Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development
Abstract
1. Introduction
2. The Biogas Industry: Current Situation and Policy Framework
2.1. Overview of the Biogas Industry Sector
2.2. Current Policy Framework for Biogas Energy Vector: The Case of the European Union
3. Biomass Feedstock: Characteristics, Potential, and Logistics
3.1. Organic Fraction of Municipal Solid Waste
3.2. Cattle Manure
3.3. Sewage Sludge
3.4. Lignocellulosic Biomass
3.5. Waste and Sewage from Agriculture-Related Industries
3.6. Algae
4. Biogas Upgrading Technologies
4.1. Water and Organic Scrubber
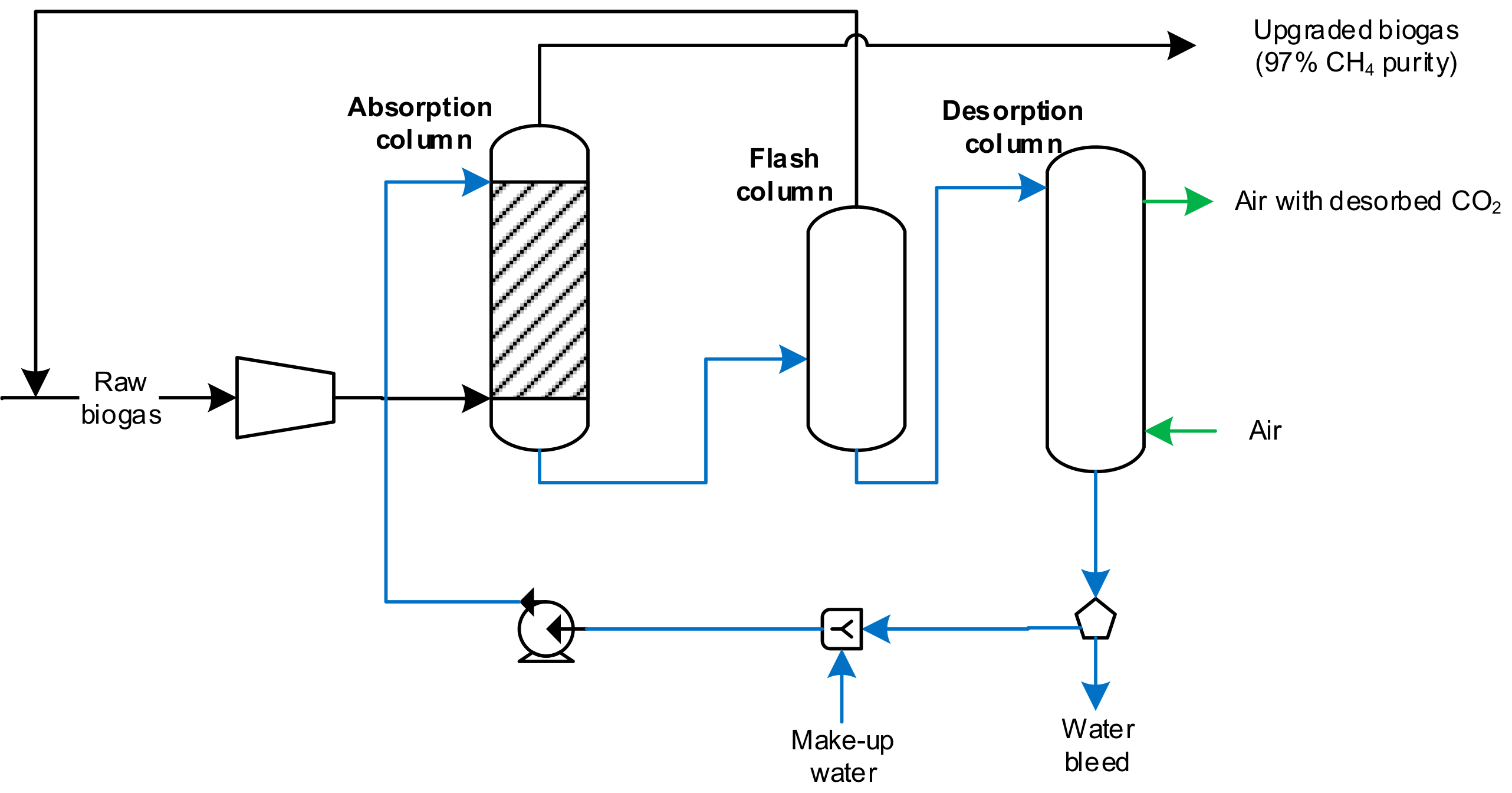
4.2. Chemical Scrubber
4.3. Pressure Swing Adsorption
4.4. Membrane Separation
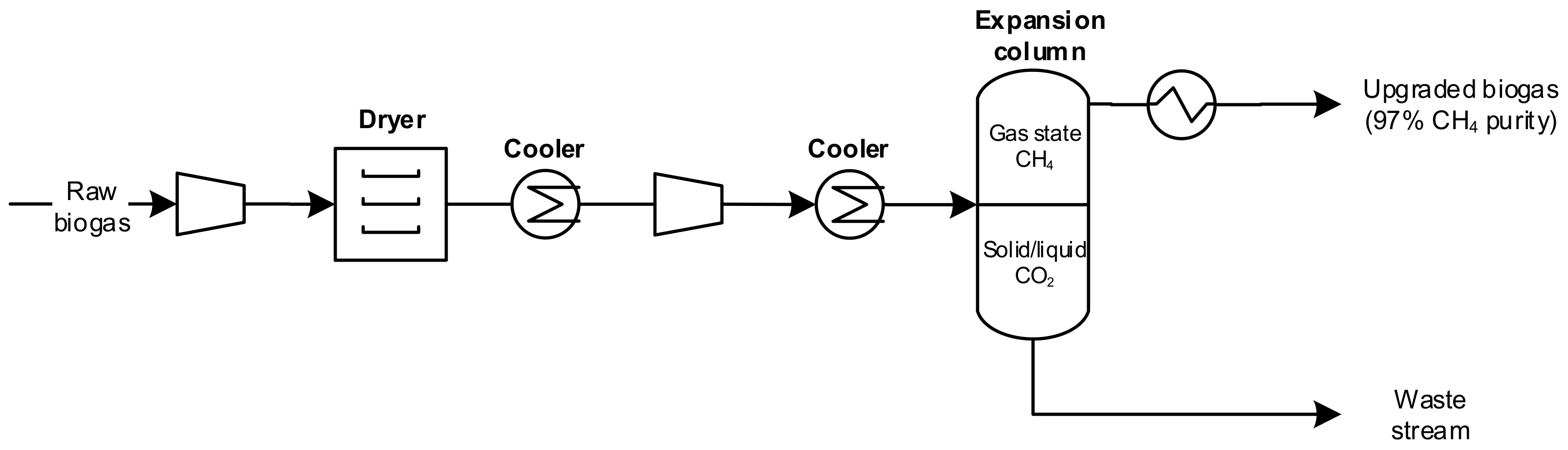
4.5. Cryogenic Separation
4.6. Cost Associated to Biogas Production and Conventional Upgrading Technologies
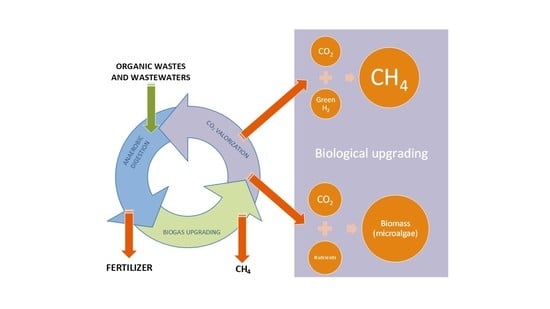
5. Biological Upgrading Development
5.1. Hydrogenotrophic Biogas Upgrading
5.2. Photosynthetic Biogas Upgrading
5.3. Microaerobic H2S Removal into Biological Upgrading Process
6. Business Models of Biogas Industry
6.1. Biogas from Agri-Food Sector
6.2. Biogas from Wastewater Sector
6.3. Biogas from Organic Fraction of Municipal Solid Waste
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EC. EU Reference Scenario 2016. Energy, Transport and GHG Emissions; Trends to 2050; European Commision: Brussels, Belgium, 2016; Available online: https://ec.europa.eu/energy/sites/ener/files/documents/20160713%20draft_publication_REF2016_v13.pdf (accessed on 22 December 2020).
- Advanced Biofuels Coalition. Leaders of Sustainable Biofuels; Advanced Biofuels Coalition: Florence, Italy, 2020; Available online: https://www.advancedbiofuelscoalition.eu/ (accessed on 22 December 2020).
- EC. Final Report. Building Up the Future. Sub Group on Advanced Biofuels. Sustainable Transport Forum; European Commision: Brussels, Belgium, 2017; Available online: https://ec.europa.eu/transparency/regexpert/index.cfm?do=groupDetail.groupDetailDoc&id=33288&no=1 (accessed on 22 December 2020).
- IEA. Integration of Biogas Systems into the Energy System. Technical Aspects of Flexible Plant Operation; International Energy Agency: Paris, France, 2020; Available online: https://www.ieabioenergy.com/wp-content/uploads/2020/09/Integration-of-biogas-systems-into-the-energy-system-Report.pdf (accessed on 22 December 2020).
- EC. Report from the Commission to the European Parliament and the Council on Progress of Clean Energy Competitiveness; European Commision: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0953&from=EN (accessed on 22 December 2020).
