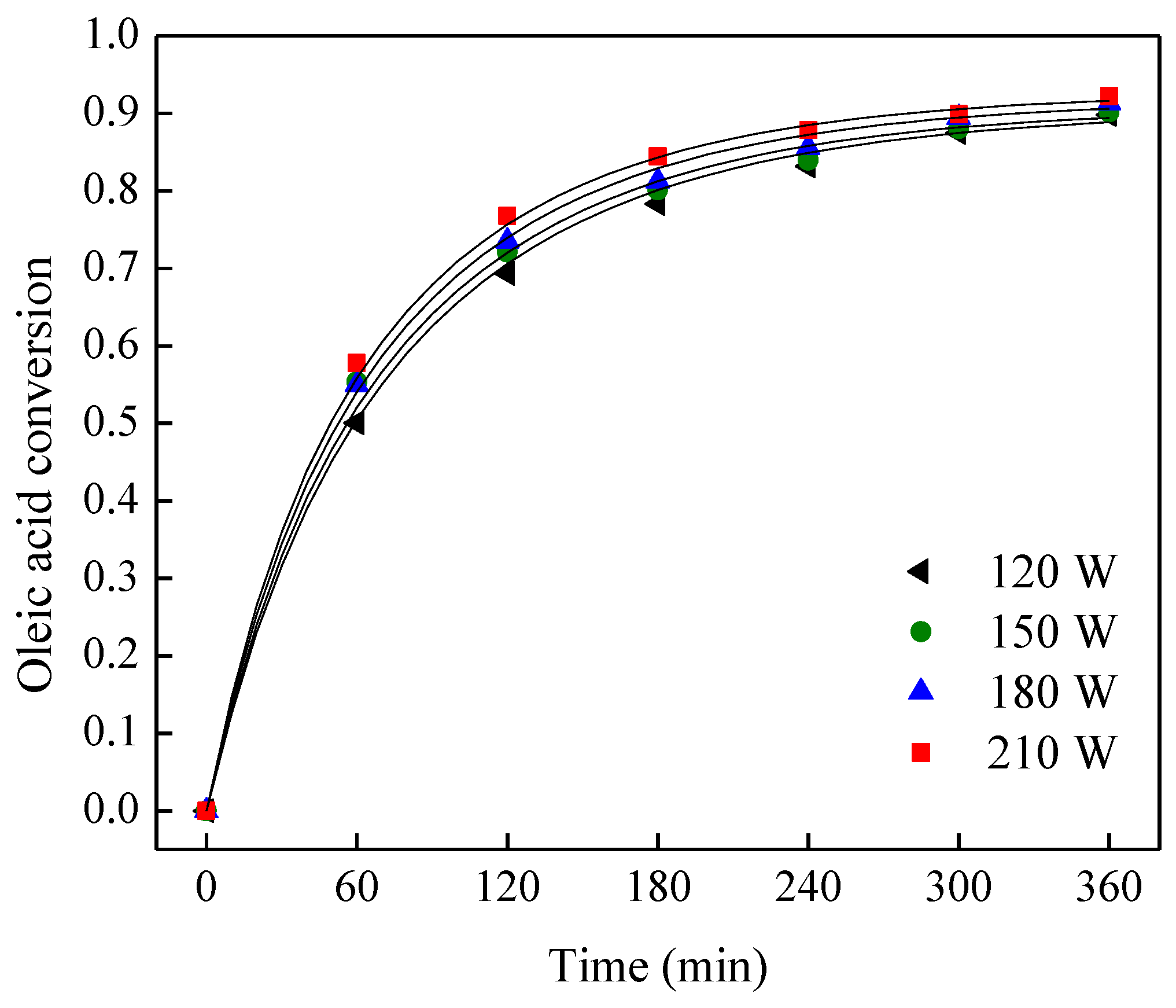
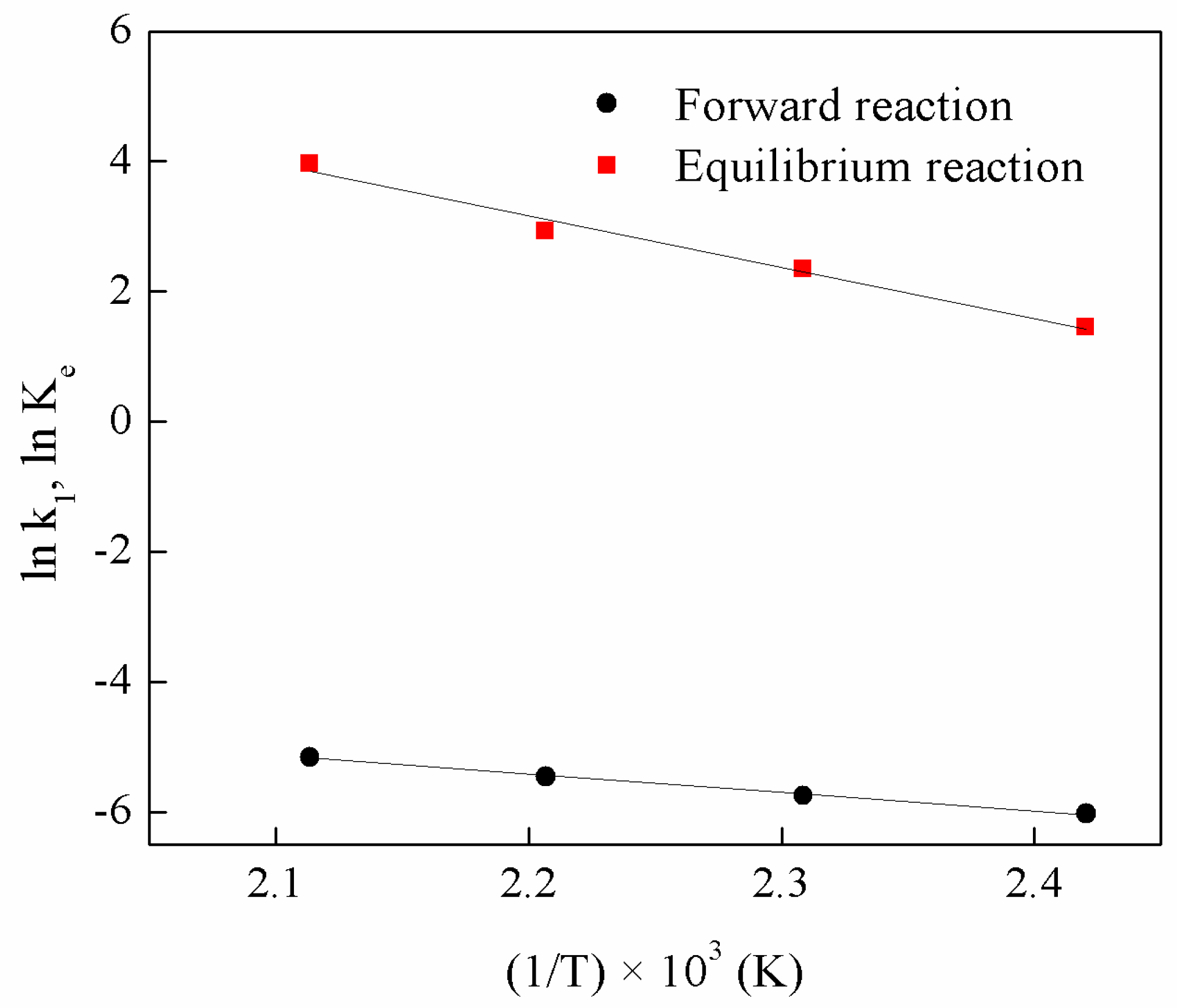

| Run | Molar Ratio of Ethanol to Oleic Acid | Temperature (K) | Microwave Power (W) | Equilibrium Constant, Ke | Forward Reaction Rate Constant, k1 (L mol−1 min−1) | R2 |
|---|---|---|---|---|---|---|
| 1 | 1:1 | 433 | 150 | 21.5253 | 6.50 × 10−3 | 0.972 |
| 2 | 2:1 | 433 | 150 | 10.3897 | 3.18 × 10−3 | 0.986 |
| 3 | 4:1 | 433 | 150 | 4.1905 | 1.59 × 10−3 | 0.993 |
| 4 | 6:1 | 433 | 150 | 2.7692 | 1.06 × 10−3 | 0.990 |
| 5 | 8:1 | 433 | 150 | 1.9294 | 0.92 × 10−3 | 0.978 |
| 6 | 2:1 | 413 | 150 | 4.2537 | 2.41 × 10−3 | 0.989 |
| 7 | 2:1 | 433 | 150 | 10.3897 | 3.18 × 10−3 | 0.986 |
| 8 | 2:1 | 453 | 150 | 18.7094 | 4.23 × 10−3 | 0.994 |
| 9 | 2:1 | 473 | 150 | 52.7582 | 5.75 × 10−3 | 0.992 |
| 10 | 2:1 | 433 | 120 | 9.8958 | 3.02 × 10−3 | 0.984 |
| 11 | 2:1 | 433 | 150 | 10.3897 | 3.18 × 10−3 | 0.986 |
| 12 | 2:1 | 433 | 180 | 12.0860 | 3.39 × 10−3 | 0.983 |
