Experimental Study of the Effect of Fuel Catalytic Additive on Specific Fuel Consumption and Exhaust Emissions in Diesel Engine
Abstract
1. Introduction
2. Research Methodology
2.1. Measurement Procedure
- Standard fuel-pure diesel, or
- Diesel fuel with catalytic additives (in a proportion of 2000: 1).
- Engine speed.
- Engine torque, transferred to engine power.
- Fuel consumption by hour, converted into specific fuel consumption.
- Concentrations of exhaust components, verified in emission values.
2.2. Fuel
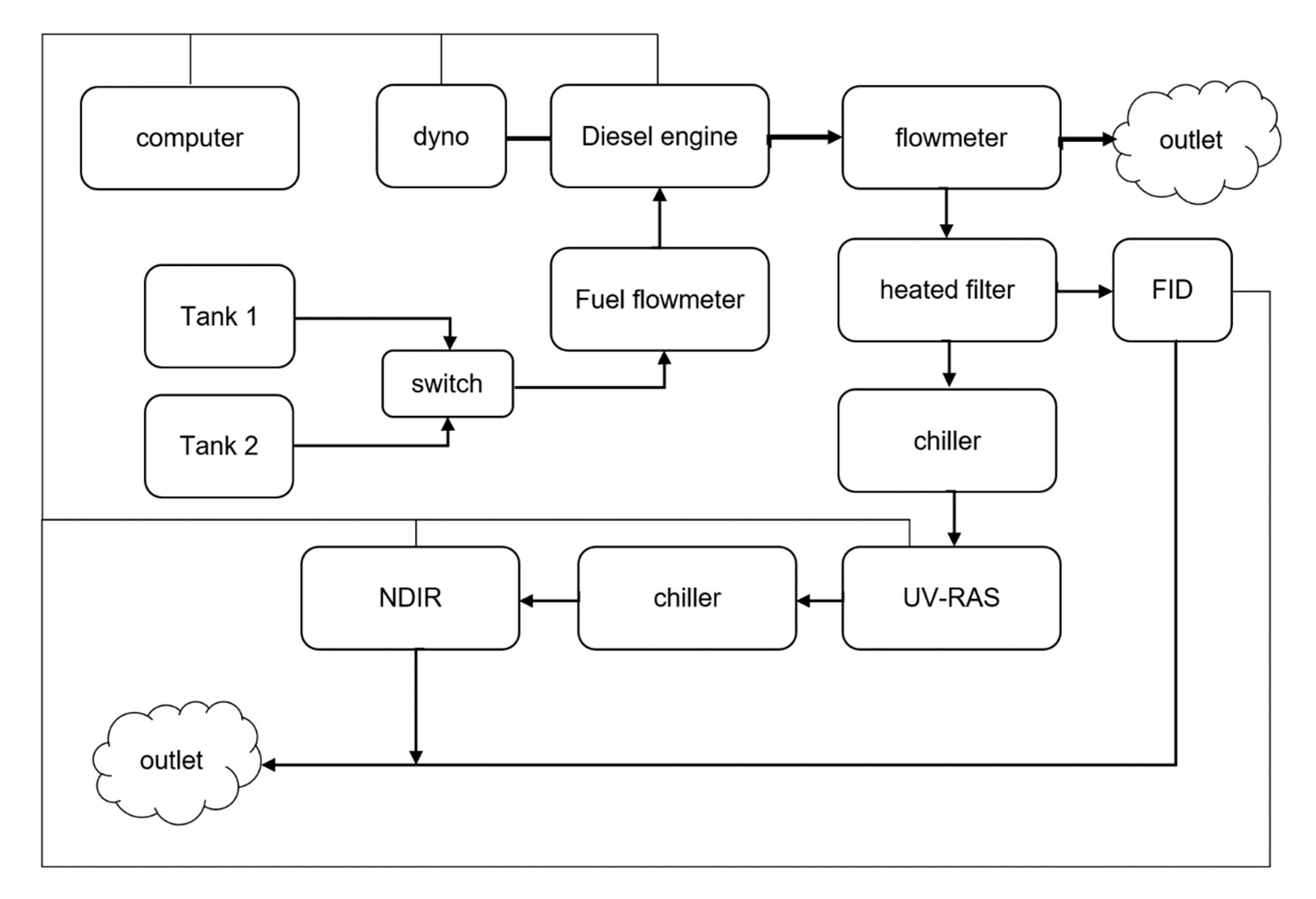
2.3. Test Stand
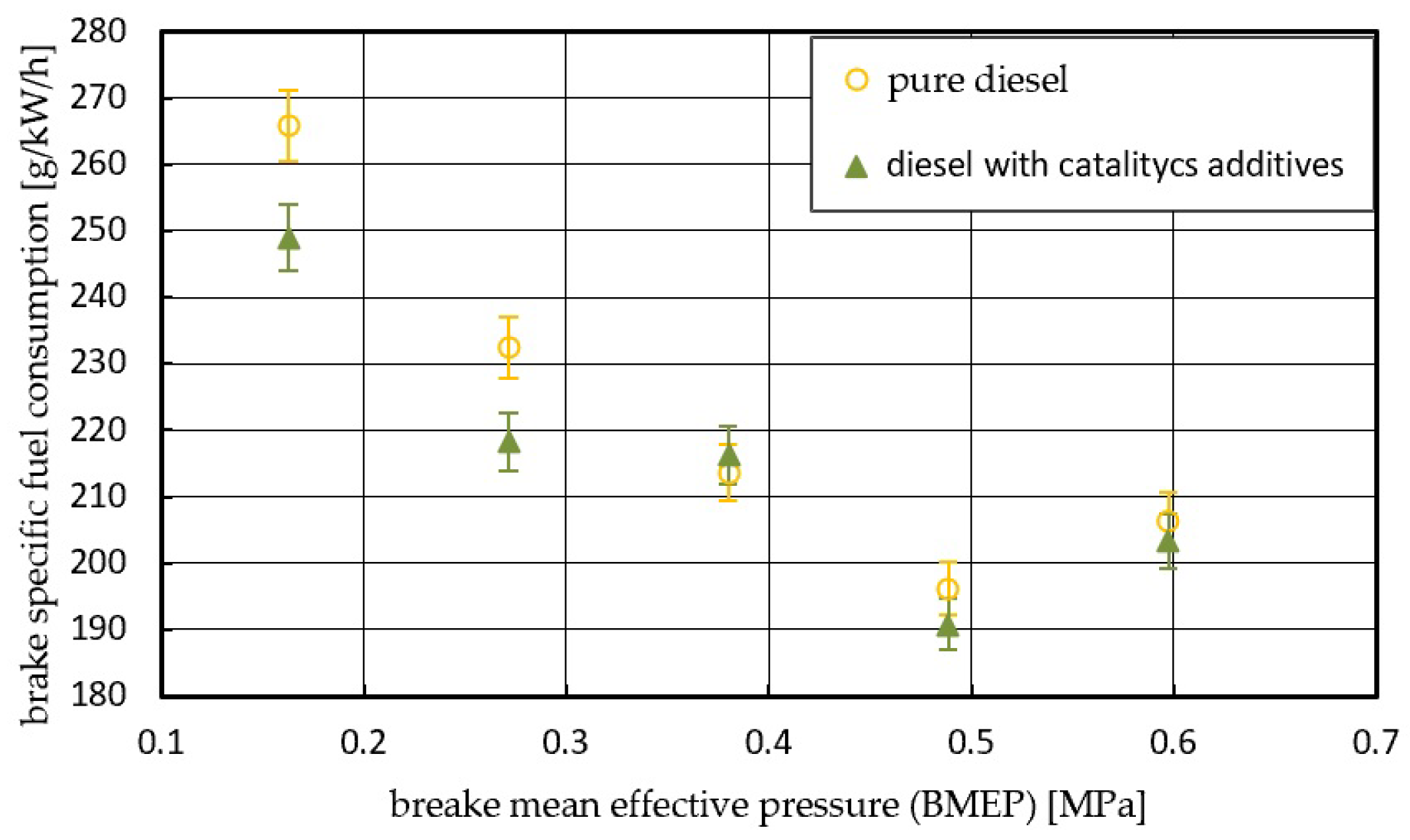
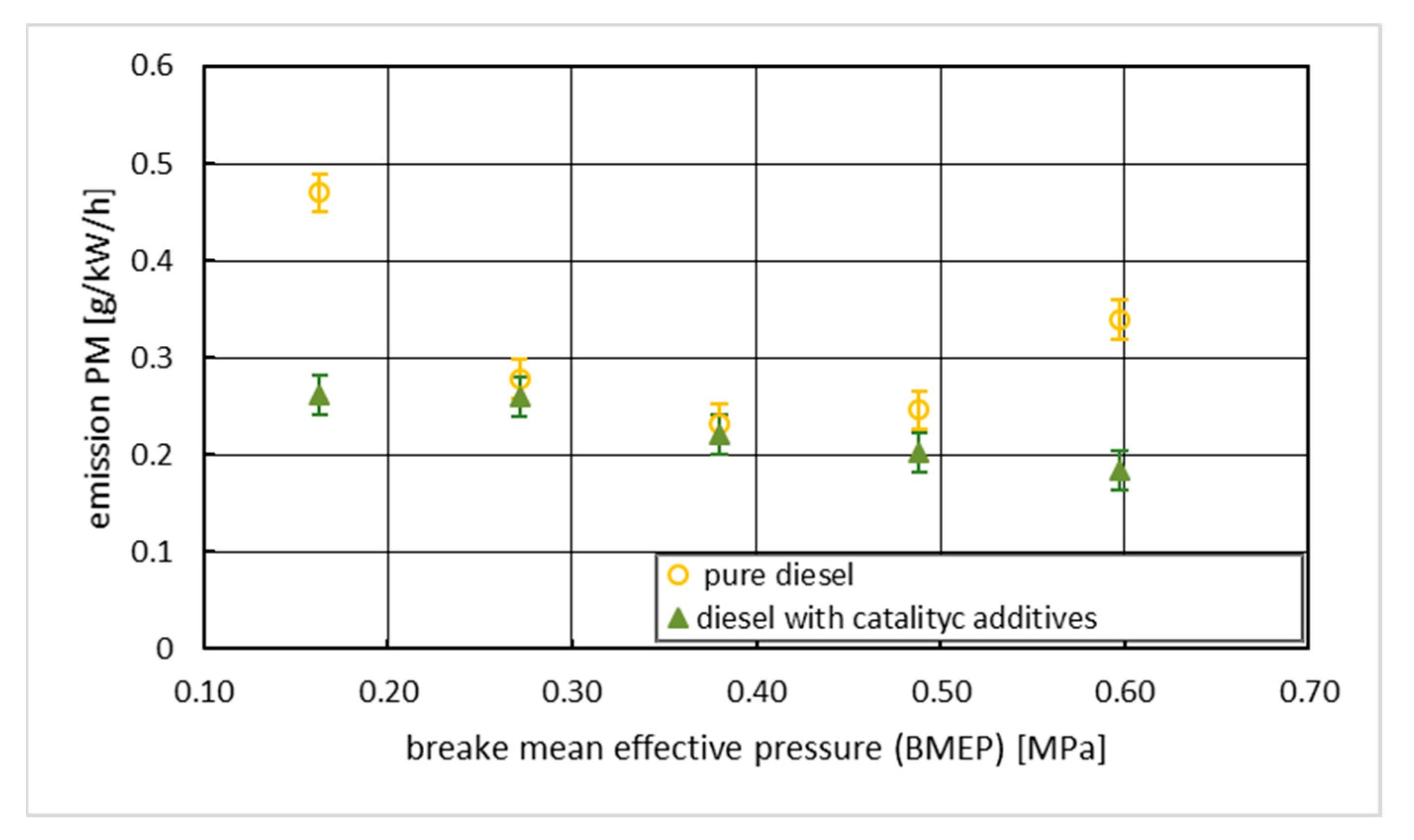
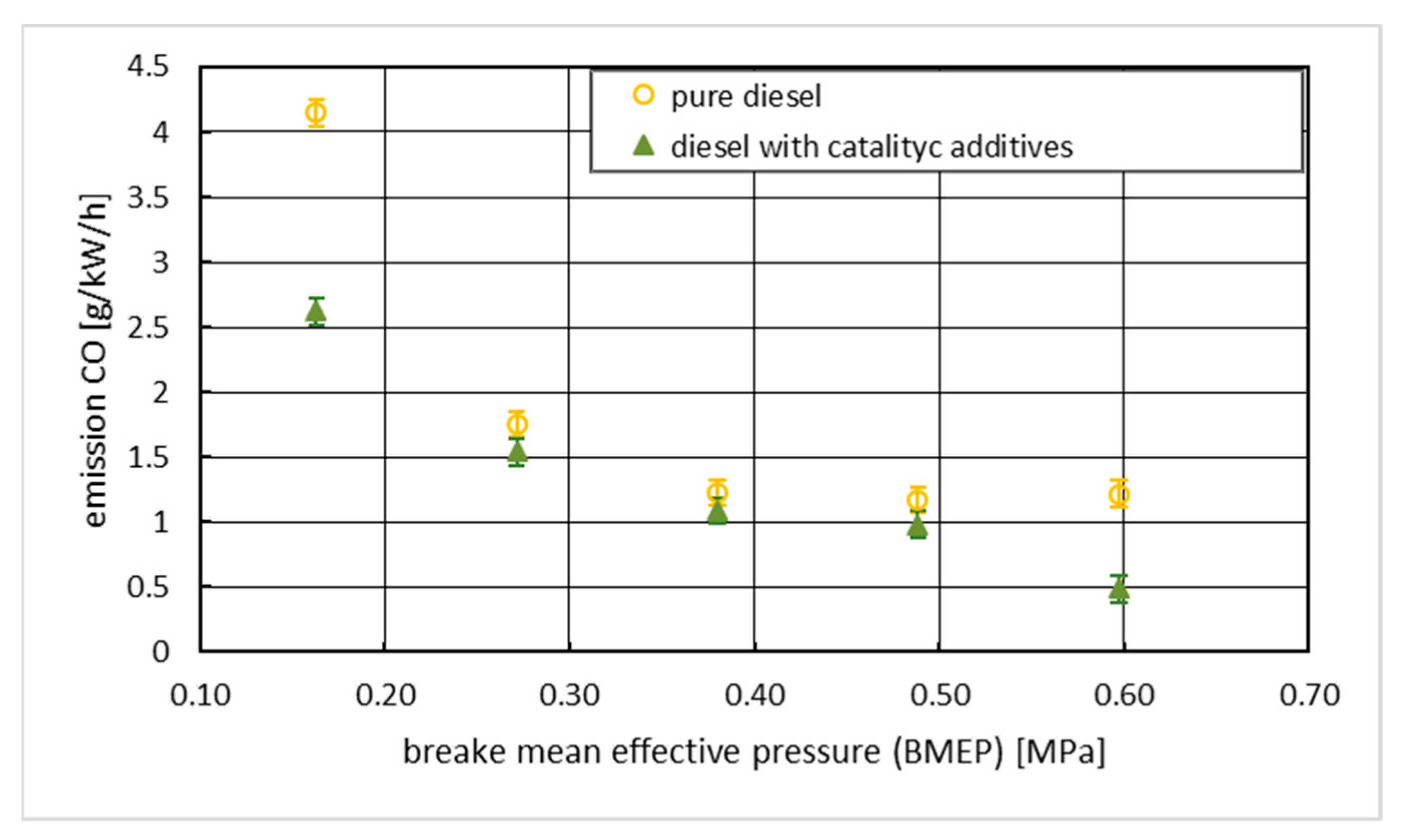
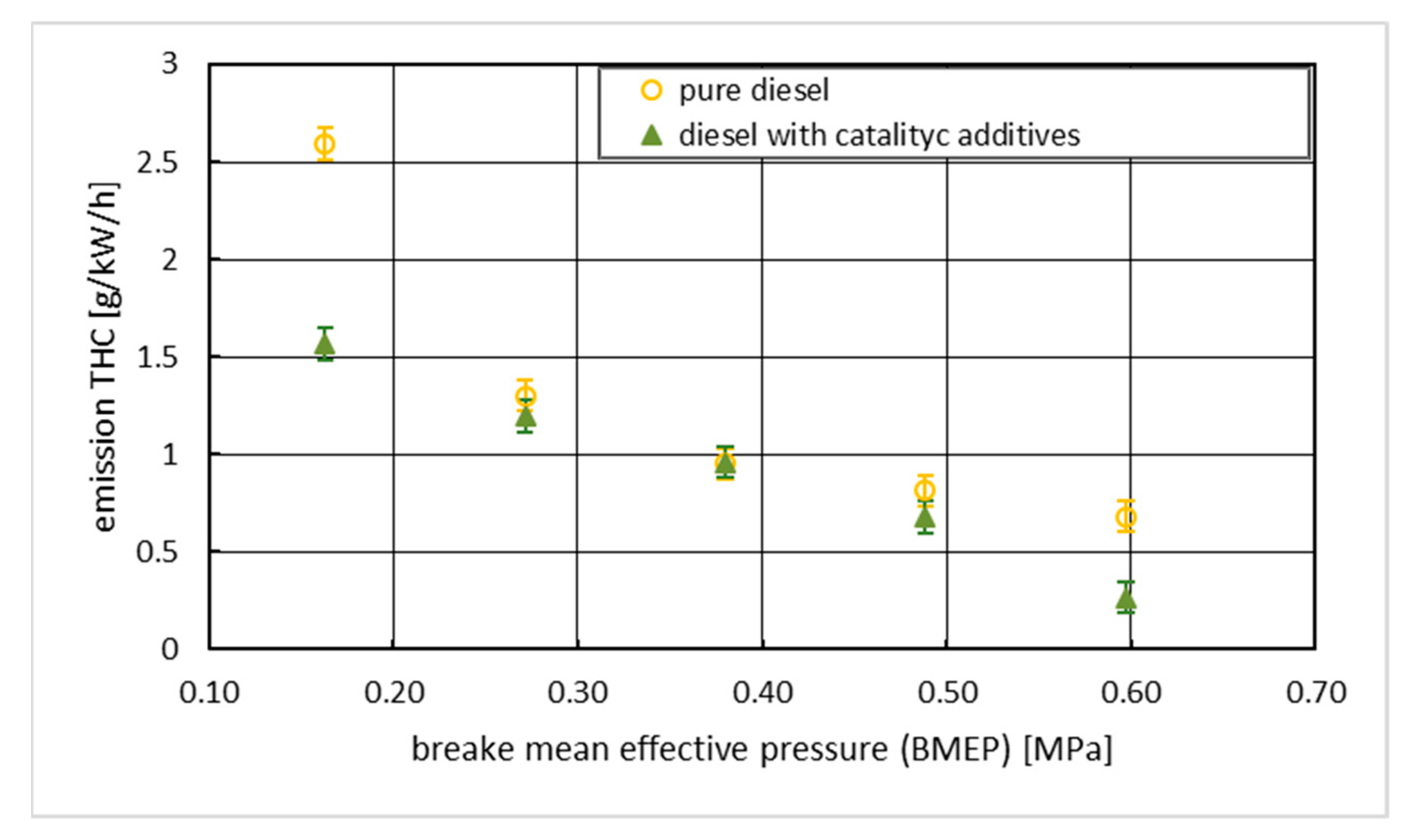
3. Results with Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D. Homogeneous combustion catalysts for efficiency improvements and emission reduction in diesel engines. In Proceedings of the 7th Asia-Pacific Conference on Combustion, Taipei, Taiwan, 24–27 May 2009. [Google Scholar]
- Popova, O.V.; Bashkatova, S.T.; Vasil’eva, E.N.; Kotin, E.B. Additives for increasing the completeness of combustion of diesel fuels. Chem. Technol. Fuels Oils 1995, 31, 88–94. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; Zhang, D. The effect of a homogeneous combustion catalyst on exhaust emissions from a single cylinder diesel engine. Appl. Energy 2013, 102, 556–562. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Y.; Zhang, D. A theoretical investigation into the effect of a homogeneous catalyst on combustion characteristics of single droplets of diesel and biodiesel. In Proceedings of the Australasian Chemical Engineering Conference, CHEMECA, Wellington, New Zealand, 23–26 September 2012; Volume 11. [Google Scholar]
