Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
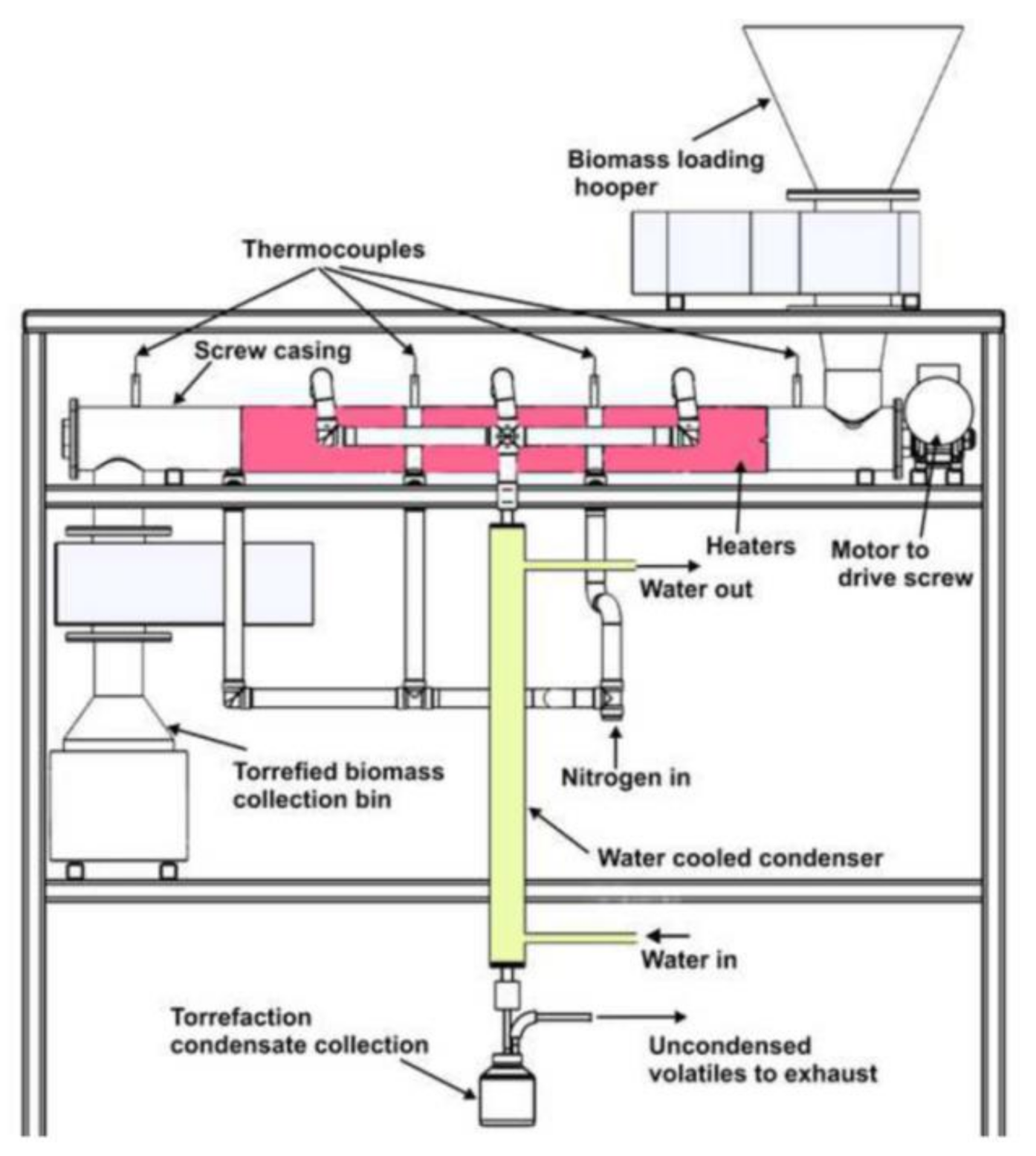
2.2. Torrefaction Experiments
2.3. Analytical Methods
2.3.1. Heating Value
2.3.2. Elemental Analysis
2.3.3. Composition Analysis
2.3.4. Moisture Analysis
2.3.5. Hydrophobicity
2.3.6. Moisture Adsorption Isotherm
- Guggenheim-Anderson-de Boer (GAB)where Meq is the moisture content (g·100 g of dry solids−1), M0 is the monolayer value (g.100 g of dry solids−1), aw is water activity (equilibrium relative humidity.100−1), and Cg and kg are constants.
- Smithwhere Meq is the moisture content (g.100 g of dry solids−1), aw water activity (equilibrium relative humidity·100−1), and a and b are constants.
- Oswinwhere Meq is the moisture content (g·100 g of dry solids−1), aw water activity (equilibrium relative humidity·100−1), and a and b are constants. Equation (9) can be written in linear form as shown in Equation (10).
2.3.7. Ash Content and Ash Meting Behavior
3. Results
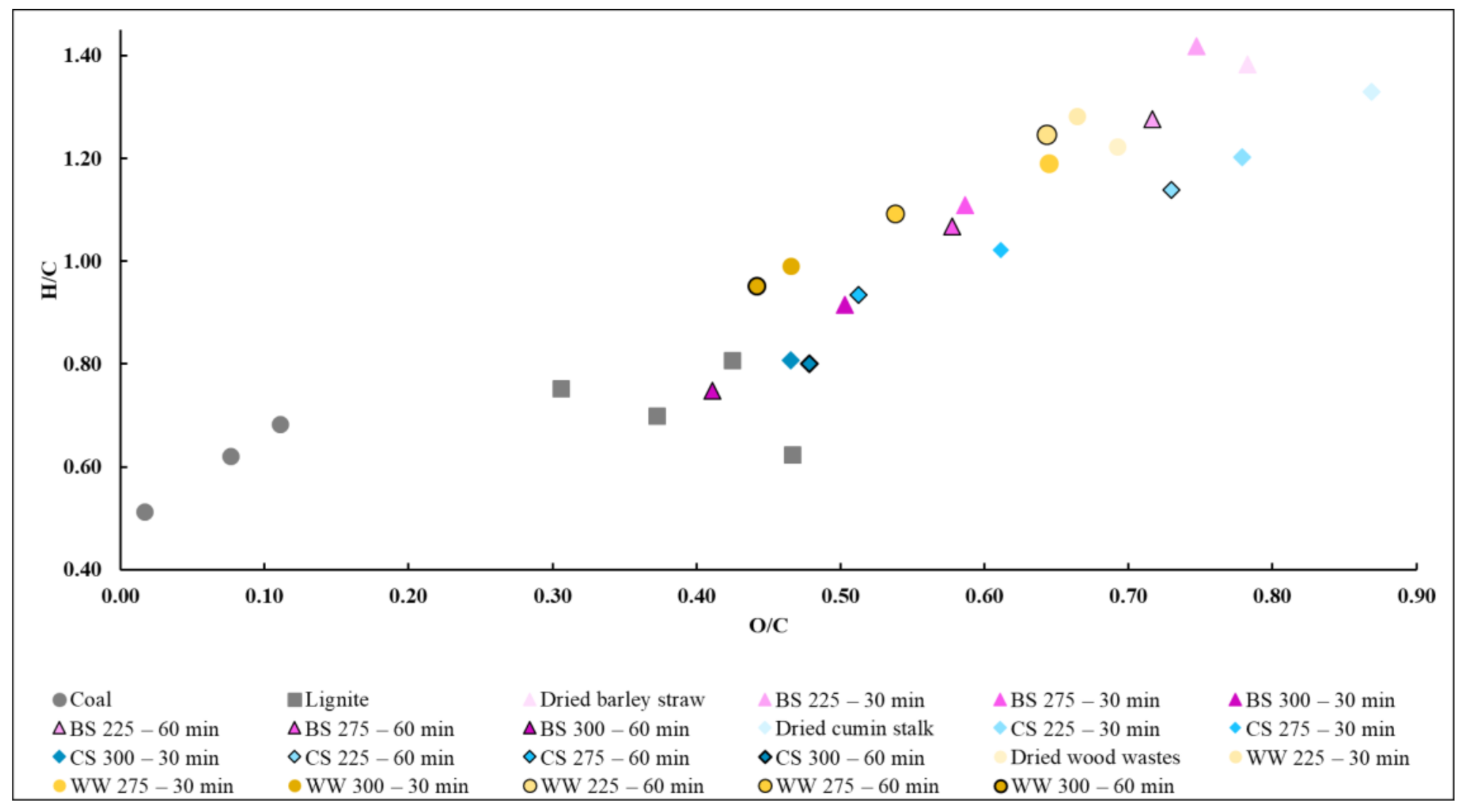
3.1. Influence of Torrefaction on Elemental Composition of Biomass
3.2. Influence of Torrefaction on the Energy Content of Biomass (Heating Value)
3.3. Influence of Torrefaction on Chemical Composition (i.e., Biomass Fibers)
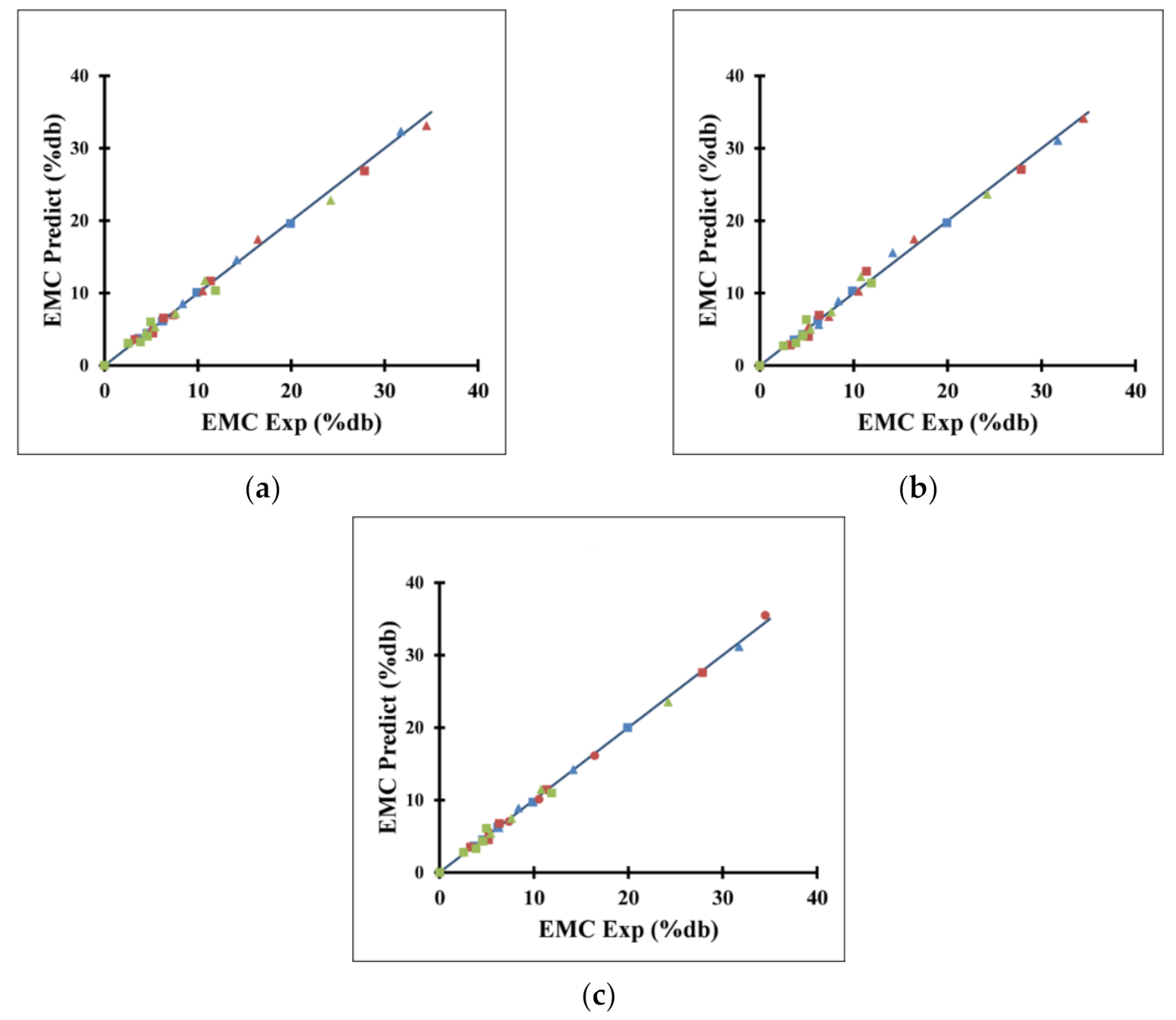
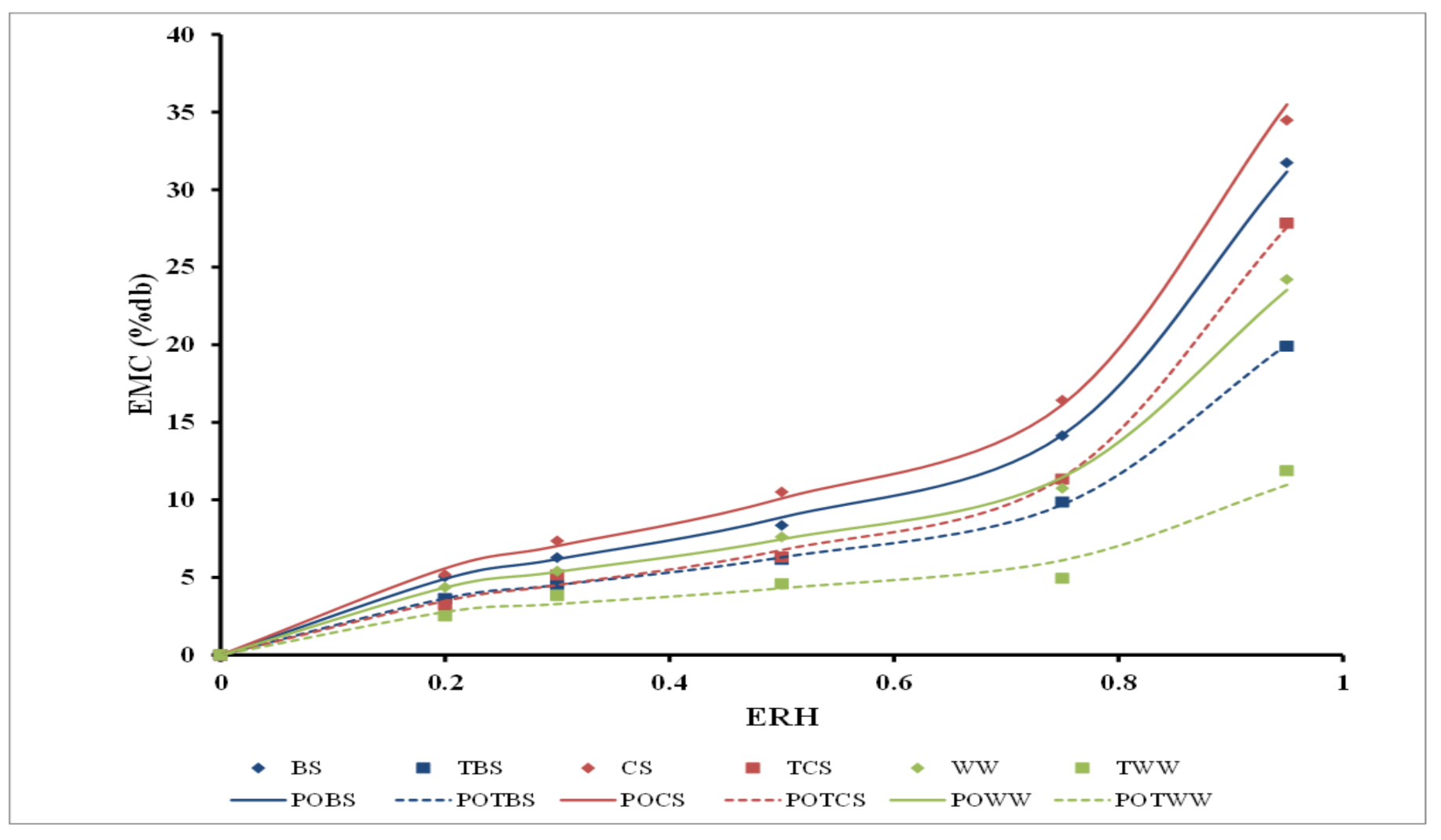
3.4. Moisture Uptake and Moisture Adsorption
3.5. Ash Content and Ash Melting Behavior
4. Discussion
4.1. Influence of Torrefaction on Elemental Composition of Biomass
4.2. Influence of Torrefaction on the Energy Content of Biomass (Heating Value)
4.3. Influence of Torrefaction on Chemical Composition (i.e., Biomass Fibers)
4.4. Moisture Uptake and Moisture Adsorption
4.5. Ash Content and Ash Melting Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savickis, J.; Zemite, L.; Zeltins, N.; Bode, I.; Jansons, L. Natural Gas and Biomethane in the European Road Transport: The Latvian Perspective. Latv. J. Phys. Tech. Sci. 2020, 57, 57–72. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O. Biomass torrefaction as a key driver for the sustainable development and decarbonization of energy production. Sustainbility 2020, 12, 922. [Google Scholar] [CrossRef] [Green Version]
- British Petroleum Company. Statistical Review of World Energy, 2020 | 69th Edition; British Petroleum Co.: London, UK, 2020. [Google Scholar]
- IEA. IEA Coal Information Overview and Statistical Report (2020); International Energy Agency: Paris, France, 2019; ISBN 9789264258631. [Google Scholar]
- Energi-Sverige Denmark and Finland Phase out Coal by 2030. Available online: https://www.energi-sverige.se/vaara-nyheter/nyheter/marknadsnyheter-2017/11/denmark-and-finland-phase-out-coal-by-2030/ (accessed on 20 September 2020).
- European Environment Agency. How Much Bioenergy Can Europe Produce without Harming the Environment? European Environment Agency: Copenhagen, Denmark, 2006. [Google Scholar]
- Zanchi, G.; Pena, N.; Bird, N. Is woody bioenergy carbon neutral? A comparative assessment of emissions from consumption of woody bioenergy and fossil fuel. GCB Bioenergy 2012, 4, 761–772. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Fahl, F.; Lugato, E.; Monforti-Ferrario, F.; Dallemand, J.F. Integrated and spatially explicit assessment of sustainable crop residues potential in Europe. Biomass Bioenergy 2019, 122, 257–269. [Google Scholar] [CrossRef]
- Statistics Estonia PM0281: Agricultural Land and Crops by Count. Available online: https://andmed.stat.ee/en/stat/majandus__pellumajandus__pellumajandussaaduste-tootmine__taimekasvatussaaduste-tootmine/PM0281/table/tableViewLayout1 (accessed on 26 April 2021).
- Rocha-Meneses, L.; Bergamo, T.F.; Kikas, T. Potential of cereal-based agricultural residues available for bioenergy production. Data Br. 2019, 23, 103829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Barta-Rajnai, E.; Skreiberg, Ø.; Khalil, R.; Czégény, Z.; Jakab, E.; Barta, Z.; Grønli, M. Effect of torrefaction on physiochemical characteristics and grindability of stem wood, stump and bark. Appl. Energy 2018, 227, 137–148. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Sebestyén, Z.; Blazsó, M.; Berényi, B.; Kumar, J.; Krishna, B.B.; Bhaskar, T.; Czégény, Z. Comparison of hydrothermal carbonization and torrefaction of azolla biomass: Analysis of the solid products. J. Anal. Appl. Pyrolysis 2020, 149, 104844. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Manatura, K. Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud. Therm. Eng. 2020, 19, 100623. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A review of biomass co-firing in North America. Renew. Sustain. Energy Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of Torrefied Biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Tapasvi, D.; Kempegowda, R.S.; Tran, K.Q.; Skreiberg, Ø.; Grønli, M. A simulation study on the torrefied biomass gasification. Energy Convers. Manag. 2015, 90, 446–457. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Jain, R.; Praveenkumar, R.; Rintala, J.; Romar, H.; Konttinen, J. Adsorption of furfural from torrefaction condensate using torrefied biomass. Chem. Eng. J. 2018, 334, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Salapa, I.; Haralampous, P.; Giakoumakis, G.; Nazos, A.; Sidiras, D. Torrefaction of barley straw for the co-production of energy and adsorbent materials. In Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering, Madrid, Spain, 16–18 August 2018; pp. 1–7. [Google Scholar]
- Nobre, C.; Dinis, T.T. Using torrefied biomass wastes as low-cost adsorbents for methylene blue. In Proceedings of the 3rd International Congress on Water, Waste and Energy Management, Rome, Italy, 18–20 July 2016; p. 499. [Google Scholar]
- Ciolkosz, D.; Desplat, J.; Schiffer, K. Raw, torrefied, and alkaline-treated biomass as a sorbent for lead in water. BioResources 2019, 14, 8530–8542. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.E.; Lee, Y.H.; Tsang, D.C.W.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef]
- Sadaka, S.; Negi, S. Improvements of biomass physical and thermochemical characteristics via torrefaction process. Environ. Prog. Sustain. Energy 2009, 28, 427–434. [Google Scholar] [CrossRef]
- Manouchehrinejad, M.; Van Giesen, I.; Mani, S. Grindability of torrefied wood chips and wood pellets. Fuel Process. Technol. 2018, 182, 45–55. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Zheng, N.-Y.; Lin, C.-S. Repurposing Washingtonia filifera petiole and Sterculia foetida follicle waste biomass for renewable energy through torrefaction. Energy 2021, 223, 120101. [Google Scholar] [CrossRef]
- Nyakuma, B.B.; Oladokun, O.; Wong, S.L.; Abdullah, T.A.T. Torrefaction of oil palm empty fruit bunch pellets: Product yield, distribution and fuel characterisation for enhanced energy recovery. Biomass Convers. Biorefinery 2021, 1–21. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterisation of renewable fuels’ torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Chen, D.; Cen, K.; Zhang, J.; Cao, X. Upgrading of biomass pellets by torrefaction and its influence on the hydrophobicity, mechanical property, and fuel quality. Biomass Convers. Biorefinery 2020, 1–10. [Google Scholar] [CrossRef]
- Kongto, P.; Palamanit, A.; Chaiprapat, S.; Tippayawong, N. Enhancing the fuel properties of rubberwood biomass by moving bed torrefaction process for further applications. Renew. Energy 2021, 170, 703–713. [Google Scholar] [CrossRef]
- Xin, S.; Huang, F.; Liu, X.; Mi, T.; Xu, Q. Torrefaction of herbal medicine wastes: Characterization of the physicochemical properties and combustion behaviors. Bioresour. Technol. 2019, 287, 121408. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Ross, A.B. Production of bio-coal, bio-methane and fertilizer from seaweed via hydrothermal carbonisation. Algal Res. 2016, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Tian, X.; Sheng, C. Moisture Sorption Isotherm of Herbaceous an Agricultural Biomass. Energy Fuels 2019, 33, 12480–12491. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Jena, K.; Chakraborty, J.P.; Sarkar, A. Energy and exergy analysis for torrefaction of pigeon pea stalk (cajanus cajan) and eucalyptus (eucalyptus tereticornis). Int. J. Hydrogen Energy 2020, 45, 18922–18936. [Google Scholar] [CrossRef]
- Nakason, K.; Pathomrotsakun, J.; Kraithong, W.; Khemthong, P.; Panyapinyopol, B. Torrefaction of agricultural wastes: Influence of lignocellulosic types and treatment temperature on fuel properties of biochar. Int. Energy J. 2019, 19, 253–266. [Google Scholar]
- Chen, W.H.; Lu, K.M.; Tsai, C.M. An experimental analysis on property and structure variations of agricultural wastes undergoing torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Barta-Rajnai, E.; Jakab, E.; Sebestyén, Z.; May, Z.; Barta, Z.; Wang, L.; Skreiberg, Ø.; Grønli, M.; Bozi, J.; Czégény, Z. Comprehensive Compositional Study of Torrefied Wood and Herbaceous Materials by Chemical Analysis and Thermoanalytical Methods. Energy Fuels 2016, 30, 8019–8030. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Lu, Q. Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Khazraie Shoulaifar, T.; Demartini, N.; Willför, S.; Pranovich, A.; Smeds, A.I.; Virtanen, T.A.P.; Maunu, S.L.; Verhoeff, F.; Kiel, J.H.A.; Hupa, M. Impact of torrefaction on the chemical structure of birch wood. Energy Fuels 2014, 28, 3863–3872. [Google Scholar] [CrossRef]
- Chen, Y.; Sharma-Shivappa, R.R.; Keshwani, D.; Chen, C. Potential of agricultural residues and hay for bioethanol production. Appl. Biochem. Biotechnol. 2007, 142, 276–290. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Wahlbom, C.F.; Gárdonyi, M.; van Zyl, W.H.; Cordero Otero, R.R.; Jönsson, L.J. Metabolic engineering of Saccharomyces cerevisiae for xylose utilization. Adv. Biochem. Eng. Biotechnol. 2001, 73, 53–84. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, J.H.; Oh, K.K.; Lee, S.J.; Lee, J.Y.; Kim, J.S.; Kim, S.W. Dilute acid pretreatment of barley straw and its saccharification and fermentation. Biotechnol. Bioprocess Eng. 2011, 16, 725–732. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Motaung, T.E.; Revaprasadu, N. Thermal degradation kinetics of sugarcane bagasse and soft wood cellulose. Materials 2017, 10, 1246. [Google Scholar] [CrossRef] [Green Version]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Torrefaction of Acacia nilotica: Oxygen Distribution and Carbon Densification Mechanism Based on In-Depth Analyses of Solid, Liquid, and Gaseous Products. Energy Fuels 2020, 34, 12586–12597. [Google Scholar] [CrossRef]
- Chiou, B.S.; Valenzuela-Medina, D.; Bilbao-Sainz, C.; Klamczynski, A.P.; Avena-Bustillos, R.J.; Milczarek, R.R.; Du, W.X.; Glenn, G.M.; Orts, W.J. Torrefaction of almond shells: Effects of torrefaction conditions on properties of solid and condensate products. Ind. Crops Prod. 2016, 86, 40–48. [Google Scholar] [CrossRef]
- Björk, H.; Rasmuson, A. Moisture equilibrium of wood and bark chips in superheated steam. Fuel 1995, 74, 1887–1890. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Yang, Q.; Chen, H. Evolution of functional groups and pore structure during cotton and corn stalks torrefaction and its correlation with hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Stratford, J.P.; Hutchings, T.R.; de Leij, F.A.A.M. Intrinsic activation: The relationship between biomass inorganic content and porosity formation during pyrolysis. Bioresour. Technol. 2014, 159, 104–111. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, C.Z.; Li, M.F.; Xiao, X. Torrefaction of corncob to produce charcoal under nitrogen and carbon dioxide atmospheres. Bioresour. Technol. 2018, 249, 348–353. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Xiao, G.; Luo, Z. Influence of torrefaction on the characteristics and pyrolysis behavior of cellulose. Energy 2017, 120, 864–871. [Google Scholar] [CrossRef]
- Hill, S.J.; Grigsby, W.J.; Hall, P.W. Chemical and cellulose crystallite changes in Pinus radiata during torrefaction. Biomass Bioenergy 2013, 56, 92–98. [Google Scholar] [CrossRef]
- Peng, J.H.; Bi, H.T.; Lim, C.J.; Sokhansanj, S. Study on density, hardness, and moisture uptake of torrefied wood pellets. Energy Fuels 2013, 27, 967–974. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Rahn, S. Effect of Torrefaction on Water Vapor Adsorption Properties and Resistance to Microbial Degradation of Corn Stover. Energy Fuels 2012, 26, 2386–2393. [Google Scholar] [CrossRef]
- Krupinska, B.; Strømmen, I.; Pakowski, Z.; Eikevik, T.M. Modeling of sorption isotherms of various kinds of wood at different temperature conditions. Dry Technol. 2007, 25, 1463–1470. [Google Scholar] [CrossRef]
- Bahar, R.; Azzouz, S.; Remond, R.; Ouertani, S.; Elaieb, M.T.; El Cafci, M.A. Moisture sorption isotherms and thermodynamic properties of Oak wood (Quercus robur and Quercus canariensis): Optimization of the processing parameters. Heat Mass Transf. und Stoffuebertragung 2017, 53, 1541–1552. [Google Scholar] [CrossRef]
- Soekarto, S.T. Keterkaitan berbagai konsep interaksi air dalam produk pangan [Interrelation on Water Interaction Concepts in Foods]--Komunikasi Singkat. J. Teknol. dan Ind. Pangan 2012, 23, 107–116. [Google Scholar] [CrossRef]
- Choudhury, D.; Sahu, J.K.; Sharma, G.D. Moisture sorption isotherms, heat of sorption and properties of sorbed water of raw bamboo (Dendrocalamus longispathus) shoots. Ind. Crops Prod. 2011, 33, 211–216. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Colin, B.; Chang, J.S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.W.J.P.; Everson, R.C.; Carrier, M.; Strydom, C.A.; Bunt, J.R. Structural and chemical modifications of typical South African biomasses during torrefaction. Bioresour. Technol. 2016, 202, 192–197. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.S.; Vital, B.R.; Carneiro, A.d.C.O.; Costa, E.V.S.; de Magalhães, M.A.; Trugilho, P.F. Structural and compositional changes in eucalyptus wood chips subjected to dry torrefaction. Ind. Crops Prod. 2017, 109, 598–602. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Wang, X.; Liu, Z.; Liu, H.; Liu, Y.; Xu, T. Study on fusion characteristics of biomass ash. Bioresour. Technol. 2010, 101, 9373–9381. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; García, J.E.C. Comparing methods for predicting the sintering of biomass ash in combustion. Fuel 2005, 84, 1893–1900. [Google Scholar] [CrossRef]
- Kopczyński, M.; Lasek, J.A.; Iluk, A.; Zuwała, J. The co-combustion of hard coal with raw and torrefied biomasses (willow (Salix viminalis), olive oil residue and waste wood from furniture manufacturing). Energy 2017, 140, 1316–1325. [Google Scholar] [CrossRef]
- Wang, Q.; Han, K.; Gao, J.; Wang, J.; Lu, C. Investigation of Maize Straw Char Briquette Ash Fusion Characteristics and the Influence of Phosphorus Additives. Energy Fuels 2017, 31, 2822–2830. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, H.; Kokko, L.; Raiko, R. The effect of torrefaction on the chlorine content and heating value of eight woody biomass samples. Biomass Bioenergy 2014, 66, 232–239. [Google Scholar] [CrossRef]
- Sá, L.C.R.; Loureiro, L.M.E.F.; Nunes, L.J.R.; Mendes, A.M.M. Torrefaction as a pretreatment technology for chlorine elimination from biomass: A case study using eucalyptus globulus labill. Resources 2020, 9, 54. [Google Scholar] [CrossRef]
- Saleh, S.B.; Flensborg, J.P.; Shoulaifar, T.K.; Sárossy, Z.; Hansen, B.B.; Egsgaard, H.; Demartini, N.; Jensen, P.A.; Glarborg, P.; Dam-Johansen, K. Release of chlorine and sulfur during biomass torrefaction and pyrolysis. Energy Fuels 2014, 28, 3738–3746. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, Y.; Lu, Z.; Gui, B.; Wei, M.; Yu, Y.; Xu, M. Release and transformation of sodium in kitchen waste during torrefaction. Energy Fuels 2014, 28, 1911–1917. [Google Scholar] [CrossRef]

| C (%) | H (%) | N (%) | S (%) | O (%) | HHV (MJ/kg) | |
|---|---|---|---|---|---|---|
| Dried barley straw | 45.96 | 5.30 | 0.71 | 0.09 | 47.94 | 18.83 |
| 225–30 min | 46.76 | 5.52 | 1.04 | 0.07 | 46.61 | 18.92 |
| 275–30 min | 52.76 | 4.88 | 1.02 | 0.09 | 41.25 | 21.22 |
| 300–30 min | 56.66 | 4.32 | 0.94 | 0.09 | 37.99 | 23.42 |
| 225–60 min | 47.98 | 5.10 | 0.99 | 0.11 | 45.82 | 19.31 |
| 275–60 min | 53.21 | 4.73 | 0.98 | 0.09 | 40.98 | 21.33 |
| 300–60 min | 61.18 | 3.81 | 1.37 | 0.13 | 33.51 | 25.10 |
| Dried cumin stalk | 43.74 | 4.79 | 0.62 | 0.05 | 50.79 | 18.12 |
| 225–30 min | 46.30 | 4.61 | 0.77 | 0.07 | 48.27 | 19.14 |
| 275–30 min | 52.15 | 4.44 | 0.79 | 0.11 | 42.51 | 21.29 |
| 300–30 min | 58.66 | 3.95 | 0.93 | 0.07 | 36.39 | 24.57 |
| 225–60 min | 47.98 | 4.55 | 0.74 | 0.06 | 46.67 | 19.63 |
| 275–60 min | 56.23 | 4.38 | 0.91 | 0.06 | 38.42 | 23.65 |
| 300–60 min | 58.00 | 3.87 | 1.04 | 0.12 | 36.97 | 24.32 |
| Dried wood wastes | 49.03 | 4.99 | 0.71 | 0.03 | 45.23 | 19.91 |
| 225–30 min | 49.79 | 5.34 | 0.42 | 0.04 | 44.41 | 20.18 |
| 275–30 min | 50.78 | 5.03 | 0.48 | 0.02 | 43.69 | 22.36 |
| 300–30 min | 58.41 | 4.82 | 0.50 | 0.02 | 36.25 | 23.74 |
| 225–60 min | 50.78 | 5.27 | 0.36 | 0.03 | 43.57 | 20.44 |
| 275–60 min | 55.08 | 5.02 | 0.41 | 0.02 | 39.48 | 22.39 |
| 300–60 min | 59.66 | 4.73 | 0.45 | 0.02 | 35.14 | 24.00 |
| Biomass/Torrefaction Condition | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Dried barley straw (BS) | 41.5 ± 0.5 | 36.3 ± 0.2 | 8.8 ± 0.6 |
| TBS 225/30 | 39.8 ± 0.6 | 32.4 ± 1.4 | 10.25 ± 0.07 |
| TBS 275/30 | 55.6 ± 0.5 | 2.35 ± 0.10 | 31.6 ± 0.2 |
| TBS 300/30 | 30.8 ± 0.7 | 3.7 ± 0.9 | 30.00 ± 0.14 |
| TBS 225/60 | 42.18 ± 0.18 | 28.33 ± 0.05 | 12.3 ± 1.0 |
| TBS 275/60 | 48 ± 2 | 2.6 ± 0.5 | 33 ± 3 |
| TBS 300/60 | 5.5 ± 0.7 | 5.00 ± 0.10 | 48.2 ± 0.3 |
| Dried cumin stalk (CS) | 44.0 ± 0.7 | 11.3 ± 0.8 | 10.86 ± 0.04 |
| TCS 225/30 | 38.8 ± 0.2 | 11.3 ± 0.6 | 17.3 ± 0.4 |
| TCS 275/30 | 24 ± 2 | 5.0 ± 0.7 | 34.1 ± 0.5 |
| TCS 300/30 | 10.4 ± 0.8 | 6.28 ± 0.13 | 36.40 ± 0.01 |
| TCS 225/60 | 39.76 ± 0.07 | 6.86 ± 0.17 | 18.2 ± 0.9 |
| TCS 275/60 | 18.1 ± 1.0 | 4.51 ± 0.04 | 33.40 ± 1.1 |
| TCS 300/60 | 9.26 ± 0.01 | 4.31 ± 0.01 | 35.30 ± 0.01 |
| Dried wood wastes (WW) | 53.5 ± 0.7 | 16.0 ± 0.7 | 20.60 ± 0.14 |
| TWW 225/30 | 49.4 ± 1.6 | 15.43 ± 0.03 | 20.70 ± 0.14 |
| TWW 275/30 | 46.5 ± 1.2 | 3.4 ± 1.2 | 35.50 ± 0.01 |
| TWW 300/30 | 34.71 ± 0.10 | 4.58 ± 0.07 | 48.7 ± 0.9 |
| TWW 225/60 | 43.3 ± 0.6 | 14.12 ± 0.10 | 25.3 ± 1.1 |
| TWW 275/60 | 46.63 ± 0.01 | 5.2 ± 1.0 | 38.3 ± 0.10 |
| TWW 300/60 | 30.5 ± 0.9 | 5.1 ± 1.0 | 55.9 ± 0.4 |
| Biomass | MC (%) | Moisture Uptake (%) |
|---|---|---|
| Dried barley straw (BS) | 8.73 ± 0.19 | 22.75 ± 0.16 |
| TBS 225/30 | 5.39 ± 0.15 | 19.0 ± 0.3 |
| TBS 275/30 | 4.3 ± 0.4 | 13.45 ± 0.03 |
| TBS 300/30 | 3.91 ± 0.13 | 12.96 ± 0.13 |
| TBS 225/60 | 5.2 ± 0.2 | 18.28 ± 0.05 |
| TBS 275/60 | 5.34 ± 0.16 | 12.9 ± 0.3 |
| TBS 300/60 | 4.46 ± 0.19 | 14.5 ± 0.8 |
| Dried cumin stalk (CS) | 9.42 ± 0.07 | 24.6 ± 0.3 |
| TCS 225/30 | 2.58 ± 0.17 | 22.0 ± 0.2 |
| TCS 275/30 | 3.48 ± 0.18 | 20.1 ± 0.4 |
| TCS 300/30 | 5.23 ± 0.16 | 22.12 ± 0.04 |
| TCS 225/60 | 2.77 ± 0.15 | 19.34 ± 0.04 |
| TCS 275/60 | 3.34 ± 0.17 | 19.45 ± 0.02 |
| TCS 300/60 | 3.89 ± 0.17 | 20.71 ± 0.13 |
| Dried wood waste (WW) | 8.871 ± 0.001 | 18.29 ± 0.12 |
| TWW 225/30 | 1.58 ± 0.17 | 13.9 ± 0.3 |
| TWW 275/30 | 1.6 ± 0.7 | 8.99 ± 0.14 |
| TWW 300/30 | 1.9 ± 0.4 | 8.46 ± 0.04 |
| TWW 225/60 | 1.86 ± 0.11 | 11.53 ± 0.10 |
| TWW 275/60 | 1.886 ± 0.004 | 8.61 ± 0.01 |
| TWW 300/60 | 2.36 ± 0.02 | 8.35 ± 0.13 |
| ERH (%) | Equilibrium Moisture Content (% db) | |||||
|---|---|---|---|---|---|---|
| BS | TBS | CS | TCS | WW | TWW | |
| 95 | 31.7 ± 0.3 | 19.9 ± 1.1 | 34.5 ± 0.4 | 27.85 ± 0.12 | 24.22 ± 0.14 | 11.88 ± 0.15 |
| 75 | 14.2 ± 0.2 | 9.87 ± 0.10 | 16.4 ± 0.4 | 11.4 ± 0.3 | 10.8 ± 1.1 | 4.95 ± 0.01 |
| 50 | 8.36 ± 0.12 | 6.17 ± 0.02 | 10.5 ± 0.3 | 6.32 ± 0.06 | 7.62 ± 0.08 | 4.59 ± 0.05 |
| 30 | 6.28 ± 0.15 | 4.55 ± 0.16 | 7.36 ± 0.15 | 5.17 ± 0.01 | 5.41 ± 0.09 | 3.9 ± 0.4 |
| 20 | 5.05 ± 0.03 | 3.65 ± 0.11 | 5.18 ± 0.12 | 3.26 ± 0.04 | 4.37 ± 0.08 | 2.53 ± 0.04 |
| Biomass | GAB | Smith | Oswin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M0 | Cg | kg | R2 | E% | a | b | R2 | E% | a | b | R2 | E% | |
| BS | 4.84 | 41.16 | 0.90 | 0.9932 | 2.62 | 2.22 | 9.63 | 0.9925 | 8.40 | 8.87 | 0.43 | 0.9977 | 2.49 |
| TBS | 3.68 | 25.15 | 0.86 | 0.9908 | 1.80 | 2.23 | 5.84 | 0.9981 | 3.17 | 6.30 | 0.39 | 0.9996 | 1.04 |
| CS | 6.80 | 9.81 | 0.84 | 0.9309 | 4.42 | 3.06 | 10.37 | 0.9971 | 4.33 | 10.10 | 0.43 | 0.9951 | 4.20 |
| TCS | 3.93 | 13.60 | 0.90 | 0.8865 | 6.68 | 0.90 | 8.74 | 0.9868 | 12.47 | 6.77 | 0.48 | 0.9898 | 5.70 |
| WW | 4.22 | 40.57 | 0.86 | 0.9094 | 5.37 | 2.49 | 7.06 | 0.9885 | 6.83 | 7.46 | 0.39 | 0.9970 | 2.57 |
| TWW | 2.31 | −49.58 | 0.82 | 0.6269 | 16.85 | 2.02 | 3.12 | 0.9452 | 13.46 | 4.31 | 0.32 | 0.9319 | 12.26 |
| Biomass | Ash Content (%) |
|---|---|
| Dried barley straw (BS) | 3.5 ± 0.2 |
| TBS 225 | 4.8 ± 0.4 |
| TBS 275 | 6.15 ± 0.10 |
| TBS 300 | 9.51 ± 0.09 |
| Dried cumin stalk (CS) | 6.86 ± 0.14 |
| TCS 225 | 7.0 ± 0.3 |
| TCS 275 | 10.2 ± 0.3 |
| TCS 300 | 13.00 ± 0.2 |
| Dried wood waste (WW) | 0.77 ± 0.06 |
| TWW 225 | 1.34 ± 0.17 |
| TWW 275 | 1.79 ± 0.08 |
| TWW 300 | 2.09 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Madissoo, M.; Pärn, L.; Virro, I.; Kikas, T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies 2021, 14, 2774. https://doi.org/10.3390/en14102774
Cahyanti MN, Doddapaneni TRKC, Madissoo M, Pärn L, Virro I, Kikas T. Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies. 2021; 14(10):2774. https://doi.org/10.3390/en14102774
Chicago/Turabian StyleCahyanti, Margareta Novian, Tharaka Rama Krishna C. Doddapaneni, Marten Madissoo, Linnar Pärn, Indrek Virro, and Timo Kikas. 2021. "Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics" Energies 14, no. 10: 2774. https://doi.org/10.3390/en14102774
APA StyleCahyanti, M. N., Doddapaneni, T. R. K. C., Madissoo, M., Pärn, L., Virro, I., & Kikas, T. (2021). Torrefaction of Agricultural and Wood Waste: Comparative Analysis of Selected Fuel Characteristics. Energies, 14(10), 2774. https://doi.org/10.3390/en14102774







