1. Introduction
Hydrogen as a carbonless and environmentally friendly fuel is considered as the future energy carrier. Its properties such as wide flammability limits, high laminar burning velocity (in air ~2.5 m/s), low ignition energy (in air ~0.02 mJ), and high lower heating value (~120 MJ/kg) point at a variety of possible hydrogen applications. However, the listed advantages of hydrogen properties might be also considered as disadvantages from the safety point of view. In the case of unintended leakage, hydrogen is easier to ignite under wider concentration conditions, and after ignition, flame will propagate faster. Additionally, the hydrogen–air flame is inherently unstable and if the conditions are favorable the hydrogen flame might go through the deflagration to detonation transition (DDT) process. As the DDT process depends on a variety of parameters (fuel concentration, congestion presence, and its geometrical configuration), it is crucial to investigate these parameters’ influence to prevent DDT in common use. As the experimental investigation is expensive in terms of time and cost, Computational Fluid Dynamics (CFD) codes have been developed to solve complex numerical problems. Nowadays, CFD analysis is applied in many branches of industry such as aerospace, automotive, power generation, manufacturing, petrochemical, process safety, turbomachinery, etc. The main advantage of using CFD simulations is to reduce the time of the designing process and, simultaneously, detailed numerical models might be used to get a better insight into the process that is difficult to investigate experimentally. DDT is one of such processes as it combines a wide range of flame propagation velocities (0–2000 m/s). Therefore, depending on the flame velocity, the interactions among chemical kinetics, flame, unburned mixture, turbulence, and shock waves are at a different level of complexity.
DDT process in hydrogen–air mixtures has been extensively investigated experimentally [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13] and numerically [
14,
15,
16,
17,
18,
19,
20,
21] over the course of 30 years. The essential condition for the planar detonation front to propagate stably is the presence of developed cellular structure, so it is possible to correlate the characteristic cell size width λ with the characteristic dimension of the channel which might be defined in various ways. For example, based on the extensive research, Dorofeev et al. [
1] provided the critical correlation of L/λ ≈ 7, where L is the characteristic dimension of the experimental setup that might be interpreted as the minimum distance for detonation formation. Dimension L is, therefore, correlated with blockage ratio and obstacle spacing value. The methodology for calculation of the L value depending on the channel geometry is described in Reference [
1]. The criterion was further confirmed by studying the blockage ratio influence on the DDT in a CH
4–air mixture [
10]. It has been also proven that DDT limits are experimental stand scale-dependent as the lower concentration limit for successful DDT was decreasing with the increase of the scale of the setup. Sample measurements of lower limits in a 30.5 cm [
11], 43 cm [
22] diameter tubes, and 2.3 m high channel [
23] gave values of 15%, 13.6%, and 12.5% of hydrogen in air, respectively. As the cost of experiments increases significantly with the scale of the experimental setup, the numerical simulations have become attractive, low-cost tools to investigate flame acceleration and transition to detonation.
One of the recent numerical codes designed and tested in a variety of scales and hydrogen–air mixtures composition is ddtFoam released by F. Ettner [
17,
24]. The code is based on an open-source freeware OPENFoam software. The main advantage of ddtFoam is its capability to capture the flame acceleration process together with the transition to detonation based on the dual-source term in the reaction progress transport equation. First term responsible for the deflagration is based on the Weller [
25] gradient combustion model, while the second term accounts for autoignition delay time. The second term is especially important as the autoignition delay time is a function of local conditions of temperature, pressure, and mixture composition. To prevent calculating ignition delay time in every time step and computational cell, the solver is using pre-calculated table of ignition delay time (IDT) prepared with Cantera software with detailed reaction mechanism of O’Conaire [
26]. The IDT table is accessed during the simulation and the solver compares the tabulated IDT (as a function of temperature, pressure, and composition) with the simulated fluid residence time at specific conditions. If the residence time excess the precalculated IDT value, the solver activates the second source term in the reaction progress transport equation. Such an approach significantly reduces the numerical cost of simulation. Similarly, the combustion products matrix as a function of the initial temperature, pressure, and composition is precalculated and accessed while the simulation progresses. Some memory saving modification for this methodology using a multiple regression model was introduced and tested in the recent paper of Malik et al. [
27]. An additional advantage of ddtFoam is the presence of a shock wave sub-grid model to better capture the progress of the shock wave induced temperature rise within a single cell. The computational cell in this model is virtually divided into shocked and non-shocked volumes and IDT is evaluated for both volumes. Such a combination leads to greater accuracy of the results at course grids. Up to date, the ddtFoam has been used to investigate the DDT process in mixtures with hydrogen concentration gradients, large scale experiments with and without adaptive mesh refinement [
28], smooth channels with mixtures of hydrogen-oxygen [
27], and channels with obstacles [
29].
