Numerical Simulation of Fluidized Bed Gasifier Coupled with Solid Oxide Fuel Cell Fed with Solid Carbon
Abstract
:1. Introduction
2. Physicochemical Model
2.1. Boudouard Gasification of Carbon
2.2. Momentum and Mass Transport in Gas–Solid Flows
2.3. Electrochemical Reaction of CO
2.4. Model Assumption
3. Simulation Method
4. Results and Discussion
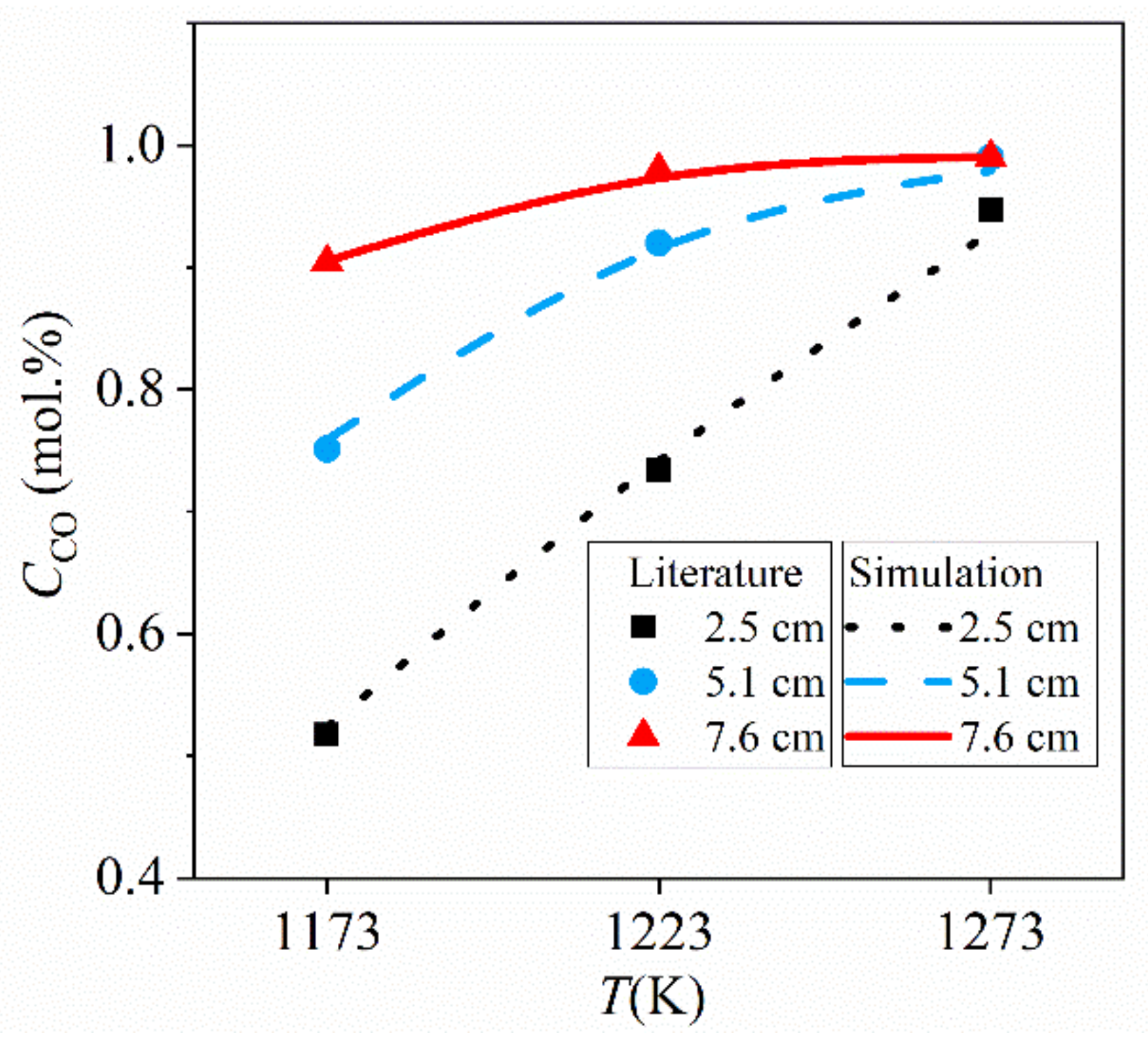
4.1. Model Validation
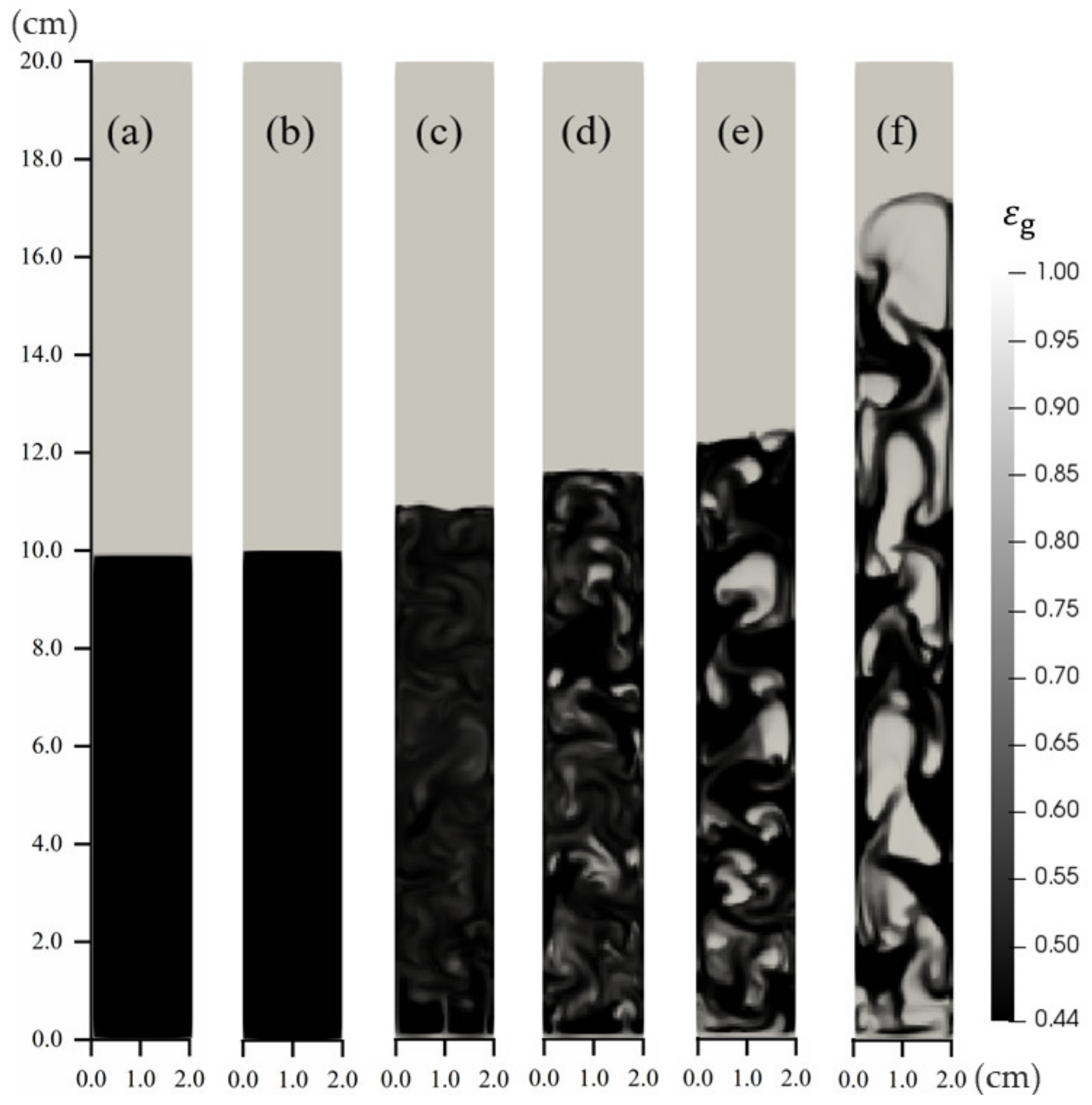
