Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids
Abstract
1. Introduction
2. Experiment
2.1. Preparation of Nanofluids
2.2. AC Breakdown Measurement
3. Results and Discussions
4. Conclusions
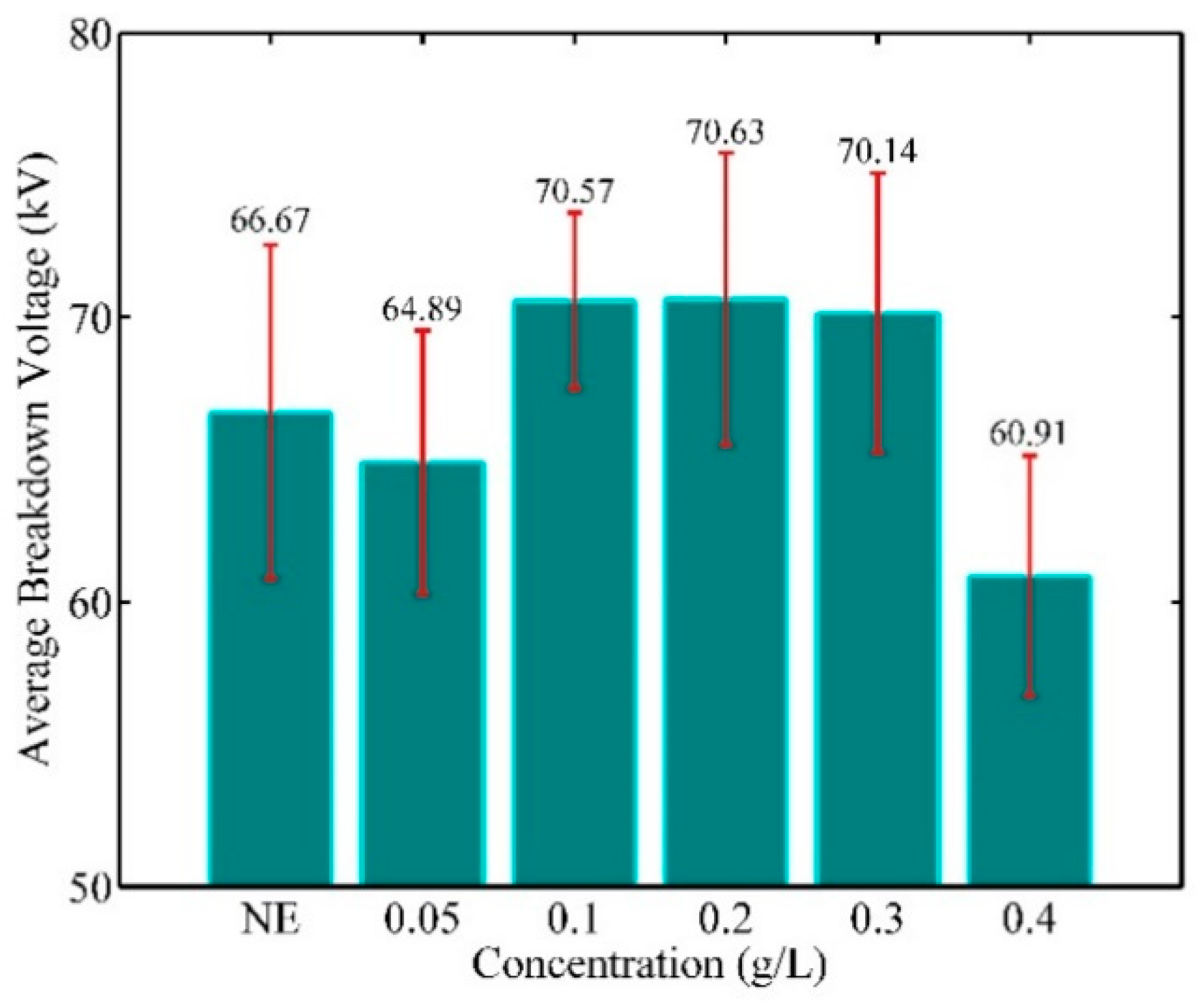
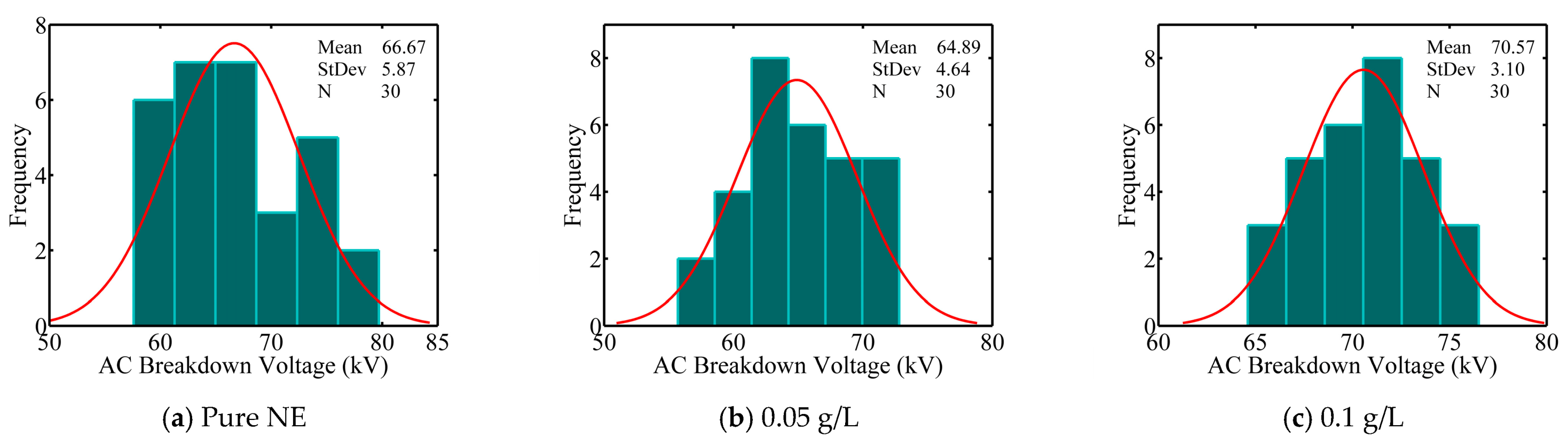