One of the first papers by Ettner [
17] described the code together with the test 2D simulations of the flame propagating in hydrogen–air mixture with concentration gradient in the channel with a variety of blockage ratios. The results showed good agreement with experiments by means of the velocity profile along the channel and pressures recorded. Additionally, the code was able to capture phenomena recorded in experiments and highly resolved simulations like Mach stem formation, DDT due to the shock wave reflection, and shock–flame interaction. Another important conclusion was that the rectangular grids finer than 2 mm did not affect the results apart from the pressure peaks sharpness in stable detonation mode. Such ddtFoam code advantage arises from the IDT and shock-wave sub-models implemented and let one use it in large-scale geometries with relatively coarse grids.
Hasslberger et al. [
28] used ddtFoam to simulate a large-scale RUT facility [
23] at the hydrogen concentration close to the lower detonability limit. The approach was to use a 3D domain with adaptive mesh refinement at the flame surface to increase accuracy and decrease the computational cost. Two experiments were simulated with very good agreement with respect to flame acceleration. The first experiment (14% H
2) was well-predicted with respect to the detonation transition placement at the reflecting surface. The second experiment (12.5% H
2) simulation did not provide a successful transition, however, the experimental detonation origin was reported [
23] to be in the flame brush area rather than due to the shock wave reflection at the wall.
The main purpose of the ddtFoam design was to address the fact that large-scale accidents simulations must be performed under resolved grids. Therefore, the sub-grid modeling of critical phenomena is necessary to provide sufficient accuracy and minimize the computational time of the simulation. Critical for DDT process phenomena recognized by Ettner [