4.2. Fluidized Bed Gasifiers
4.2.1. Effects of CO2 Inlet Velocity
4.2.2. Effects of Activity of Carbon Fuel and CO2 Inlet Velocity
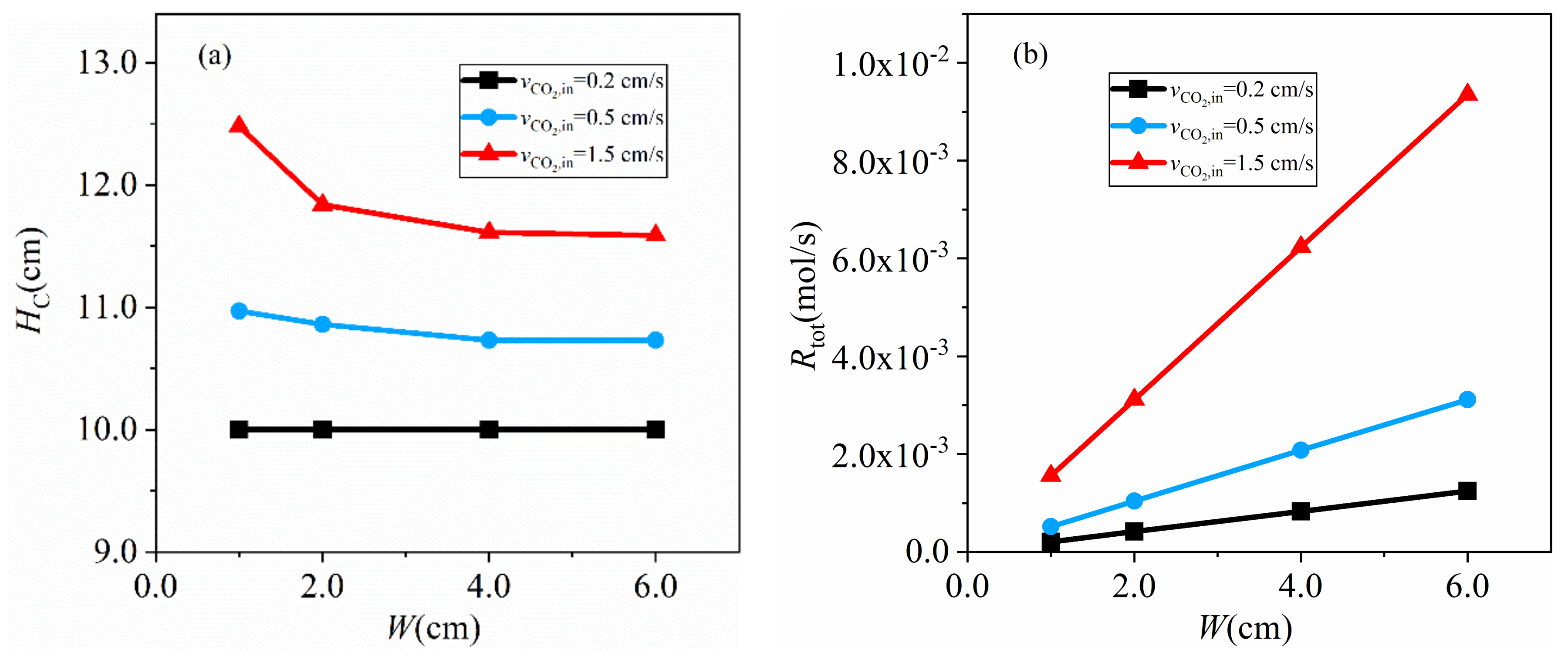
4.2.3. Effects of Channel Width
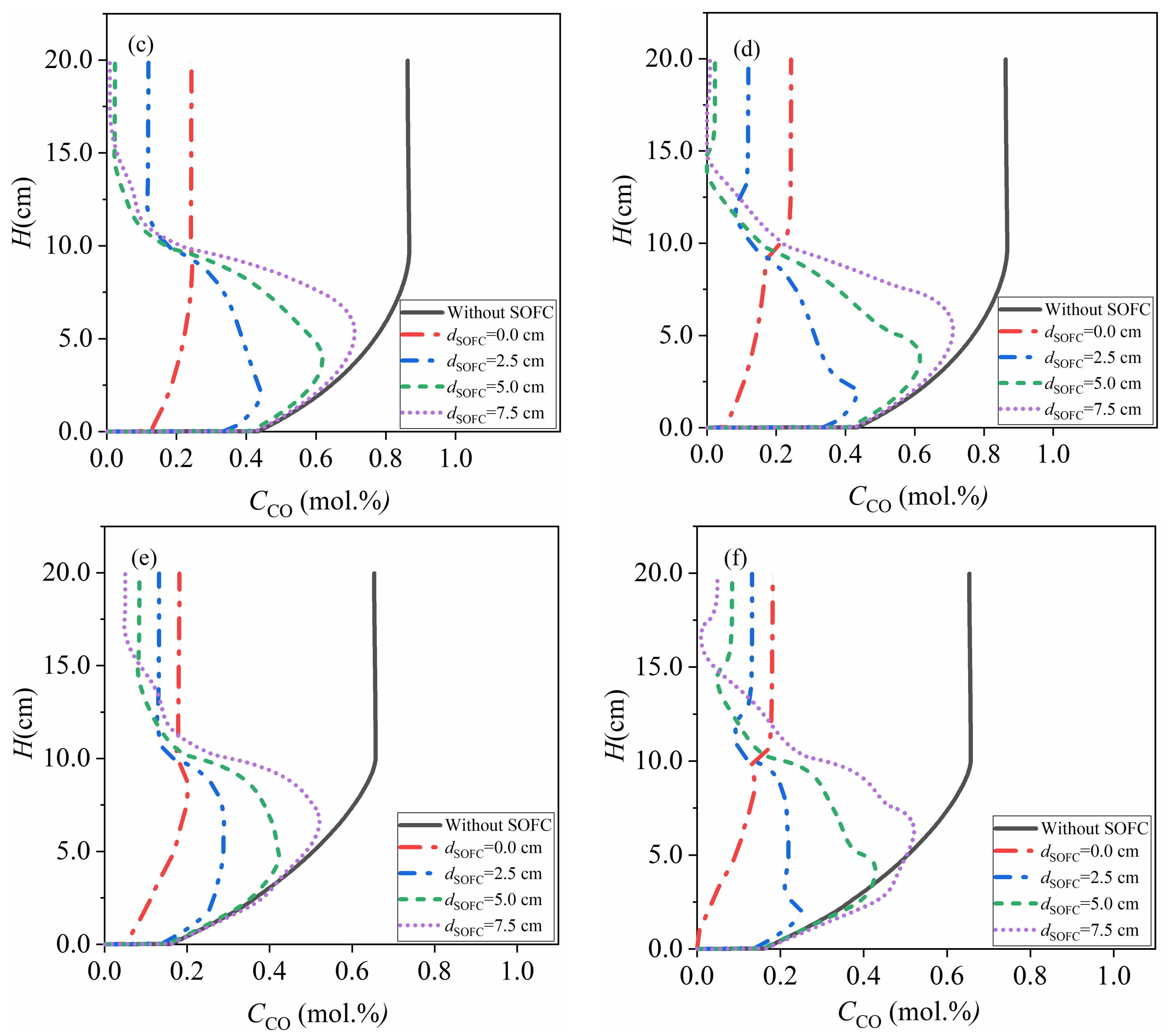
4.3. Fluidized Bed Gasifiers Coupled with SOFC
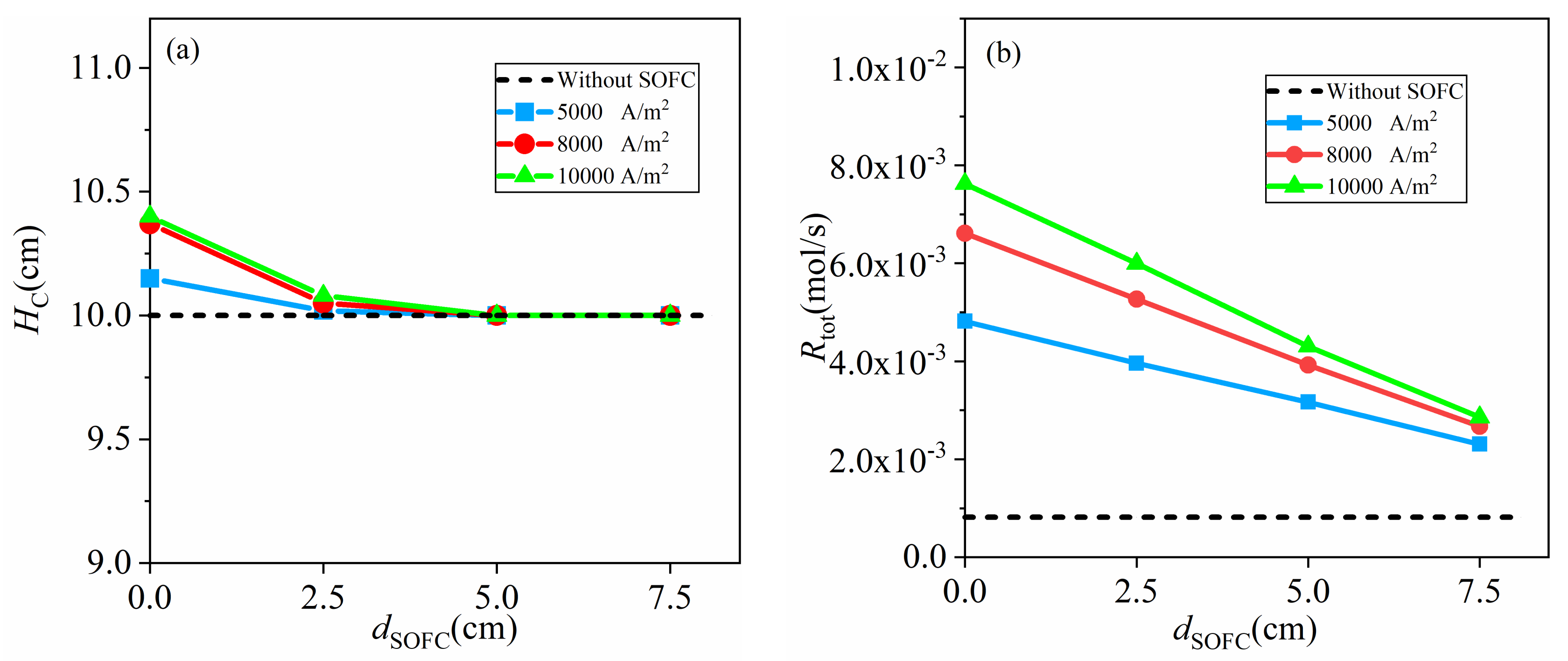
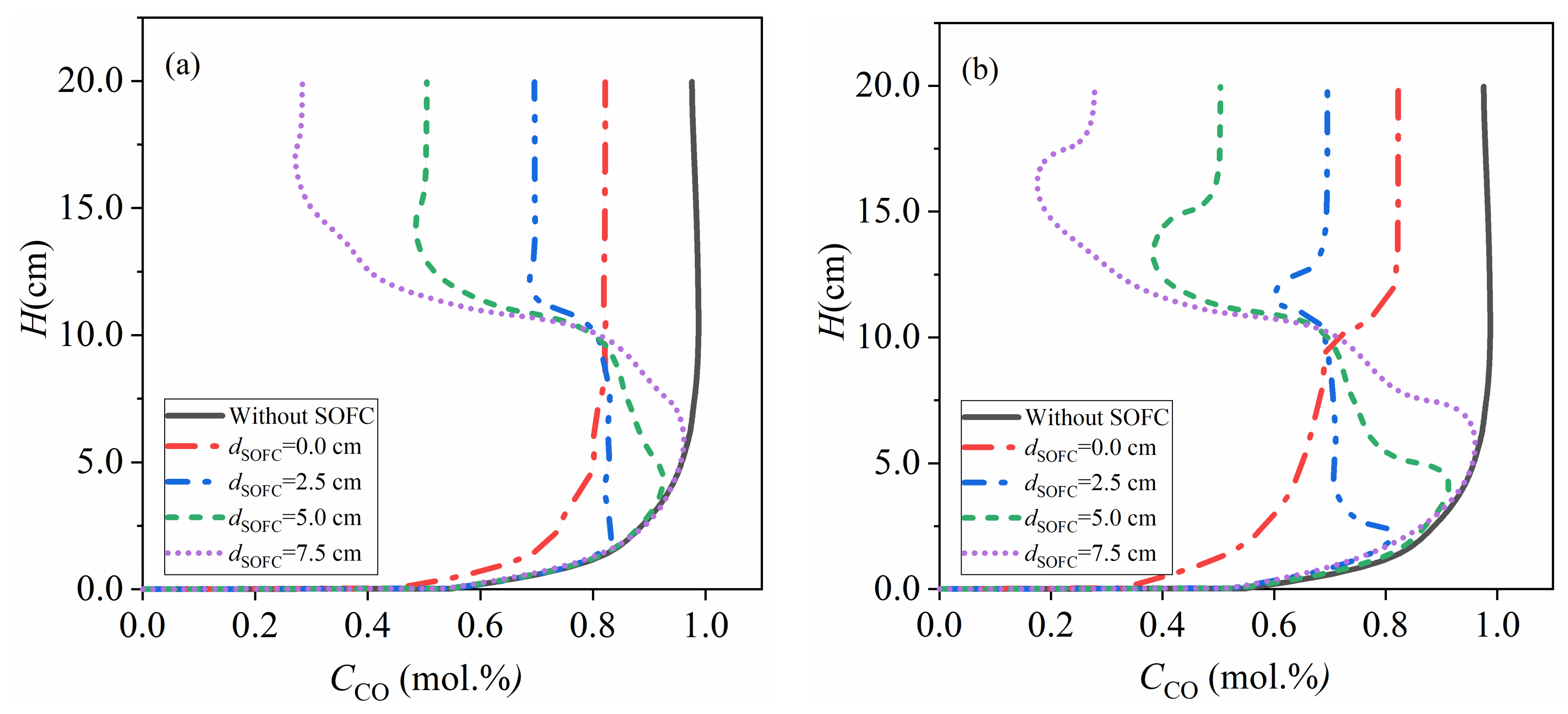
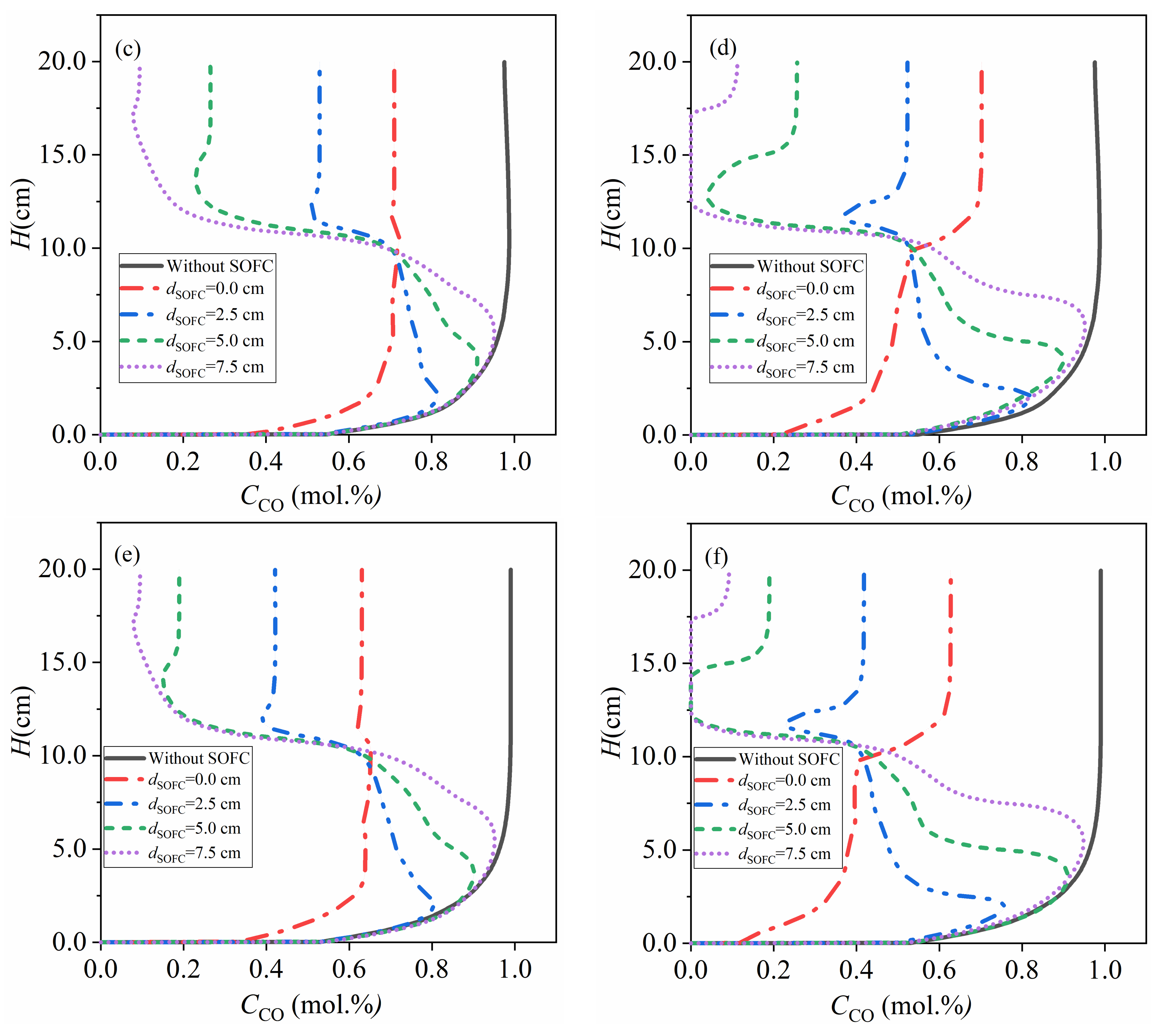
4.3.1. Low Activity Carbon fuel with = 7.2 × 10−6 mol/m2