| 13 | 2:1 | 433 | 210 | 13.8671 | 3.59 × 10−3 | 0.997 |
| Forward Reaction | Equilibrium Reaction | ||||
|---|---|---|---|---|---|
| Activation Energy (kJ mol−1) | Pre-Exponential Factor (L mol−1 min−1) | R2 | Molar Reaction Heat (kJ mol−1) | Pre-Exponential Factor | R2 |
| 23.59 | 2.27 | 0.997 | 65.98 | 9.07 × 108 | 0.985 |
| Forward Reaction | Equilibrium Reaction | ||||
|---|---|---|---|---|---|
(L mol−1 min−1) | (L mol−1 min−1 W−1) | R2 | (W−1) | R2 | |
| 1.5282 | 4.40 × 10−3 | 0.997 | 3.69 × 108 | 4.11 × 106 | 0.951 |
| Catalyst | Catalyst Loading (%, w/w) | Conversion (%) | References |
|---|---|---|---|
| H2SO4 | 1 | 99.9 | [16] |
| HZSM-5 | 20 | 80 | [20] |
| Eversa Transform lipase | 11.98 | 96.73 | [24] |
| Catalyst free (supercritical methanol) | - | 97.0 | [29] |
| Catalyst free (microwave irradiation) | - | 35 | [58] |
| Catalyst free (traditional heating) | - | 67.13 | This study |
| Catalyst free (microwave irradiation) | - | 97.62 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.C.; Wang, F.-M.; Dinh, K.K.; Pham, T.T.; Juan, H.-Y.; Nguyen, N.P.; Ong, H.C.; Su, C.-H. Microwave-Assisted Noncatalytic Esterification of Fatty Acid for Biodiesel Production: A Kinetic Study. Energies 2020, 13, 2167. https://doi.org/10.3390/en13092167
Nguyen HC, Wang F-M, Dinh KK, Pham TT, Juan H-Y, Nguyen NP, Ong HC, Su C-H. Microwave-Assisted Noncatalytic Esterification of Fatty Acid for Biodiesel Production: A Kinetic Study. Energies. 2020; 13(9):2167. https://doi.org/10.3390/en13092167
Chicago/Turabian StyleNguyen, Hoang Chinh, Fu-Ming Wang, Kim Khue Dinh, Thanh Truc Pham, Horng-Yi Juan, Nguyen Phuong Nguyen, Hwai Chyuan Ong, and Chia-Hung Su. 2020. "Microwave-Assisted Noncatalytic Esterification of Fatty Acid for Biodiesel Production: A Kinetic Study" Energies 13, no. 9: 2167. https://doi.org/10.3390/en13092167