17] were shock wave reflection and triggering conditions for a successful transition to detonation. Both were addressed by sub-grid modeling described earlier in this chapter. As sub-grid modeling demands validation for a variety of scales the present work might be considered as a next step to validate the code with experimental data and point at possible, further improvements. The main aim of this study was to assess the capabilities of ddtFoam code to capture the limits of hydrogen in the air for which successful transition to detonation might be observed. The numerical results are compared to the experimentally recorded values measured for three different obstacle spacing configurations with a fixed blockage ratio of 0.5. The comparison was made for such parameters as pressure profiles, maximum overpressures, run-up distance, and DDT limits.
2. Experimental Setup Description and Results
The experimental setup has been previously used for the investigation of the scale effect of the obstacle-filled 2-m-long channel and the results are reported in the paper of Teodorczyk [
30]. The current setup has been extended to 4 m to observe further flame acceleration, the transition to detonation, and stable detonation. The tube consisted of two 2-m-long sections with dimensions of 0.08 × 0.11 m in cross-section (H × W). The obstacles were made of aluminum 10 mm thick and 40 mm high, which provided a constant blockage ratio of 0.5 for all obstacles spacing configurations. The spacing S between obstacles was equal to H, 2H, 3H. Initial conditions for the H
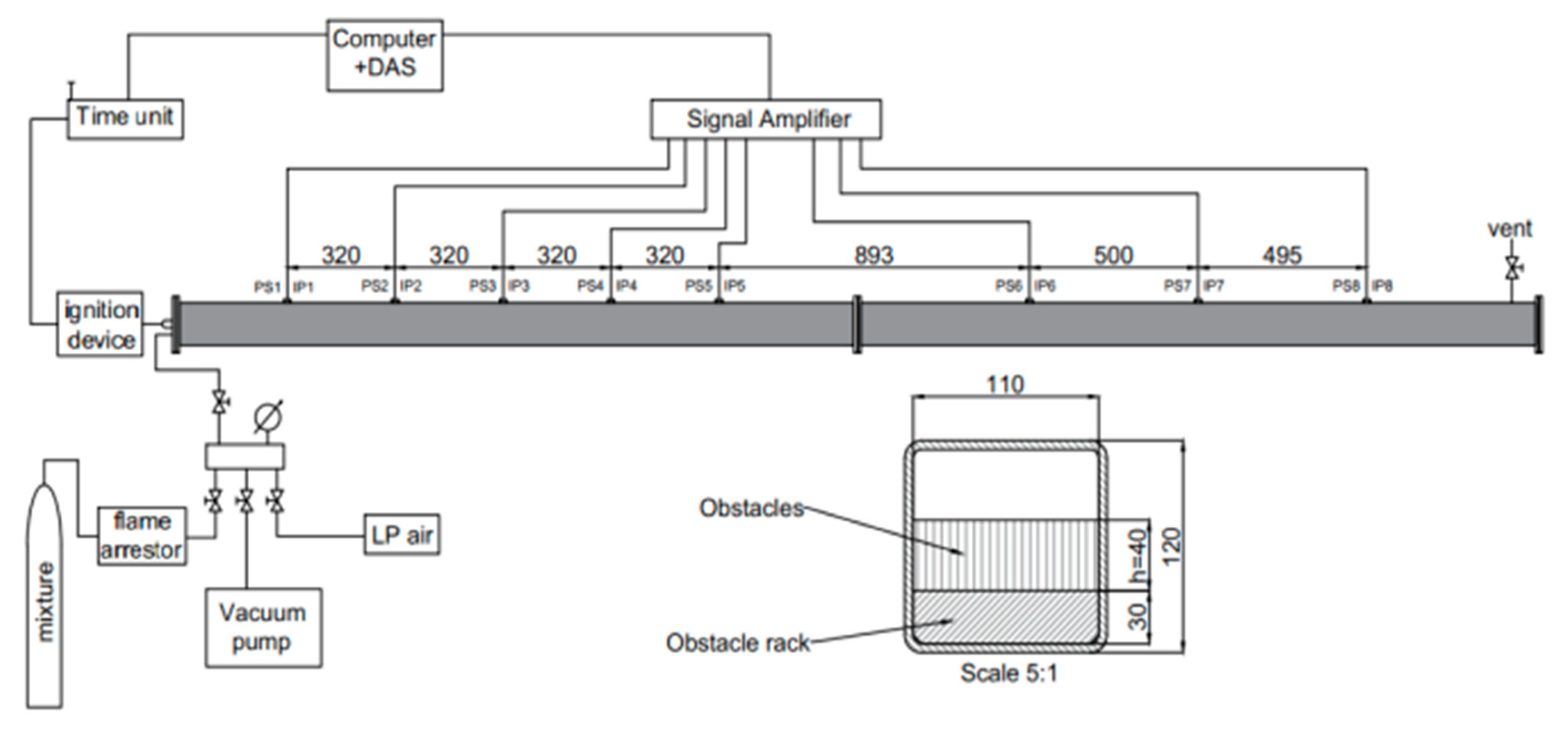
2–air mixture were T = 295 ± 3K and P = 0.1 MPa. The hydrogen concentration in air was within 15–55% [vol./vol.], which corresponds to an equivalence ratio of 0.42–2.92. The total number of experiments was nearly 300. The number of experiments for each mixture composition and geometrical configuration was 10–13 for most of the tests, three configurations were tested with 4, 5, or 7 single experiments. The weak ignition source was a spark plug placed at one end of the channel at its half-height middle-width. The measurements included 8 PCB 113B24 type pressure sensors (PS1–PS8) with a measurement range of 6.7 MPa and 8 in-house made ion probes (IP1–IP8). Sensors were placed in pairs along the tube to indicate pressure wave and the following flame. Velocity profile was measured based on the time-of-arrival method (ToA). The scheme of the experimental setup is presented in
Figure 1. The tube cross-section for all obstacles spacing considered is presented in
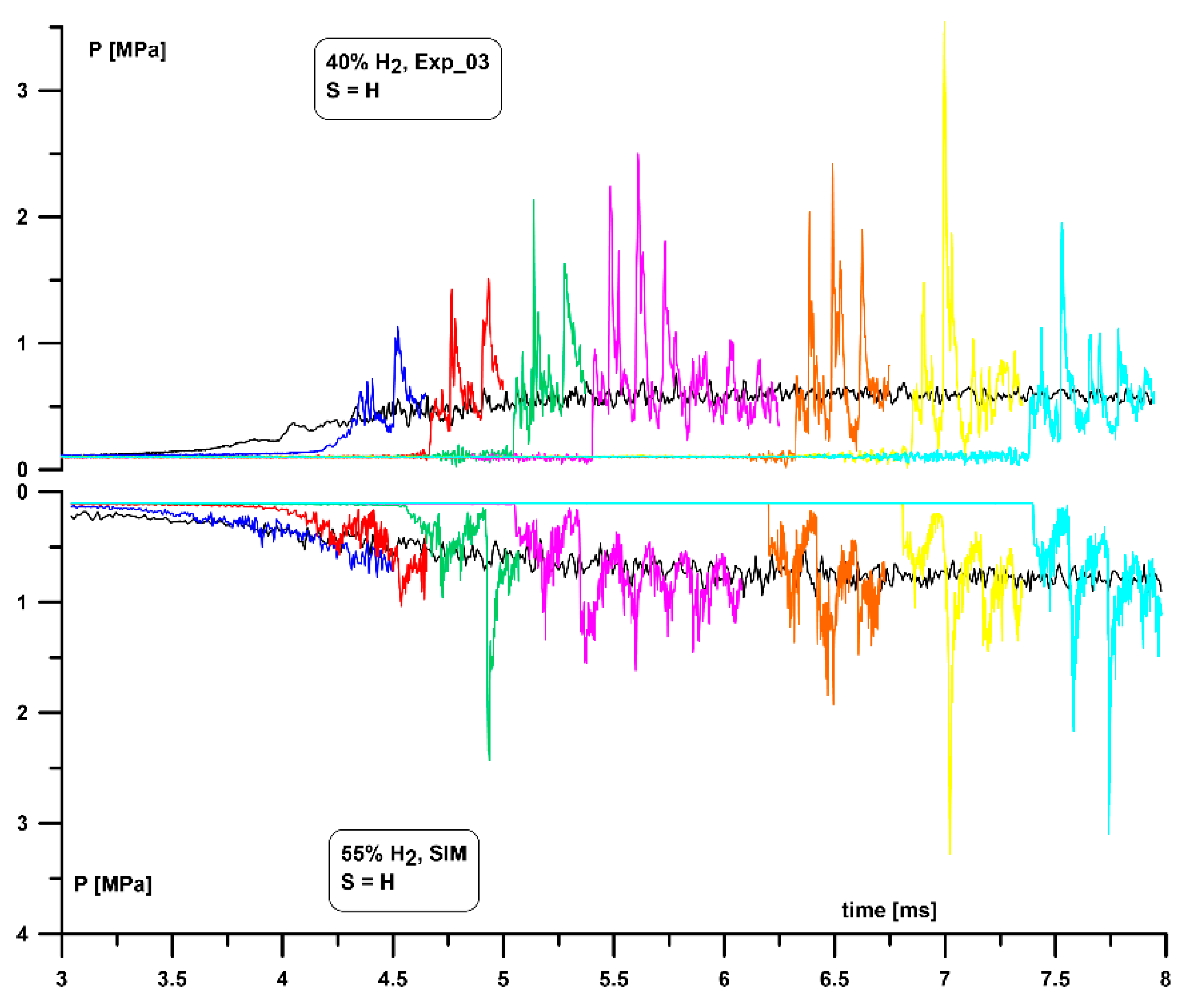
Figure 2. An example graph of the pressure sensors and ion probes profiles is presented in
Figure 3 in which each of 8 colors corresponds to a consecutive set of sensors. The same color scheme applies to pressure profiles presented in this paper. The clearly visible largest delay between signals from PS5 and PS6 is due to the most distant (893 mm) placement between both sensors. More detailed experimental setup description is available in References [
30,
31].
The main results of the experimental research [
31] are summarized in
Table 1 and in
Figure 4 with DDT limits for all spacings and with the mean velocities measured at the end of the detonation tube. The assumption was that the mixture is within the DDT limits if at least one test proved transition to detonation in any of the repeated experimental sets. The limits of DDT were, therefore, extreme mixtures for such experimental sets. What is clear from
Figure 4 that with the obstacles spacing increase the DDT limits get wider and simultaneously mean detonation velocities increase.