- Allegue, L.B.; Hinge, J. Biogas and Bio-Syngas Upgrading; Danish Technological Institute: Taastrup, Denmark, 2012; Available online: https://www.teknologisk.dk/_/media/52679_Report-Biogas%20and%20syngas%20upgrading.pdf (accessed on 22 December 2020).
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuelsbioprod. Biorefin. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Anneli Petersson, A.W. Biogas Upgrading Technologies—Developments and Innovations; International Energy Agency: Paris, France, 2009; Available online: https://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/upgrading_rz_low_final.pdf (accessed on 22 December 2020).
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.R.; Ángeles, R.; Marín, D.; Díaz, I.; Colzi, A.; Posadas, E.; Lebrero, R.; Muñoz, R. Biogas purification and upgrading technologies. In Biogas: Fundamentals, Process, and Operation; Tabatabaei, M., Ghanavati, H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 239–276. [Google Scholar]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy, 69th ed.; BP: Middlesex, UK, 2020; Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed on 20 April 2021).
- IEA. Outlook for Biogas and Biomethane. Prospects for Organic Growth; International Energy Agency: Paris, France, 2020; Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth (accessed on 22 December 2020).
- European Biogas Assosiation. Insitutional Web Page; European Biogas Association: Brussels, Belgium, 2020; Available online: https://www.europeanbiogas.eu/ (accessed on 22 December 2020).
- EBA. EBA Statistical Report 2020; European Biogas Association: Brussels, Belgium, 2020; Available online: https://www.europeanbiogas.eu/eba-statistical-report-2020/ (accessed on 20 April 2021).
- Fachverband. Biogas Market Data in Germany 2019/2020; Fachverband Biogas: Freising, Germany, 2020; Available online: https://www.biogas.org/edcom/webfvb.nsf/id/EN-German-biogas-market-data/$file/20-07-23_Biogasindustryfigures-2019-2020_english.pdf (accessed on 1 March 2021).
- EBA. EBA Statistical Report 2018; European Biogas Association: Brussels, Belgium, 2018; Available online: https://www.europeanbiogas.eu/wp-content/uploads/2019/11/EBA_report2018_abriged_A4_vers12_220519_RZweb.pdf (accessed on 22 December 2020).
- Surendra, K.C.; Takara, D.; Hashimoto, A.G.; Khanal, S.K. Biogas as a sustainable energy source for developing countries: Opportunities and challenges. Renew. Sustain. Energy Rev. 2014, 31, 846–859. [Google Scholar] [CrossRef]
- IEA. Advanced Biofuels—Potential for Cost Reduction; International Energy Agency: Paris, France, 2020; Available online: https://www.ieabioenergy.com/blog/publications/new-publication-advanced-biofuels-potential-for-cost-reduction/ (accessed on 22 December 2020).
- EBA. The ’European Biomethane Map 2020’ Shows a 51% Increase of Biomethane Plants in Europe in Two Years; European Biogas Association: Brussels, Belgium, 2020; Available online: https://www.europeanbiogas.eu/wp-content/uploads/2020/06/Biomethane-map_Press-Release_EBA_GiE.pdf (accessed on 1 March 2021).
- EU. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources; Publications Office of the European Union: Luxembourg, 2018; Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 1 November 2020).
- EC. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment Updated Bioeconomy Strategy; European Commision: Brussels, Belgium, 2018; Available online: https://op.europa.eu/en/publication-detail/-/publication/edace3e3-e189-11e8-b690-01aa75ed71a1/language-en/format-PDF/source-149755478 (accessed on 22 December 2020).
- EC. Circular Economy Action Plan. For a Cleaner and More Competitive Europe; European Commision: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/environment/circular-economy/pdf/new_circular_economy_action_plan.pdf (accessed on 22 December 2020).
- SAF. Web Page; Sustainable Agribusiness Forum: Kyiv, Ukraine, 2020; Available online: https://saf.org.ua/en/news/655/ (accessed on 22 December 2020).
- Repele, M.; Udrene, L.; Bazbauers, G. Support mechanisms for biomethane production and supply. Energy Procedia 2017, 113, 304–310. [Google Scholar] [CrossRef]
- EurObserv’ER. Biofuels Barometer 2020; EurObserv’ER: Paris, France, 2020; Available online: https://www.eurobserv-er.org/biofuels-barometer-2020/ (accessed on 22 December 2020).
- Monteny, G.-J.; Bannink, A.; Chadwick, D. Greenhouse gas abatement strategies for animal husbandry. Agric. Ecosyst. Environ. 2006, 112, 163–170. [Google Scholar] [CrossRef]
- Scheftelowitz, M.; Thrän, D. Unlocking the energy potential of manure—An assessment of the biogas production potential at the farm level in Germany. Agriculture 2016, 6, 20. [Google Scholar] [CrossRef]
- Akyürek, Z. Potential of biogas energy from animal waste in the Mediterranean region of Turkey. J. Energy Syst. 2018, 160–167. [Google Scholar] [CrossRef]
- Tessele, F.; van Lier, J. Anaerobic digestion and the circular economy. Water E J. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Cecchi, F.; Bolzonella, D.; Pavan, P.; Macé, S.; Mata-Alvarez, J. Anaerobic digestion of the organic fraction of municipal solid waste for methane production: Research and industrial application. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 463–472. [Google Scholar]
- Curry, R.; Pérez-Camacho, M.N.; Brennan, R.; Gilkinson, S.; Cromie, T.; Foster, P.; Smyth, B.; Orozco, A.; Groom, E.; Murray, S.; et al. Quantification of anaerobic digestion feedstocks for a regional bioeconomy. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2018, 171, 94–103. [Google Scholar] [CrossRef]
- Steffen, R.; Szolar, O.; Braun, R. Feedstocks for Anaerobic Digestion; Department of Agrobiotechnology Tulln, University of Natural Resources and Life Science: Vienna, Austria, 1998; Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.487.370&rep=rep1&type=pdf (accessed on 20 December 2020).
- Frigon, J.-C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: A comparative review. Biofuelsbioproducts Biorefining 2010, 4, 447–458. [Google Scholar] [CrossRef]
- Frigon, J.-C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Himanshu, H.; Murphy, J.D.; Grant, J.; O’Kiely, P. Synergies from co-digesting grass or clover silages with cattle slurry in in vitro batch anaerobic digestion. Renew. Energy 2018, 127, 474–480. [Google Scholar] [CrossRef]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef]
- Wahid, R.; Nielsen, S.F.; Hernandez, V.M.; Ward, A.J.; Gislum, R.; Jørgensen, U.; Møller, H.B. Methane production potential from Miscanthus sp.: Effect of harvesting time, genotypes and plant fractions. Biosyst. Eng. 2015, 133, 71–80. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane production from lignocellulose: Biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Naran, E.; Toor, U.A.; Kim, D.-J. Effect of pretreatment and anaerobic co-digestion of food waste and waste activated sludge on stabilization and methane production. Int. Biodeterior. Biodegrad. 2016, 113, 17–21. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants: Status and future trends. Appl. Biochem. Biotechnol. 2003, 109, 95–106. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Giordano, A.; Liotta, F.; Panico, A.; Pirozzi, F. Anaerobic co-digestion of organic wastes. Rev. Environ. Sci. Bio/Technol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2019, 25, 1–17. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012; Available online: https://openknowledge.worldbank.org/handle/10986/17388 (accessed on 20 December 2020).
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef]
- FAO. World Livestock: Transforming the Livestock Sector through the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: http://www.fao.org/3/CA1201EN/ca1201en.pdf (accessed on 20 December 2020).
- Meat_Atlas. Facts and Fi Gures about the ANIMALS we Eat. Berlin, Germany; Heinrich Böll Foundation and Friends of the Earth Europe: Brussels, Belgium, 2014; Available online: https://www.foeeurope.org/sites/default/files/publications/foee_hbf_meatatlas_jan2014.pdf (accessed on 20 December 2020).
- FAO. Meat Sources; Food and Agriculture organization of the United Nations: Rome, Italy, 2014; Available online: http://www.fao.org/ag/againfo/themes/es/meat/backgr_sources.html (accessed on 20 December 2020).
- Ogbuewu, I.P.; Odoemenam, V.U.; Obikaonu, H.O.; Opara, M.N.; Emenalom, O.O.; Uchegbu, M.C.; Okoli, I.C.; Esonu, B.O.; Iloeje, M.U. The growing importance of neem (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: A review. Res. J. Med. Plant 2011, 5, 230–245. [Google Scholar] [CrossRef]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166–167, 514–531. [Google Scholar] [CrossRef]
- Van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Velthof, G.L.; Lesschen, J.P.; Webb, J.; Pietrzak, S.; Miatkowski, Z.; Pinto, M.; Kros, J.; Oenema, O. The impact of the Nitrates Directive on nitrogen emissions from agriculture in the EU-27 during 2000–2008. Sci. Total Environ. 2014, 468–469, 1225–1233. [Google Scholar] [CrossRef]
- Webb, J.; Sommer, S.G.; Kupper, T.; Groenestein, K.; Hutchings, N.J.; Eurich-Menden, B.; Rodhe, L.; Misselbrook, T.H.; Amon, B. Emissions of ammonia, nitrous oxide and methane during the management of solid manures. In Agroecology and Strategies for Climate Change; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 67–107. [Google Scholar]
- Nielsen, H.B.; Mladenovska, Z.; Westermann, P.; Ahring, B.K. Comparison of two-stage thermophilic (68 °C/55 °C) anaerobic digestion with one-stage thermophilic (55 °C) digestion of cattle manure. Biotechnol. Bioeng. 2004, 86, 291–300. [Google Scholar] [CrossRef]
- Tallou, A.; Haouas, A.; Jamali, M.Y.; Atif, K.; Amir, S.; Aziz, F. Review on cow manure as renewable energy. In Smart Village Technology: Concepts and Developments; Patnaik, S., Sen, S., Mahmoud, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 341–352. [Google Scholar]
- Issah, A.-A.; Kabera, T.; Kemausuor, F. Biogas optimisation processes and effluent quality: A review. Biomass Bioenergy 2020, 133, 105449. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Frison, A.; Raga, R.; Angelidaki, I. Improving methane production from digested manure biofibers by mechanical and thermal alkaline pretreatment. Bioresour. Technol. 2016, 216, 545–552. [Google Scholar] [CrossRef]
- Meneses-Quelal, O.; Velázquez-Martí, B. Pretreatment of animal manure biomass to improve biogas production: A review. Energies 2020, 13, 3573. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Trobajo, R.; Caiola, N.; Ibáñez, C.; Fabregat, A.; Bengoa, C. Biogas production from sewage sludge and microalgae co-digestion under mesophilic and thermophilic conditions. Renew. Energy 2015, 75, 374–380. [Google Scholar] [CrossRef]
- Bachmann, N. Sustainable Biogas Production in Municipal Wastewater Treatment Plants; International Energy Agency: Paris, France, 2015; Available online: https://task37.ieabioenergy.com/files/daten-redaktion/download/Technical%20Brochures/Wastewater_biogas_grey_web-1.pdf (accessed on 22 December 2020).
- VSA. Energie in ARA, Leitfaden zur Energieoptimierung auf Abwasserreinigungsanlagen; Handbuch im Auftrag des Bundesamtes für Energie und des Verband Schweizer Abwasser: Glattbrugg, Switzerland, 2010; Available online: https://vsa.ch/Mediathek/energie-in-ara/ (accessed on 20 December 2020).
- Zhang, H.J. Sludge Treatment to Increase Biogas Production. Trita-LWR Degree Project 10–20 [Degree Project]; Royal Institute of Technology (KTH): Stockholm, Sweden, 2010. [Google Scholar]
- Al Seadi, T.; Lukehurst, C. Quality Management of Digestate from Biogas Plants Used as Fertiliser; IEA Bioenergy: Paris, France, 2012; Available online: https://www.ieabioenergy.com/wp-content/uploads/2012/05/digestate_quality_web_new.pdf (accessed on 20 December 2020).
- Matsakas, L.; Rova, U.; Christakopoulos, P. Strategies for enhanced biogas generation through anaerobic digestion of forest material—An overview. BioResources 2016, 11, 5482–5499. [Google Scholar]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef]
- Garcìa-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Vacuum pyrolysis of softwood and hardwood biomass. J. Anal. Appl. Pyrolysis 2007, 78, 104–116. [Google Scholar] [CrossRef]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Sequential parametric optimization of methane production from different sources of forest raw material. Front. Microbiol. 2015, 6, 1163. [Google Scholar] [CrossRef]
- Salehian, P.; Karimi, K.; Zilouei, H.; Jeihanipour, A. Improvement of biogas production from pine wood by alkali pretreatment. Fuel 2013, 106, 484–489. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Available online: http://www.fao.org/3/mb060e/mb060e00.pdf (accessed on 20 December 2020).
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; bin Ujang, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental sustainability of anaerobic digestion of household food waste. J. Environ. Manag. 2019, 236, 798–814. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.W.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wu, S.; Zhang, W.; Dong, R. Effects of organic loading rate and effluent recirculation on the performance of two-stage anaerobic digestion of vegetable waste. Bioresour. Technol. 2013, 146, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.; Luongo Malave, A.C.; Bernardi, M.; Fino, D. Energy efficacy used to score organic refuse pretreatment processes for hydrogen anaerobic production. Waste Manag. 2013, 33, 2225–2233. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Lay, C.-H.; Vo, T.-P.; Lin, P.-Y.; Abdul, P.M.; Liu, C.-M.; Lin, C.-Y. Anaerobic hydrogen and methane production from low-strength beverage wastewater. Int. J. Hydrogen Energy 2019, 44, 14351–14361. [Google Scholar] [CrossRef]
- Ma, B.; Peng, Y.; Zhang, S.; Wang, J.; Gan, Y.; Chang, J.; Wang, S.; Wang, S.; Zhu, G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013, 129, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.S.; Harada, H.; Viraraghavan, T. Low-strength wastewater treatment by a UASB reactor. Bioresour. Technol. 1996, 55, 187–194. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Song, W.; Su, H.; Ding, L.; Lin, R.; Lu, H.; Liu, J.; Zhou, J.; Cen, K. Fermentative hydrogen production using algal biomass as feedstock. Renew. Sustain. Energy Rev. 2015, 51, 209–230. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.-P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuelsbioprod. Biorefin. 2012, 6, 205–218. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef]
- Polakovičová, G.; Kušnír, P.; Nagyová, S.; Mikulec, J. Process integration of algae production and anaerobic digestion. Chem. Eng. Trans. 2012, 29, 1129–1134. [Google Scholar]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Effect of organic loading rate on anaerobic digestion of thermally pretreated Scenedesmus sp. biomass. Bioresour. Technol. 2013, 129, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour. Technol. 2013, 131, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Schwede, S.; Rehman, Z.-U.; Gerber, M.; Theiss, C.; Span, R. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour. Technol. 2013, 143, 505–511. [Google Scholar] [CrossRef]
- Ayala-Parra, P.; Liu, Y.; Field, J.A.; Sierra-Alvarez, R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew. Energy 2017, 108, 410–416. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Improved performance of anaerobic digestion of Spirulina maxima algal biomass by addition of carbon-rich wastes. Biotechnol. Lett. 1983, 5, 677–682. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef]
- IEA. Insitutional Web Page; International Energy Agency: Paris, France, 2020; Available online: http://task37.ieabioenergy.com/plant-list.html (accessed on 22 December 2020).
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Posadas, E.; Toledo-Cervantes, A.; Lebrero, R.; Muñoz, R. Influence of alkalinity and temperature on photosynthetic biogas upgrading efficiency in high rate algal ponds. Algal Res. 2018, 33, 284–290. [Google Scholar] [CrossRef]
- Bauer, F.; Persson, T.; Hulteberg, C.; Tamm, D. Biogas upgrading—Technology overview, comparison and perspectives for the future. Biofuelsbioprod. Biorefin. 2013, 7, 499–511. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Chapter 33—Biogas Upgrading: Current and Emerging Technologies. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: London, UK, 2019; pp. 817–843. [Google Scholar]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Makaruk, A.; Miltner, M.; Harasek, M. Membrane biogas upgrading processes for the production of natural gas substitute. Sep. Purif. Technol. 2010, 74, 83–92. [Google Scholar] [CrossRef]
- Navigant. Gas for Climate. The Optimal Role for Gas in a Net-Zero Emissions Energy System; Navigant: Utrecht, The Netherlands, 2019; Available online: https://www.europeanbiogas.eu/wp-content/uploads/2019/11/GfC-study-The-optimal-role-for-gas-in-a-net-zero-emissions-energy-system.pdf (accessed on 22 December 2020).
- IEA. Average Costs of Biogas Production Technologies Per Unit of Energy Produced (Excluding Feedstock); International Energy Agency: Paris, France, 2018; Available online: https://www.iea.org/data-and-statistics/charts/average-costs-of-biogas-production-technologies-per-unit-of-energy-produced-excluding-feedstock-2018 (accessed on 22 December 2020).
- Ardolino, F.; Cardamone, G.F.; Parrillo, F.; Arena, U. Biogas-to-biomethane upgrading: A comparative review and assessment in a life cycle perspective. Renew. Sustain. Energy Rev. 2020, 139, 110588. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; Guerras, L.S.; Martín, M. Optimal technology selection for the biogas upgrading to biomethane. J. Clean. Prod. 2020, 267, 122032. [Google Scholar] [CrossRef]
- Niederbacher, M. Biometano Agricolo e Industriale: La Nuovasfida per l’Autotrazione; BTS Biogas: Verona, Italy, 2017. [Google Scholar]
- Satinover, S.J.; Schell, D.; Borole, A.P. Achieving high hydrogen productivities of 20 L/L-day via microbial electrolysis of corn stover fermentation products. Appl. Energy 2020, 259, 114126. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, D.; Xu, M.; Su, Y.; Ma, J.; Angelidaki, I.; Zhang, Y. Optimization of a newly developed electromethanogenesis for the highest record of methane production. J. Hazard. Mater. 2020, 407, 124363. [Google Scholar] [CrossRef]
- Viessmann. Power-to-Gas Plant Put into Operation; Viessmann: Allendor, Germany, 2015; Available online: https://www.viessmann.ae/content/dam/vi-brands/AE/Press/Press%20PDF/Power-to-gas_plant_put_into_operation.pdf/_jcr_content/renditions/original.media_file.download_attachment.file/Power-to-gas_plant_put_into_operation.pdf (accessed on 1 March 2021).
- Electrochaea. Market Opportunity; Electrochaea GMbH: Planegg, Germany, 2021; Available online: http://www.electrochaea.com/market/ (accessed on 1 March 2021).
- Wulf, C.; Zapp, P.; Schreiber, A. Review of power-to-X demonstration projects in Europe. Front. Energy Res. 2020, 8, 191. [Google Scholar] [CrossRef]
- Vo, T.T.Q.; Wall, D.M.; Ring, D.; Rajendran, K.; Murphy, J.D. Techno-economic analysis of biogas upgrading via amine scrubber, carbon capture and ex-situ methanation. Appl. Energy 2018, 212, 1191–1202. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological methanation of CO2 in a novel biofilm plug-flow reactor: A high rate and low parasitic energy process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Dupnock, T.L.; Deshusses, M.A. Detailed investigations of dissolved hydrogen and hydrogen mass transfer in a biotrickling filter for upgrading biogas. Bioresour. Technol. 2019, 290, 121780. [Google Scholar] [CrossRef] [PubMed]
- Porté, H.; Kougias, P.G.; Alfaro, N.; Treu, L.; Campanaro, S.; Angelidaki, I. Process performance and microbial community structure in thermophilic trickling biofilter reactors for biogas upgrading. Sci. Total Environ. 2019, 655, 529–538. [Google Scholar] [CrossRef]
- Jensen, M.B.; Strübing, D.; de Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Koch, K.; Kofoed, M.V.W. Stick or leave—Pushing methanogens to biofilm formation for ex situ biomethanation. Bioresour. Technol. 2019, 291, 121784. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Ding, J.; Dai, K.; van Loosdrecht, M.C.M.; Zeng, R.J. Stable acetate production in extreme-thermophilic (70 °C) mixed culture fermentation by selective enrichment of hydrogenotrophic methanogens. Sci. Rep. 2014, 4, 5268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Campanaro, S.; Treu, L.; Kougias, P.G.; Angelidaki, I. Novel ecological insights and functional roles during anaerobic digestion of saccharides unveiled by genome-centric metagenomics. Water Res. 2019, 151, 271–279. [Google Scholar] [CrossRef]
- Burkhardt, M.; Jordan, I.; Heinrich, S.; Behrens, J.; Ziesche, A.; Busch, G. Long term and demand-oriented biocatalytic synthesis of highly concentrated methane in a trickle bed reactor. Appl. Energy 2019, 240, 818–826. [Google Scholar] [CrossRef]
- Ullrich, T.; Lindner, J.; Bär, K.; Mörs, F.; Graf, F.; Lemmer, A. Influence of operating pressure on the biological hydrogen methanation in trickle-bed reactors. Bioresour. Technol. 2018, 247, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-type injection system as a potential H2 mass transfer technology for full-scale in situ biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. H2 addition through a submerged membrane for in-situ biogas upgrading in the anaerobic digestion of sewage sludge. Bioresour. Technol. 2019, 280, 1–8. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Zhang, X.; Gong, H.; Xia, Y.; Holmes, D.E. Application of in-situ H2-assisted biogas upgrading in high-rate anaerobic wastewater treatment. Bioresour. Technol. 2020, 299, 122598. [Google Scholar] [CrossRef] [PubMed]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Berg Olesen, C.; Feilberg, A.; Kofoed, M.V.W. Parameters affecting acetate concentrations during in-situ biological hydrogen methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid biogas upgrading in a two-stage thermophilic reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Díaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Kim, S.; Mostafa, A.; Im, S.; Lee, M.-K.; Kang, S.; Na, J.-G.; Kim, D.-H. Production of high-calorific biogas from food waste by integrating two approaches: Autogenerative high-pressure and hydrogen injection. Water Res. 2021, 194, 116920. [Google Scholar] [CrossRef]
- Bahr, M.; Diaz, I.; Dominguez, A.; Gonzalez Sanchez, A.; Munoz, R. Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ. Sci. Technol. 2014, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Estrada, J.M.; Lebrero, R.; Muñoz, R. A comparative analysis of biogas upgrading technologies: Photosynthetic vs physical/chemical processes. Algal Res. 2017, 25, 237–243. [Google Scholar] [CrossRef]
- Ángeles, R.; Arnaiz, E.; Gutiérrez, J.; Sepúlveda-Muñoz, C.A.; Fernández-Ramos, O.; Muñoz, R.; Lebrero, R. Optimization of photosynthetic biogas upgrading in closed photobioreactors combined with algal biomass production. J. Water Process Eng. 2020, 38, 101554. [Google Scholar] [CrossRef]
- Rodero, M.d.R.; Carvajal, A.; Arbib, Z.; Lara, E.; de Prada, C.; Lebrero, R.; Muñoz, R. Performance evaluation of a control strategy for photosynthetic biogas upgrading in a semi-industrial scale photobioreactor. Bioresour. Technol. 2020, 307, 123207. [Google Scholar] [CrossRef]
- Marín, D.; Carmona-Martínez, A.A.; Blanco, S.; Lebrero, R.; Muñoz, R. Innovative operational strategies in photosynthetic biogas upgrading in an outdoors pilot scale algal-bacterial photobioreactor. Chemosphere 2021, 264, 128470. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tsapekos, P.; Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Rafiee, S.; Angelidaki, I. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 2017, 4, 741–750. [Google Scholar] [CrossRef]
- Ramos, I.; Pérez, R.; Fdz-Polanco, M. Microaerobic desulphurisation unit: A new biological system for the removal of H2S from biogas. Bioresour. Technol. 2013, 142, 633–640. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Qi, D.; Ding, Y.; Zhao, Z. Review on microaeration-based anaerobic digestion: State of the art, challenges, and prospectives. Sci. Total Environ. 2020, 710, 136388. [Google Scholar] [CrossRef]
- Ramos, I.; Peña, M.; Fdz-Polanco, M. Where does the removal of H2S from biogas occur in microaerobic reactors? Bioresour. Technol. 2014, 166, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Romero, M. Start-up of microaerobic removal of H2S from biogas in full-scale digestion of sewage sludge. In Proceedings of the 15th IWA World Conference on Anaerobic Digestion (AD-15), Beijing, China, 17–20 October 2017. [Google Scholar]
- Díaz, I.; Ramos, I.; Fdz-Polanco, M. Economic analysis of microaerobic removal of H2S from biogas in full-scale sludge digesters. Bioresour. Technol. 2015, 192, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, J.G.D.S.; de Araújo, M.H.P.; dos Santos, A.B.; da Silva, M.E.R.; Firmino, P.I.M. Redox mediator, microaeration, and nitrate addition as engineering approaches to enhance the biotransformation of antibiotics in anaerobic reactors. J. Hazard. Mater. 2021, 403, 123932. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef]
- GMI. Market Opportunities for Anaerobic Digestion of Livestock and Agro-Industrial Waste in India; Global Methane Initiative: Washington, DC, USA, 2020; Available online: https://globalmethane.org/documents/MarketOpportunitiesForADInIndiaMarket/Business%20Models%20and%20Case%20Studies.pdf (accessed on 22 December 2020).
- Skovsgaard, L.; Jacobsen, H.K. Economies of scale in biogas production and the significance of flexible regulation. Energy Policy 2017, 101, 77–89. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- EPA. Agstar Project Development Handbook. A Handbook for Developing Anaerobic Digestion/Biogas Systems on Farms in the United States; United States Enviromantal Protection Agency: Washington, DC, USA, 2020. Available online: https://www.epa.gov/sites/production/files/2014-12/documents/agstar-handbook.pdf (accessed on 22 December 2020).
- Alessi, A.; Lopes, A.d.C.P.; Müller, W.; Gerke, F.; Robra, S.; Bockreis, A. Mechanical separation of impurities in biowaste: Comparison of four different pretreatment systems. Waste Manag. 2020, 106, 12–20. [Google Scholar] [CrossRef]
- Fan, Y.V.; Klemeš, J.J.; Lee, C.T.; Perry, S. Anaerobic digestion of municipal solid waste: Energy and carbon emission footprint. J. Environ. Manag. 2018, 223, 888–897. [Google Scholar] [CrossRef]
- Micolucci, F.; Gottardo, M.; Cavinato, C.; Pavan, P.; Bolzonella, D. Mesophilic and thermophilic anaerobic digestion of the liquid fraction of pressed biowaste for high energy yields recovery. Waste Manag. 2016, 48, 227–235. [Google Scholar] [CrossRef] [PubMed]
- EC. Optimal Use of Biogas from Waste Streams. An. Assessment of the Potential of Biogas from Digestion in the EU beyond 2020; European Commision: Brussels, Belgium, 2016; Available online: https://ec.europa.eu/energy/sites/ener/files/documents/ce_delft_3g84_biogas_beyond_2020_final_report.pdf (accessed on 22 December 2020).












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. https://doi.org/10.3390/en14102742
Iglesias R, Muñoz R, Polanco M, Díaz I, Susmozas A, Moreno AD, Guirado M, Carreras N, Ballesteros M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies. 2021; 14(10):2742. https://doi.org/10.3390/en14102742
Chicago/Turabian StyleIglesias, Raquel, Raúl Muñoz, María Polanco, Israel Díaz, Ana Susmozas, Antonio D. Moreno, María Guirado, Nely Carreras, and Mercedes Ballesteros. 2021. "Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development" Energies 14, no. 10: 2742. https://doi.org/10.3390/en14102742
APA StyleIglesias, R., Muñoz, R., Polanco, M., Díaz, I., Susmozas, A., Moreno, A. D., Guirado, M., Carreras, N., & Ballesteros, M. (2021). Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies, 14(10), 2742. https://doi.org/10.3390/en14102742