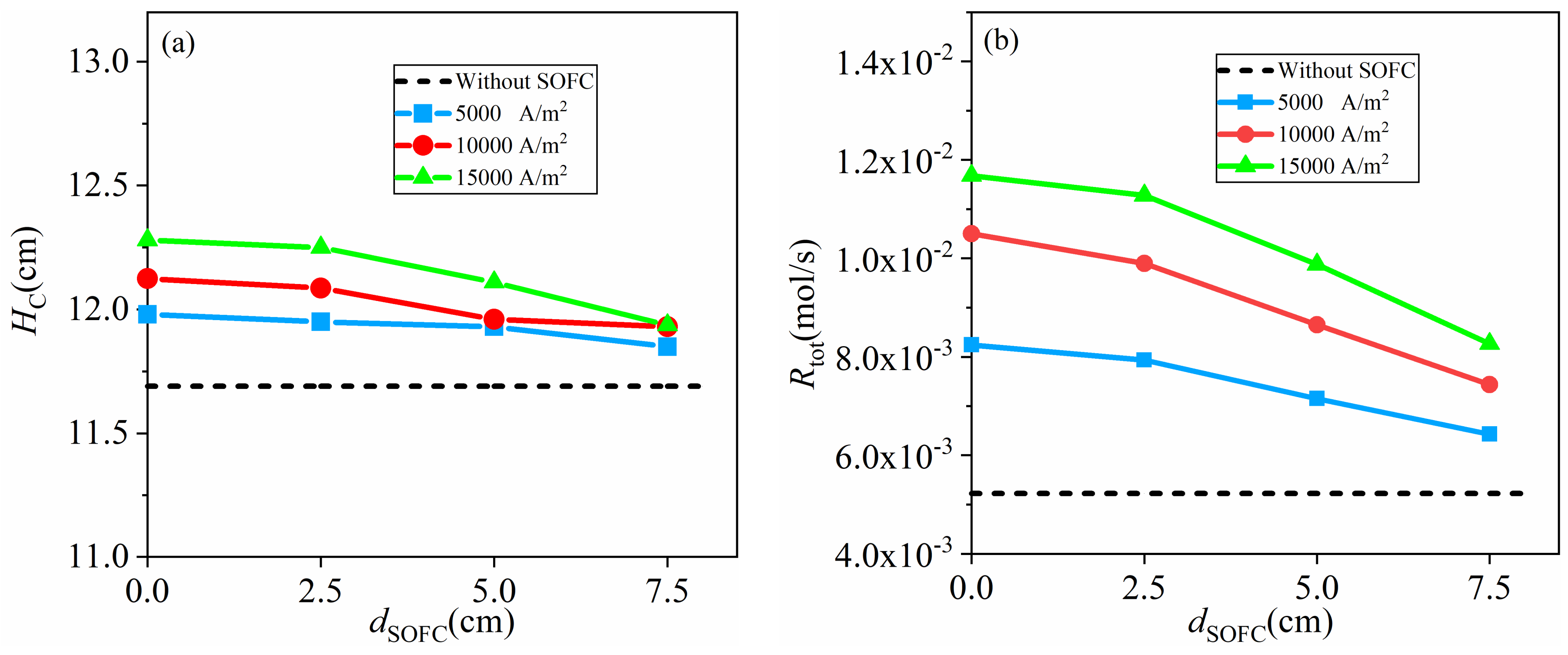
4.3.2. High Activity Carbon Fuel with = 7.2 × 10−5 mol/m2
5. Conclusions
- (1)
- results calculated based on the fluidized bed gasifiers model agree well with the experimental tests, validating its reliability;
- (2)
- the total gasification rate rises with the increase of inlet velocity, carbon activity and channel width, while too high an inlet velocity reduces CO concentration in the channel;
- (3)