3. Numerical Simulations Description
Numerical simulations were performed with the use of ddtFoam code initially described in the Introduction chapter. The ddfFoam solves the unsteady and compressible Navier-Stokes equations density-based. Harten, Lax and van Leer (HLLC) Riemann scheme [
32] is used to solve convective terms with multidimensional slope limiters [
33,
34] suitable for compressible high Mach number. More details of the numerical code schemes can be found in References [
17,
24,
33]. The numerical domain was 2D representing the longitudinal cross-section of the experimental detonation tube channel. The numerical channel was, therefore, 4 m in length and 0.080 m in height. The numerical mesh was orthogonal and structural and contained approximately 320,000 cells, 1 mm by 1 mm each. In total, 36 simulations were performed for various hydrogen in air concentrations in the range of 15–65% H
2 in air, which corresponds to equivalence ratio 0.42–4.42. Initial temperature and pressure were set to 293 K and 101,325 Pa, respectively. The walls were considered as non-slip and adiabatic. The numerical ignition spot was in a form of 25 mm radius area patch filled in with hot combustion products. The center of the ignition spot was at the same position as in experiments, at the center of the channel height at one end of the tube. During the simulations, temperature and pressure numerical sensors were placed at the same position as in experiments. The pre-calculated table of ignition delay time was prepared based on the detailed reaction mechanism of O’Conaire [
26] and Cantera software calculations [
35]. For turbulence modeling, k-omega SST (Shear Stress Transport) turbulence model has been chosen. This modeling approach combines the advantages of
k-ε and
k-ω as in regions near walls it behaves like
k-ω and switches to
k-ε in a free stream region. Wall function utilized was OPENFOAM built-in function KqRWallFunction and omegaWallFunction appropriate for high re-flows. Turbulent kinetic energy
k and specific dissipation rate
ω were initialized according to Ettner’s settings [
17]. The time step of the simulation was adaptive according to Courant-Friedlich-Lewy (CFL) condition at the maximum possible value of 0.5. The simulation time of a single case was around 70 h on a 28 CPU workstation.
5. Discussion
The numerical and experimental DDT limits values are summarized in
Table 2. The lower DDT limits obtained numerically are very similar for all considered geometries and very close to 20% H
2 in air. The upper DDT limits in simulations differ and are in the range of 50–62.5% H
2 depending on the obstacle spacing. The best match between experimental and numerical limits is for case S = 3H, which is also confirmed by means of the RUD comparison (see
Figure 17). The maximum overpressures recorded were also similar and within 1.8–2.5 MPa range for both, experiments and simulations, in case S = 3H. The worst match is observed for case S = H. This effect might be explained by means of the cyclic initiation and attenuation of real detonation front in a more congested configuration. This type of cyclic process called quasi-detonation is a result of more dense congestion, which leads to higher momentum losses, diffraction of the detonation wave behind the obstacle, and, therefore, lower mean detonation velocity observed in experiments comparing to the ideal C-J velocity.
Simultaneously, such detonation behavior is not observed in numerical simulations as the implemented adiabatic flow model is less sensitive to the heat and momentum losses, which are of importance in real reactive flow. Additionally, simulations do not include some effects of the channel geometry (for lower obstacles spacing) on initiation, cellular structure development, and further stable propagation or attenuation of detonation. In the case of lower obstacles spacing, the interaction of obstacles on real 3-dimensional detonation front structure is the strongest, which has been confirmed by experiments. In less congested geometries, the detonation is more prone to develop and propagate stably, which is expressed by higher mean experimental velocities and wider DDT limits. Simultaneously, in less congested geometries, the transition to detonation was observed due to the reflection at the obstacles surface [
31], which is the main mechanism triggering detonation in simulations. Due to that fact one might conclude that ddtFoam is more suitable to simulate geometries with a relatively low level of congestion and for more congested geometries the code will provide conservative results with some safety margin. Considering the flame acceleration process, pressure profiles, and overpressure values along the congested channel, it must be concluded that these parameters are well represented for all the considered geometrical configurations, especially for the fast deflagration propagation regime. RUDs are numerically predicted as a U-shaped curve with the best match to the experimental curve for S = 3H configuration. Discrepancies might be observed in stable detonation front velocity, which is overpredicted with respect to ideal C-J detonation, and the change between deflagration and detonation regime of combustion is very sharp. These features arise from the implemented specific autoignition model. The main ‘trigger’ to switch simulation to detonation is the sufficiently low ignition delay time. Therefore, numerical detonation once initiated will propagate if the unburned mixture will be available independently on the channel blockage ratio. This code feature does not seem to be critical as properly predicted flame acceleration process together with overpressures and RUD are of greater importance. The ddtFoam code is not equipped with a numerical mechanism attenuating initially stable detonation, as a result, the numerical quasi-detonation process is not possible to be observed. This fact is especially visible in comparison between experimental and numerical results for case S = H. That code feature might be one of the future code improvement subjects. Additionally, the numerical model is not able to predict cellular detonation front structure; therefore, it omits all the effects arising from this characteristic detonation dimension. This feature might be also a subject for further code development.