- Zeller, H.W.; Westphal, T.E. Effectiveness of Iron-Based Fuel Additives for Diesel Soot Control; Bureau of Mines: Washington, DC, USA, 1992. [Google Scholar]
- Zhu, M.; Ma, Y.; Zhang, D. Effect of a homogeneous combustion catalyst on combustion characteristics and fuel efficiency of biodiesel in a diesel engine. In Proceedings of the International Conference on Applied Energy, Suzhou, China, 5–8 July 2012; pp. 960–967, This is an example of an ordered list. [Google Scholar]
- Du, C.J.; Kracklauer, J.; Kittelson, D.B. Influence of an Iron Fuel Additive on Diesel Combustion. SAE Tech. Paper Ser. 1998, 980536. [Google Scholar] [CrossRef]
- Bobolev, V.K.; Gen, M.Y.; Mal’tsev, V.M.; Melesov, G.P.; Pokhil, P.F.; Seleznev, V.A.; Stasenko, A.N.; Chuiko, S.V. Mechanism of action of iron catalysts on the combustion of composite systems. Combust. Explos. Shock Waves 1971, 7, 317–324. [Google Scholar] [CrossRef]
- Howard, J.B.; Kausch, W.J. Soot control by fuel additive. Prog. Energy Combust. Sci. 1980, 6, 263–276. [Google Scholar] [CrossRef]
- Otto, K.; Bartosiewicz, L.; Shelef, M. Effects of calcium, strontium, and barium as catalysts and sulphur scavengers in the steam gasification of coal chars. Fuel 1979, 58, 565–572. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Wang, Y.; Yu, X.; Smallbone, A.; Dong, C.; Roskilly, A.P. Comparative study of using multi-wall carbon nanotube and two different sizes of cerium oxide nanopowders as fuel additives under various diesel engine conditions. Fuel 2019, 256, 115904. [Google Scholar] [CrossRef]
- Zhang, J.; Nazarenko, Y.; Zhang, L.; Calderon, L.; Lee, K.; Garfunkel, E.; Schwander, S.; Tetley, T.D.; Chung, K.F.; Porter, A.E.; et al. Impacts of a nanosized ceria additive on diesel engine emissions of particulate and gaseous pollutants. Environ. Sci. Technol. 2013, 47, 13077–13085. [Google Scholar] [CrossRef]
- Pandey, A.K.; Nandgaonkar, M.; Suresh, S.; Varghese, A. The Effect of Cerium Oxide Nano Particles Fuel Additive on Performance, Combustion, NOx Reduction and Nano Particle Emission of Karanja and Jatropha Biodiesel in a Military 585 kW CIDI Engine. SAE Tech. Paper 2019. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Najafi, B.; Mirsalim, S.M.; Gharehghani, A.; Mohammadi, P.; Aghbashlo, M.; Ghaffari, A.; Khounani, Z.; Shojaei, T.R.; et al. A novel emulsion fuel containing aqueous nano cerium oxide additive in diesel–biodiesel blends to improve diesel engines performance and reduce exhaust emissions: Part I—Experimental analysis. Fuel 2017, 207, 741–750. [Google Scholar] [CrossRef]
- Leach, F.C.P.; Davy, M.; Terry, B. Combustion and emissions from cerium oxide nanoparticle dosed diesel fuel in a high speed diesel research engine under low temperature combustion (LTC) conditions. Fuel 2020, 119636. [Google Scholar] [CrossRef]
- Keskin, A.; Guru, M.; Altiparmak, D. Influence of tall oil biodiesel with Mg and Mo based fuel additives on diesel engine performance and emission. Biosour. Technol. 2008, 99, 6434–6438. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.T.; McKinnon, D.L.; Martin, J.R.; Pavlich, D.A. A diesel particulate regeneration system using a copper fuel additive. SAE Tech. Paper Ser. 1993, 930131. [Google Scholar] [CrossRef]
- Fanick, E.; Valentiine, J. Emissions reduction performance of a bimetallic platinum/cerium fuel borne catalyst with several diesel particulate filters on different sulfur fuels. SAE Tech. Paper Ser. 2001. [Google Scholar] [CrossRef]
- Jelles, S.J.; Makkee, M.; Moulijn, J.A. Ultra low dosage of platinum and cerium fuel additives in diesel particulate control. Top. Catal. 2001, 16–17, 269–273. [Google Scholar] [CrossRef]
- Kelso, D.T.; Epperly, W.; Hart, M. Effects of platinum fuel additive on diesel emissions and efficiency. SAE Tech. Paper Ser. 1990, 901492. [Google Scholar] [CrossRef]