- in the range this study involved, a higher coupling extent and inlet velocity of CO2 result in a larger total gasification rate and maximum current density, because of enhanced mass transport by expansion of the carbon bed, back-mixing, solid mixing and gas mixing. The gasification rate rises as high as 28 times that of the fixed bed gasifiers with the same activity of carbon and the maximum current density increases from <5000 A/m2 to >15,000 A/m2 due to the coupling between fluidized bed gasifiers and SOFC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cao, D.X.; Sun, Y.; Wang, G.L. Direct carbon fuel cell: Fundamentals and recent developments. J. Power Sources 2007, 167, 250–257. [Google Scholar] [CrossRef]
- Nease, J.; Adams, T.A. Comparative life cycle analyses of bulk-scale coal-fueled solid oxide fuel cell power plants. Appl. Energy 2015, 150, 161–175. [Google Scholar] [CrossRef]
- Rady, A.C.; Giddey, S.; Badwal, S.P.S.; Ladewig, B.P.; Bhattacharya, S. Review of fuels for direct carbon fuel cells. Energy Fuel 2012, 26, 1471–1488. [Google Scholar] [CrossRef]
- Yu, F.; Han, T.; Wang, Z.; Xie, Y.; Wu, Y.; Jin, Y.; Yang, N.; Xiao, J.; Kawi, S. Recent progress in direct carbon solid oxide fuel cell: Advanced anode catalysts, diversified carbon fuels, and heat management. Int. J. Hydrog. Energy 2021, 46, 4283–4300. [Google Scholar] [CrossRef]
- Gur, T.M. Perspectives on oxygen-based coal conversion towards zero-carbon power generation. Energy 2020, 196, 117074. [Google Scholar] [CrossRef]
- Chen, K.K.; Han, Y.; Tong, Z.; Gasda, M.; Ho, W.S.W. Membrane processes for CO2 removal and fuel utilization enhancement for solid oxide fuel cells. J. Membr. Sci. 2021, 620, 118846. [Google Scholar] [CrossRef]
- Gur, T.M. Progress in carbon fuel cells for clean coal technology pipeline. Int. J. Energy Res. 2016, 40, 13–29. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A.; Munnings, C. A comprehensive review of direct carbon fuel cell technology. Prog. Energy Combust. Sci. 2012, 38, 360–399. [Google Scholar] [CrossRef]
- Rady, A.C.; Giddey, S.; Kulkarni, A.; Badwal, S.P.S.; Bhattacharya, S. Direct carbon fuel cell operation on brown coal with a Ni-GDC-YSZ Anode. Electrochim. Acta 2015, 178, 721–731. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, N.; Fang, Y.H.; Fan, H.; