Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
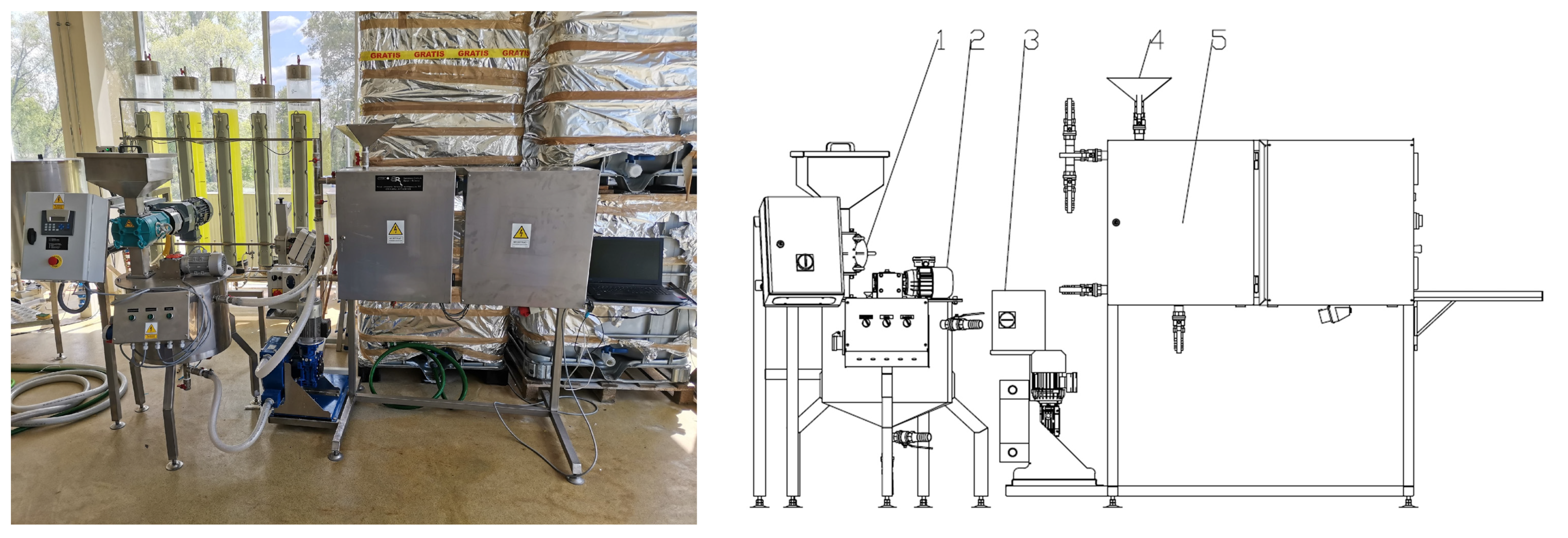
2.2. Equipment
2.3. Pretreatment
2.4. Determination of Cellulose, Hemicellulose, and Lignin
2.5. Analytical Methods
3. Results and Discussion
3.1. Pretreatment Efficiency
3.2. Methane Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Zhang, X.; Liu, Q.; Zhang, Q.; Chen, L.; Ma, L. A review of conversion of lignocellulose biomass to liquid transport fuels by integrated refining strategies. Fuel Process. Technol. 2020, 208, 106485. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kubicek, C.P.; Berrin, J.G.; Wilson, D.W.; Couturier, M.; Berlin, A.; Filho, E.X.F.; Ezeji, T. Fungal Enzymes for Bio-Products from Sustainable and Waste Biomass. Trends Biochem. Sci. 2016, 41, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Kupchik, M.P.; Sereda, K.; Vorobiev, E. Electrostimulated thermal permeabilisation of potato tissues. Biosyst. Eng. 2008, 99, 76–80. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Haberl Meglič, S.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Buchmann, L.; Mathys, A. Perspective on Pulsed Electric Field Treatment in the Bio-based Industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portu, J.; López, R.; López, N.; Santamaría, P.; Garde-Cerdán, T.; López-Alfaro, I. Inactivation of wine-associated microbiota by continuous pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2015, 29, 187–192. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Nierop Groot, M.N.; Nederhoff, A.L.; van Boekel, M.A.J.S.; Matser, A.M.; Mastwijk, H.C. Pulsed electric field processing of different fruit juices: Impact of pH and temperature on inactivation of spoilage and pathogenic micro-organisms. Int. J. Food Microbiol. 2014, 173, 105–111. [Google Scholar] [CrossRef]
- Kotnik, T. Lightning-triggered electroporation and electrofusion as possible contributors to natural horizontal gene transfer. Phys. Life Rev. 2013, 10, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of?) knowledge. Biochim. Biophys. Acta Gen. Subj. 2005, 1724, 270–280. [Google Scholar] [CrossRef]
- Teissié, J.; Eynard, N.; Gabriel, B.; Rols, M.P. Electropermeabilization of cell membranes. Adv. Drug Deliv. Rev. 1999, 35, 3–19. [Google Scholar] [CrossRef]
- Dellarosa, N.; Tappi, S.; Ragni, L.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Metabolic response of fresh-cut apples induced by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 38, 356–364. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C.; Prochnow, A. Particle Size Reduction during Harvesting of Crop Feedstock for Biogas Production I: Effects on Ensiling Process and Methane Yields. Bioenergy Res. 2012, 5, 926–936. [Google Scholar] [CrossRef]
- Dahunsi, S.O. Mechanical pretreatment of lignocelluloses for enhanced biogas production: Methane yield prediction from biomass structural components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Ebowski, M.D. Application of hydrodynamic cavitation for improving methane fermentation of sida hermaphrodita silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef] [Green Version]
- Lizasoain, J.; Trulea, A.; Gittinger, J.; Kral, I.; Piringer, G.; Schedl, A.; Nilsen, P.J.; Potthast, A.; Gronauer, A.; Bauer, A. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The influence of dilute sulfuric acid pretreatment on biogas production form wheat plant. Int. J. Green Energy 2016, 13, 1129–1134. [Google Scholar] [CrossRef]
- Khor, W.C.; Rabaey, K.; Vervaeren, H. Low temperature calcium hydroxide treatment enhances anaerobic methane production from (extruded) biomass. Bioresour. Technol. 2015, 176, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Kong, X.; Du, J.; Ye, X.; Xi, Y.; Jin, H.; Zhang, M.; Guo, D. Enhanced methane production from wheat straw with the assistance of lignocellulolytic microbial consortium TC-5. Bioresour. Technol. 2018, 263, 33–39. [Google Scholar] [CrossRef]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic waste valorisation strategy through enzyme and biogas production. Bioresour. Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Sergent, M.; Raouche, S.; Carrere, H. Influence of white-rot fungus Polyporus brumalis BRFM 985 culture conditions on the pretreatment efficiency for anaerobic digestion of wheat straw. Biomass Bioenergy 2018, 110, 75–79. [Google Scholar] [CrossRef]
- Xu, W.; Fu, S.; Yang, Z.; Lu, J.; Guo, R. Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour. Technol. 2018, 259, 18–23. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Ostermeier, R.; Giersemehl, P.; Siemer, C.; Töpfl, S.; Jäger, H. Influence of pulsed electric field (PEF) pre-treatment on the convective drying kinetics of onions. J. Food Eng. 2018, 237, 110–117. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Dadan, M.; Rybak, K.; Lojkowski, W.; Chudoba, T.; Witrowa-Rajchert, D. The effect of pulsed electric field on drying kinetics, color, and microstructure of carrot. Dry. Technol. 2016, 34, 1286–1296. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.D.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, β-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Onwude, D.I.; Hashim, N.; Janius, R.; Abdan, K.; Chen, G.; Oladejo, A.O. Non-thermal hybrid drying of fruits and vegetables: A review of current technologies. Innov. Food Sci. Emerg. Technol. 2017, 43, 223–238. [Google Scholar] [CrossRef]
- Hartyáni, P.; Dalmadi, I.; Cserhalmi, Z.; Kántor, D.B.; Tóth-Markus, M.; Sass-Kiss, Á. Physical-chemical and sensory properties of pulsed electric field and high hydrostatic pressure treated citrus juices. Innov. Food Sci. Emerg. Technol. 2011, 12, 255–260. [Google Scholar] [CrossRef]
- Vervoort, L.; van der Plancken, I.; Grauwet, T.; Timmermans, R.A.H.; Mastwijk, H.C.; Matser, A.M.; Hendrickx, M.E.; van Loey, A. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice: Part II: Impact on specific chemical and biochemical quality parameters. Innov. Food Sci. Emerg. Technol. 2011, 12, 466–477. [Google Scholar] [CrossRef]