- Krutzsch, B.; Wenninger, G. Effect of sodium- and lithium-based fuel additives on the regeneration efficiency of diesel particulate filters. SAE Tech. Paper Ser. 1992, 922188. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Y.; Zhang, D. An experimental study of the effect of a homogeneous combustion catalyst on fuel consumption and smoke emission in a diesel engine. Energy 2011, 36, 6004–6009. [Google Scholar] [CrossRef]
- Cho, S.-H.; Yoo, J.-I.; Turley, A.T.; Miller, C.A.; Linak, W.P.; Wendt, J.O.; Huggins, F.E.; Gilmour, M.I. Relationships between composition and pulmonary toxicity of prototype particles from coal combustion and pyrolysis. Proc. Combust. Inst. 2009, 32, 2717–2725. [Google Scholar] [CrossRef]
- Fairbairn, E.A.; Keller, A.A.; Madler, L. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef]
- Falugi, C.; Aluigi, M.G.; Chiantore, M.C. Toxicity of metal oxide nanoparticles in immune cells of the sea urchin. Mar. Environ. Res. 2012, 76, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of a Homogeneous Combustion Catalyst on the Nanostructure and Oxidative Properties of Soot from Biodiesel Combustion in a Compression Ignition Engine; Corpus ID: 56163840; Elsevier BV: Amsterdam, The Netherlands, 2015; Volume 35, pp. 1947–1954. [Google Scholar]
- Platt, R.A. Reduction in Greenhouse Gas Emissions by Application of a Combustion Catalyst; Fuel Technology Pty Ltd.: Kewdale, Western Australia, 1999. [Google Scholar]
- Zhang, D.; Ma, Y.; Zhu, M. Nanostructure and oxidative properties of soot from a compression ignition engine: The effect of a homogeneous combustion catalyst. Proc. Combust. Inst. 2013, 34, 1869–1876. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Y.; Zhang, D. Effect of a homogeneous combustion catalyst on the combustion characteristics and fuel efficiency in a diesel engine. Appl. Energy 2012, 91, 166–172. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, M.; Ma, Y.; Millin, N. Homogeneous Combustion Catalysts for Efficiency Improvements and Emission Reduction in Diesel Engines; Handbook of Clean Energy Systems by John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Staude, S.; Hecht, C.; Wlokas, I.; Schulz, C.; Atakan, B. Experimental and numerical investigation of FeCO5 to a laminar premixed hydrogen flame. Z. Phys. Chem. 2009, 223, 639–649. [Google Scholar] [CrossRef]
- Linteris, G.T.; Babushok, V.I. Promotion or inhibition of hydrogen-air ignition by iron containing compounds. Proc. Combust. Inst. 2009, 32, 2535–2542. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.L. Promotion of CO oxidization and inhibition of NO formation by gaseous iron species during hightemperature off-gas combustion. Energy Fuels 2011, 25, 967–974. [Google Scholar] [CrossRef]
- Park, K.; Bae, G.T.; Shin, K.S. The addition effect of FeCO5 on methane ignition. Bull. Korean Chem. Soc. 2002, 23, 175–176. [Google Scholar]
- Stratakis, G.A.; Stamatelos, A.M. Thermogravimetric analysis of soot emitted by a modern diesel engine run on catalystdoped fuel. Combust. Flame 2003, 132, 157–169. [Google Scholar] [CrossRef]
- Kim, S.H.; Fletcher, R.A.; Zachariah, M.R. Understanding the difference in oxidative properties between flame and diesel soot nanoparticles: The role of metals. Environ. Sci. Technol. 2005, 39, 4021–4026. [Google Scholar] [CrossRef]
- Song, J.H.; Wang, J.G.; Boehman, A.L. The role of fuelborne catalyst in diesel particulate oxidation behavior. Combust. Flame 2006, 146, 73–84. [Google Scholar] [CrossRef]
- Heidari-Maleni, A.; Gundoshmian, T.M.; Jahanbakhshi, A.; Ghobadian, B. Performance improvement and exhaust emissions reduction in diesel engine through the use of graphene quantum dot (GQD) nanoparticles and ethanol-biodiesel blends. Fuel 2020, 267, 117116. [Google Scholar] [CrossRef]
- ProOne Fuel Maximizer—Safety Data Sheet. Available online: www.pro-one.us (accessed on 25 March 2015).
- Kim, H.J.; Lee, S.H.; Kwon, S.I.; Park, S.; Lee, J.; Keel, J.H.; Lee, J.T.; Park, S. Investigation of the Emission Characteristics of Light-Duty Diesel Vehicles in Korea Based on EURO-VI Standards According to Type of After-Treatment System. Energies 2020, 13, 4936. [Google Scholar] [CrossRef]
- Azad, A.K.; Rasul, M.G.; Sharma, S.C.; Khan, M.M.K. The Lubricity of Ternary Fuel Mixture Blends as a Way to Assess Diesel Engine Durability. Energies 2018, 11, 33. [Google Scholar] [CrossRef]
- Doosan, Daewoo—Marine Diesel Engine. Available online: http://iv.doosaninfracore.com (accessed on 5 July 2018).


| Ingredient | Proportion wt% |
|---|---|
| Naphtha Petroleum | 60–100% |
| Naphthalene | 3–7% |
| Organometallic Compounds | 1–5% |
| 1,2,4-Trimethylbenzene | 0.1–1% |
| Engine Speed, rpm | Brake Mean Effective Pressure, MPa | ||||||
|---|---|---|---|---|---|---|---|
| 0.05 | 0.16 | 0.27 | 0.38 | 0.49 | 0.60 | 0.71 | |
| 1000 | 1 | 2 | 3 | 4 | 5 | 6 | - |
| 1200 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 1400 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 1600 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 1800 | 28 | 29 | 30 | 31 | 32 | 33 | - |
| 2000 | 34 | 35 | 36 | 37 | 38 | 39 | - |
| Parameter | Test Methods | Unit | Quality Requirements of Decree | Pure Diesel | Fuel with Catalyst 22.3 mg/L |
|---|---|---|---|---|---|
| Density at 15 °C | PN-EN ISO 12185: 2002, | kg/m3 | 800.0 ÷ 840.0 | 810.5 | 810.5 |
| Sulfur content | PN-EN ISO 20846: 2012 | mg/kg | max.10.0 | 4.8 | 4.8 |
| Water content | PN-EN ISO 12937: 2005 | mg/kg | max. 200 | 50 | 30 |
| Cold filter plugging point | PN-EN 116: 2015-09 | °C | max. −32 | −37 | −38 |
| Cloud point | PN-EN ISO 3015: 1997 | °C | max. −22 | −38 | −39 |
| Flashpoint | PN-EN ISO 2719: 20016-08 | °C | over 55.0 | 60.5 | 60 |
| Kinematic viscosity at 40 °C | PN-EN ISO 3104: 2004 | mm2/s | 1.500 ÷ 4.000 | 1.708 | 1.647 |
| Polycyclic aromatic Hydrocarbons | PN-EN ISO 12916: 2016-03 | %(m/m) | max. 8.0 | 1.4 | 1.3 |
| Cetane number | PN-EN ISO 5165: 2003 | - | min. 51.0 | 53 | 53.1 |
| Lubricity HFRR | PN-EN ISO 12156-1: 2016-04 | µm | max. 460 | 420 | 430 |
| Oxidation stability | PN-EN ISO 15751: 2014 -05 | h | min. 20.0 | >30 | >30 |
| Oxidation stability | PN-EN ISO 12205: 2011 + Ap1: 2013 | g/m3 | max. 25 | 4 | <2 |
| Copper strip corrosion (Cu/3 h/50 °C) | PN-EN ISO 2160: 2004 | class 1 | class 1 | 1a | 1a |
| FAME content | PN-EN ISO 14078: 2014-06 | %(v/v) | max. 7.0 | <0.5 | <0.5 |
| Total contamination | PN -EN ISO 12662: 2014-05 | mg/kg | max. 24.0 | <12 | <12 |
| Carbon residue on 10% distillation residue | PN-EN ISO 10370: 2014-12 | %(m/m) | max. 0.30 | <0.10 | <0.10 |
| Ash content | PN-EN ISO 6245: 2008 | %(m/m) | max. 0.010 | <0.001 | <0.001 |
| Cetane index | PN-EN ISO 4264: 201O/A1: 2013 | - | min. 46.0 | 50.7 | 51 |
| Manganese content | PN-EN ISO 16576: 2014-12 | mg/l | max. 2.0 | <0.5 | <0.5 |
| Distillation-recovered at 180 °C | PN-EN ISO 3405: 2012 | %(v/v) | max. 10.0 | 0.5 | 0.7 |
| Distillation-recovered at 340 °C | PN-EN ISO 3405: 2012 | %(v/v) | min. 95 | 98 | 98.5 |
| Parameter | Specification |
|---|---|
| Bore × Stroke | 123 mm × 155 mm |
| Cylinders arrangement | In line, 6 |
| Displacement | 11.051 dm3 |
| Fuel system | Diesel, Direct injection |
| Governor type of injection pump | Mechanical variable speed (R.Q.V) |
| Injection timing (B.T.D.C) | 16° ± 1° |
| Fuel injection nozzle opening pressure | 1st: 16 MPa, 2nd: 22 MPa |
| Mean effective pressure | 1.303 MPa |
| Rating output | 235 kW @ 2000 rpm |
| Specific fuel consumption @ rated power | 218 g/kWh |
| Emission standard | Stage IIIa |
| Parameters | Unit | Range | Accuracy |
|---|---|---|---|
| CO | Vol, % | 0–10 | ±0.01 |
| PM | ppm | 0–100% | ±1 |
| THC | ppm | 0–10,000 | ±1 |
| NOx | ppm | 0–500 | ±1 |
| Average Specific Fuel Consumption g/kWh +/−1% | |
|---|---|
| Pure diesel | 209 |
| Diesel with FPC | 208 |
| Relative difference, % | 0.5 |
| Average Emissions, g/kWh | ||||
|---|---|---|---|---|
| NOx | PM | CO | THC | |
| Pure diesel | 7.32 | 0.18 | 1.29 | 0.89 |
| Diesel with FPC | 7.41 | 0.15 | 1.16 | 0.82 |
| Relative difference, % | +1.2 | −16.7 | −10.1 | −7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczyk, M.; Sroka, Z.J.; Krakowian, K.; Wlostowski, R. Experimental Study of the Effect of Fuel Catalytic Additive on Specific Fuel Consumption and Exhaust Emissions in Diesel Engine. Energies 2021, 14, 54. https://doi.org/10.3390/en14010054
Tkaczyk M, Sroka ZJ, Krakowian K, Wlostowski R. Experimental Study of the Effect of Fuel Catalytic Additive on Specific Fuel Consumption and Exhaust Emissions in Diesel Engine. Energies. 2021; 14(1):54. https://doi.org/10.3390/en14010054
Chicago/Turabian StyleTkaczyk, Marcin, Zbigniew J. Sroka, Konrad Krakowian, and Radoslaw Wlostowski. 2021. "Experimental Study of the Effect of Fuel Catalytic Additive on Specific Fuel Consumption and Exhaust Emissions in Diesel Engine" Energies 14, no. 1: 54. https://doi.org/10.3390/en14010054
APA StyleTkaczyk, M., Sroka, Z. J., Krakowian, K., & Wlostowski, R. (2021). Experimental Study of the Effect of Fuel Catalytic Additive on Specific Fuel Consumption and Exhaust Emissions in Diesel Engine. Energies, 14(1), 54. https://doi.org/10.3390/en14010054