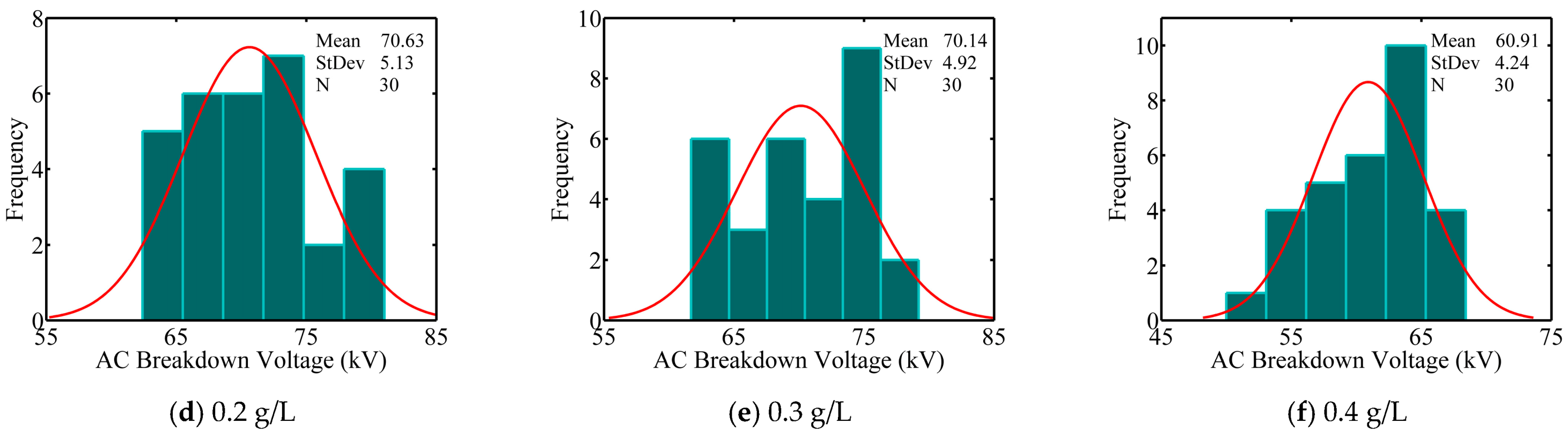
- In natural ester-based ZnO nanofluids, breakdown voltages decrease for 0.05 and 0.4 g/L concentrations and increase for 0.1 to 0.3 g/L concentrations. The best improvement in breakdown voltage is of about 5%; it is obtained with a concentration of 0.1 g/L ZnO.
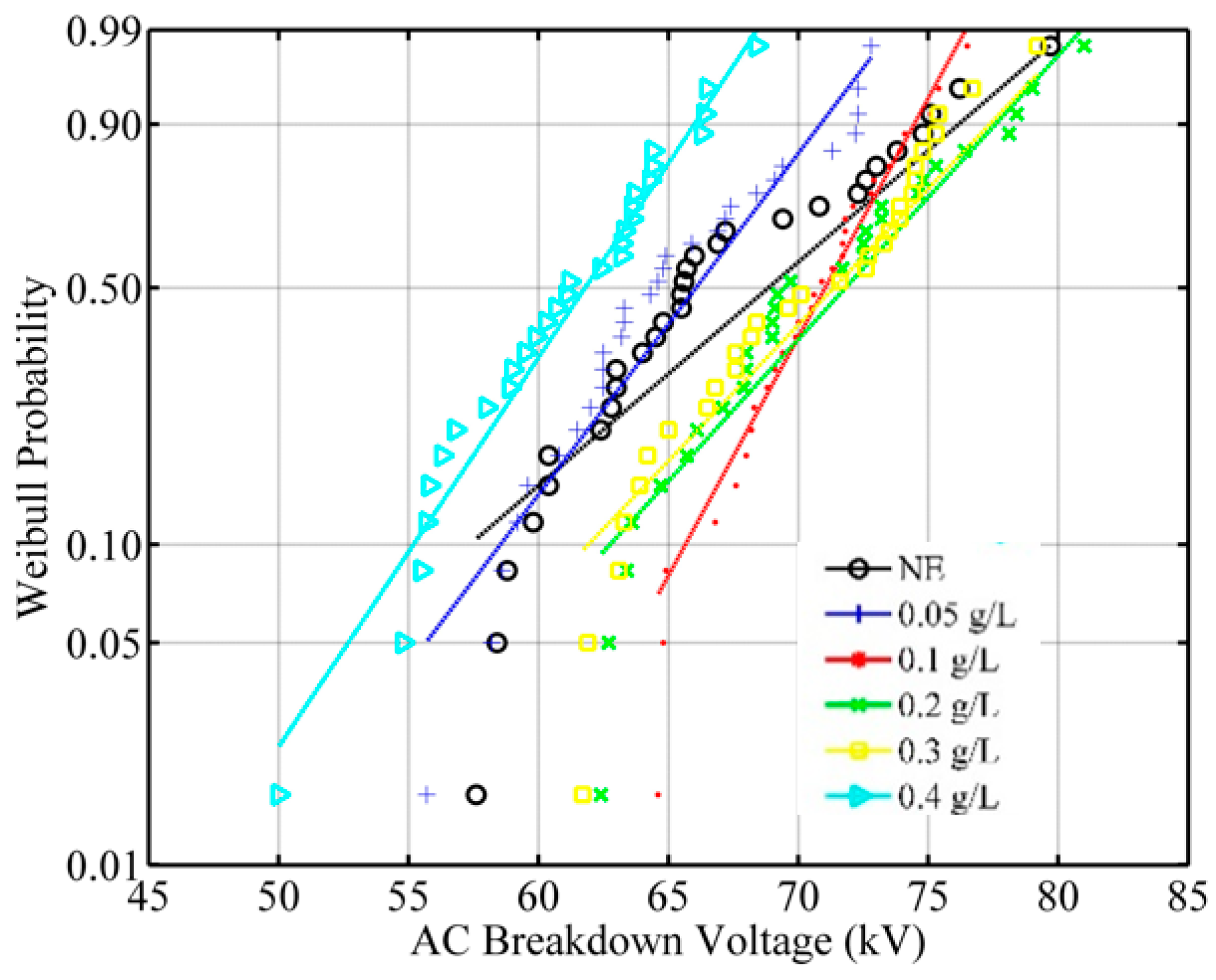
- Breakdown voltage data of all samples comply with normal distribution. Using these distribution functions, risks of 1%, 10% and 50% probabilities of breakdown voltages are calculated. The best improvement in 1% probability withstand voltage is in 0.1 g/L ZnO nanofluid. At this concentration, the probability of breakdown voltage increases by 22.7%. The improvement at concentrations of 0.2 and 0.3 g/L for the same probability is 13.2% and 11.1%, respectively. For 10% probability, the best performance is also in the 0.1 g/L concentration. At this probability, the development of the breakdown voltage is 13%.
- This increase in breakdown voltages of ZnO-added natural esters provides the opportunity to design power system equipment, especially transformers, in smaller dimensions and to meet the increasing demand.
- It is thought that the critical value of the amount of nanoparticles is exceeded at a concentration of 0.4 g/L. Increasing nanoparticle concentration beyond this value reveals the implication of a tunnelling/bridging mechanism that leads to a reduction in breakdown voltages.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fofana, I. 50 years in the development of insulating liquid. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.; Li, C. A review on properties, opportunities, and challenges of transformer oil-based nanofluids. J. Nanomater. 2016, 8371560, 1–23. [Google Scholar] [CrossRef]
- Wang, M.; Vandermaar, A.J.; Srivastava, K.D. Review of condition assessment of power transformers in service. IEEE Electr. Insul. Mag. 2002, 18, 12–25. [Google Scholar] [CrossRef]
- Reffas, A.; Idir, O.; Ziani, A.; Moulai, H.; Nacer, A.; Khelfane, I.; Ouagueni, M.; Beroual, A. Influence of thermal ageing and electrical discharges on uninhibited olive oil properties. IET Sci. Meas. Technol. 2016, 10, 711–718. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. AC dielectric strength of synthetic ester-based Fe3O4, Al2O3 and SiO2 nanofluids—Conformity with normal and Weibull distributions. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 625–633. [Google Scholar] [CrossRef]
- Stockton, D.P.; Bland, J.R.; McClanahan, T.; Wilson, J.; Harris, D.L.; McShane, P. Natural ester transformer fluids: Safety, reliability & environmental performance. In Proceedings of the IEEE Petroleum and Chemical Industry Technical Conference, Calgary, AB, Canada, 17–19 September 2007. [Google Scholar] [CrossRef]
- Beroual, A.; Khaled, U.; Noah, P.S.M.; Sitorus, H. Comparative study of breakdown voltage of mineral, synthetic and natural oils and based mineral oil mixtures under AC and DC voltages. Energies 2017, 10, 511. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. Statistical investigation of AC dielectric strength of natural ester oil-based Fe3O4, Al2O3, and SiO2 nano-fluids. IEEE Access 2019, 7, 60594–60601. [Google Scholar] [CrossRef]
- Mahanta, D.K.; Laskar, S. Electrical insulating liquid: A review. J. Adv. Dielectr. 2017, 7, 1730001–1/9. [Google Scholar] [CrossRef]
- Ahmad, F.; Khan, A.A.; Khan, Q.; Hussain, M.R. State-of-art in nano-based dielectric oil: A review. IEEE Access 2019, 7, 13396–13410. [Google Scholar] [CrossRef]
- Sitorus, H.B.H.; Setiabudy, R.; Bismo, S.; Beroual, A. Jatropha curcas methyl ester oil obtaining as vegetable insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2021–2028. [Google Scholar] [CrossRef]
- Jaroszewski, M.; Beroual, A.; Golebiowski, D. Effect of temperature on dielectric loss factor of biodegradable transformer oil. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 11–13 September 2018. [Google Scholar] [CrossRef]
- Tenbohlen, S.; Koch, M.; Vukovic, D.; Weinlader, A.; Baum, J.; Harthun, J.; Schafer, M.; Barker, S.; Fotscher, R.; Dohnal, D.; et al. Application of vegetable oil-based insulating fluids to hermetically sealed power transformers. Cigre Session 2008, 42, 24–29. [Google Scholar]
- Fofana, I.; Wasserberg, V.; Borsi, H.; Gockenbach, E. Challenge of mixed insulating liquids for use in high-voltage transformers. 1. Investigation of mixed liquids. IEEE Electr. Insul. Mag. 2002, 18, 18–31. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995; pp. 66–74. [Google Scholar]
- Khaled, U.; Beroual, A. AC dielectric strength of mineral oil-based Fe3O4 and Al2O3 nanofluids. Energies 2018, 11, 3505. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. Comparative study on the AC breakdown voltage of transformer mineral oil with transformer oil-based Al2O3 nanofluids. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 10–13 September 2018. [Google Scholar] [CrossRef]
- Azizie, N.A.; Hussin, N. Preparation of vegetable oil-based nanofluid and studies on its insulating property: A review. J. Phys. Conf. Ser. 2020, 1432, 012025. [Google Scholar] [CrossRef]
- Chen, J.; Sun, P.; Sima, W.; Shao, Q.; Ye, L.; Li, C. A promising nano-insulating-oil for industrial application: Electrical properties and modification mechanism. Nanomaterials 2019, 9, 788. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Yin, H.; Huang, Z.; Wang, Q.; Liu, L.; Sun, J.; He, J. Analysis of dielectric properties and breakdown characteristics of vegetable insulating oil with modified by ZnO nanoparticles. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application (ICHVE), Athens, Greece, 10–13 September 2018. [Google Scholar] [CrossRef]
- Hanai, M.; Hosomi, S.; Kojima, H.; Hayakawa, N.; Okubo, H. Dependence of TiO2 and ZnO nanoparticle concentration on electrical insulation characteristics of insulating oil. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013; pp. 780–783. [Google Scholar] [CrossRef]
- Bakrutheen, M.; Karthik, R.; Madavan, R. Investigation of critical parameters of insulating mineral oil using semiconductive nanoparticles. In Proceedings of the International Conference on Circuits, Power and Computing Technologies (ICCPCT), Nagercoil, Indie, 20–21 March 2013; pp. 294–299. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. Rsc Adv. 2017, 7, 12599. [Google Scholar] [CrossRef]
- IEC 60156. Insulating Liquids—Determination of the Breakdown Voltage at Power Frequency—Test Method; IEC 60156 Ed. 2; International Electrotechnical Commission (IEC): Geneva, Switzerland, 1995. [Google Scholar]
- Anderson, T.W.; Darling, D.A. A test of goodness of fit. J. Am. Stat. Assoc. 1954, 49, 765–769. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 19, 591–611. [Google Scholar] [CrossRef]
- Sitorus, H.B.H.; Beroual, A.; Setiabudy, R.; Bismo, S. Statistical analysis of AC and DC breakdown voltage of JMEO (jatropha methyl ester oil), mineral oil and their mixtures. In Proceedings of the IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017. [Google Scholar] [CrossRef]
- Martin, D.; Wang, Z.D. Statistical analysis of the AC breakdown voltages of ester based transformer oils. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1044–1050. [Google Scholar] [CrossRef]
- Jian-quan, Z.; Yue-fan, D.; Mu-tian, C.; Cheng-rong, L.; Xiao-xin, L.; Yu-zhen, L. AC and lightning breakdown strength of transformer oil modified by semiconducting nanoparticles. In Proceedings of the 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 652–654. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]


| Property | MIDEL eN 1204 |
|---|---|
| Density at 20 °C (g/cm3) | 0.92 |
| Kinematic viscosity at 40 °C (mm2/s) | 8 |
| Pour temperature (°C) | −31 |
| Flash Point (°C) | >315 |
| Fire Point (°C) | >350 |
| Total acid number (mg KOH/g) | 0.04 |
| Water content (ppm) | 50 |
| Dissipation factor at 90 °C | <0.1 |
| W | p-Value | Conformity of Weibull Distribution | |
|---|---|---|---|
| NE | 0.5449 | 0.1611 | Accepted |
| 0.05 g/L | 0.3345 | 0.5149 | Accepted |
| 0.1 g/L | 0.1782 | 0.9109 | Accepted |
| 0.2 g/L | 0.2860 | 0.6007 | Accepted |
| 0.3 g/L | 0.7278 | 0.0516 | Accepted |
| 0.4 g/L | 0.3842 | 0.3957 | Accepted |
| W | p-Value | Conformity of Normal Distribution | |
|---|---|---|---|
| NE | 0.9516 | 0.1866 | Accepted |
| 0.05 g/L | 0.9626 | 0.3608 | Accepted |
| 0.1 g/L | 0.9756 | 0.7005 | Accepted |
| 0.2 g/L | 0.9654 | 0.4231 | Accepted |
| 0.3 g/L | 0.9385 | 0.0830 | Accepted |
| 0.4 g/L | 0.9670 | 0.4601 | Accepted |
| BDV Probability | NE BDV (kV) | Type of NFs | BDV (kV) | Change (%) |
|---|---|---|---|---|
| %1 | 51.17 | 0.05 g/L | 54.16 | 5.9 |
| 0.1 g/L | 62.78 | 22.7 | ||
| 0.2 g/L | 57.92 | 13.2 | ||
| 0.3 g/L | 56.83 | 11.1 | ||
| 0.4 g/L | 51.02 | −0.3 | ||
| %10 | 58.52 | 0.05 g/L | 59.12 | 1.0 |
| 0.1 g/L | 66.27 | 13.2 | ||
| 0.2 g/L | 63.72 | 8.9 | ||
| 0.3 g/L | 62.94 | 7.5 | ||
| 0.4 g/L | 55.43 | −5.3 | ||
| %50 | 67.55 | 0.05 g/L | 65.20 | −3.5 |
| 0.1 g/L | 70.55 | 4.4 | ||
| 0.2 g/L | 70.85 | 4.9 | ||
| 0.3 g/L | 70.45 | 4.3 | ||
| 0.4 g/L | 60.85 | −9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duzkaya, H.; Beroual, A. Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids. Energies 2021, 14, 99. https://doi.org/10.3390/en14010099
Duzkaya H, Beroual A. Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids. Energies. 2021; 14(1):99. https://doi.org/10.3390/en14010099
Chicago/Turabian StyleDuzkaya, Hidir, and Abderrahmane Beroual. 2021. "Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids" Energies 14, no. 1: 99. https://doi.org/10.3390/en14010099
APA StyleDuzkaya, H., & Beroual, A. (2021). Statistical Analysis of AC Dielectric Strength of Natural Ester-Based ZnO Nanofluids. Energies, 14(1), 99. https://doi.org/10.3390/en14010099