- González-Arenzana, L.; López-Alfaro, I.; Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Microbial inactivation and MLF performances of Tempranillo Rioja wines treated with PEF after alcoholic fermentation. Int. J. Food Microbiol. 2018, 269, 19–26. [Google Scholar] [CrossRef]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ki, D.; Parameswaran, P.; Rittmann, B.E.; Torres, C.I. Effect of pulsed electric field pretreatment on primary sludge for enhanced bioavailability and energy capture. Environ. Eng. Sci. 2015, 32, 831–837. [Google Scholar] [CrossRef]
- Salerno, M.B.; Lee, H.-S.; Parameswaran, P.; Rittmann, B.E. Using a Pulsed Electric Field as a Pretreatment for Improved Biosolids Digestion and Methanogenesis. Proc. Water Environ. Fed. 2012, 2008, 2005–2018. [Google Scholar] [CrossRef]
- Lindmark, J.; Lagerkvist, A.; Nilsson, E.; Carlsson, M.; Thorin, E.; Dahlquist, E.; Lindmark, J.; Nilsson, E.; Thorin, E.; Dahlquist, E.; et al. Evaluating the Effects of Electroporation Pre-treatment on the Biogas Yield from Ley Crop Silage. Appl. Biochem. Biotechnol. 2014, 174, 2616–2625. [Google Scholar] [CrossRef]
- Đurđica, K.; Davor, K.; Slavko, R.; Daria, J.; Robert, S.; Marina, T. Electroporation of harvest residues for enhanced biogas production in anaerobic co-digestion with dairy cow manure. Bioresour. Technol. 2019, 274, 215–224. [Google Scholar]
- Safavi, S.M.; Unnthorsson, R. Methane yield enhancement via electroporation of organic waste. Waste Manag. 2017, 66, 61–69. [Google Scholar] [CrossRef]
- Lee, I.S.; Rittmann, B.E. Effect of low solids retention time and focused pulsed pre-treatment on anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 2542–2548. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Qin, X.; Wu, Q.; Zhao, Y.; Bai, S.; Peng, W.; Feng, B. Effect of high-voltage pulsed electric field (HPEF) pretreatment on biogas production rates of hybrid Pennisetum by anaerobic fermentation. Nat. Gas Ind. B 2018, 5, 48–53. [Google Scholar] [CrossRef]



| Method | Pretreatment Type | Pretreatment Conditions | Biomass | Methane Fermentation Conditions | Increased Methane Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Physical | Mechanical pulverization | Pulverization, particle sizes of 33 to 6 mm | Sorghum hybrid | 35 °C, 30 days | 11–13 | [18] |
| Grinding/ Milling | Mechanical | Elephant grass, Mexican sunflower, Siam weed | 37 °C, 30 days | 22 | [19] | |
| Hydrodynamic cavitation | 4 kW, 5 min, 2800 RPM | Sida hermaphrodita silage | 37 °C, 40 days | 30 | [20] | |
| Physico- chemical | Steam explosion | 160 °C for 2 min | Corn stover | 37.5 °C, 49 days | 22 | [21] |
| Hydrothermal | 175 °C | Napier grass | 35 °C, 42 days | 35 | [22] | |
| Chemical | Acid pretreatment | Dilute H2SO4 (1%), 121 °C, 10–120 min | Wheat plant | 37 °C, 30 days | 15.5 | [23] |
| Alkali pretreatment | Ca(OH)2 (7.5%), 10 °C, 20 h | Maize straw, grass, sprout stem | 37 °C, 30 days | 37 | [24] | |
| Organic Solvent | NMMO, 120 °C, 3 h | Wheat straw | 37 °C, 40 days | 11 | [25] | |
| Biological | Microbial consortium | Microbial consortium TC-5 | Wheat straw | 37 °C 30 days | 22.2 | [26] |
| 45 °C 35 days | 36.3 | |||||
| Fungal pretreatment | Pleurotus eryngii | Corn stover | 40 days, 30 °C | 19 | [27] | |
| Polyporus brumalis | Wheat straw | 36 °C, 57 days | 52 | [28] | ||
| Bacterial pretreatment | Bacillus subtilis | Corn straw | 37 °C, 51 days | 17.35 | [29] |
| Parameters | Value |
|---|---|
| Hydration [%] | 57.38 |
| Total solids [%] | 42.62 |
| Volatile solids [% TS] | 95.80 |
| Total carbon (TC) [mg C/g TS] | 445.50 |
| Total organic carbon (TOC) [mg C/g TS] | 422.37 |
| Total nitrogen (TN) [mg N/g TS] | 13.42 |
| C/N | 31.47 |
| Duration of PEF Pretreatment (s) | Cellulose (% TS) | Lignin (% TS) | Hemicellulose (% TS) | Glucose Yield (mg/g TS) |
|---|---|---|---|---|
| 0 (control) | 21.04 ± 0.78 | 20.02 ± 0.51 | 3.14 ± 0.09 | 50.60 ± 0.77 |
| 30 | 20.07 ± 0.36 | 20.27 ± 0.65 | 3.10 ± 0.35 | 51.42 ± 0.25 |
| 60 | 19.91 ± 1.90 | 19.87 ± 0.66 | 2.95 ± 0.20 | 51.45 ± 0.46 |
| 90 | 20.06 ± 0.76 | 19.79 ± 0.75 | 3.07 ± 0.36 | 51.11 ± 0.33 |
| 120 | 19.69 ± 0.57 | 19.74 ± 0.67 | 3.17 ± 0.29 | 51.57 ± 0.65 |
| 150 | 19.68 ± 0.70 | 19.48 ± 0.28 | 2.94 ± 0.06 | 52.18 ± 0.31 |
| 180 | 19.50 ± 0.87 | 18.77 ± 0.36 | 2.68 ± 0.23 | 52.85 ± 0.26 |
| 210 | 19.55 ± 0.76 | 19.22 ± 0.48 | 2.85 ± 0.14 | 52.79 ± 0.32 |
| 240 | 19.58 ± 0.80 | 19.26 ± 0.60 | 2.88 ± 0.03 | 52.76 ± 0.31 |
| 270 | 19.62 ± 0.76 | 19.36 ± 0.45 | 2.86 ± 0.11 | 52.78 ± 0.22 |
| Disintegration Time (s) | Energy Input (Wh/gTS) | Increase in Energy due to PEF Disintegration | Energy Gain (Wh/g TS) |
|---|---|---|---|
| 0 | - | - | - |
| 30 | 0.058 ± 0.005 | 0.113 ± 0.068 | 0.054 ± 0.068 |
| 60 | 0.117 ± 0.005 | 0.166 ± 0.035 | 0.049 ± 0.036 |
| 90 | 0.175 ± 0.007 | 0.188 ± 0.019 | 0.013 ± 0.019 |
| 120 | 0.233 ± 0.009 | 0.386 ± 0.031 | 0.153 ± 0.031 |
| 150 | 0.292 ± 0.006 | 0.426 ± 0.018 | 0.135 ± 0.018 |
| 180 | 0.350 ± 0.006 | 0.585 ± 0.009 | 0.235 ± 0.009 |
| 210 | 0.408 ± 0.005 | 0.554 ± 0.043 | 0.146 ± 0.044 |
| 240 | 0.467 ± 0.005 | 0.559 ± 0.029 | 0.092 ± 0.029 |
| 270 | 0.525 ± 0.006 | 0.568 ± 0.049 | 0.043 ± 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwarc, D.; Szwarc, K. Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage. Energies 2021, 14, 119. https://doi.org/10.3390/en14010119
Szwarc D, Szwarc K. Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage. Energies. 2021; 14(1):119. https://doi.org/10.3390/en14010119
Chicago/Turabian StyleSzwarc, Dawid, and Karolina Szwarc. 2021. "Use of a Pulsed Electric Field to Improve the Biogas Potential of Maize Silage" Energies 14, no. 1: 119. https://doi.org/10.3390/en14010119






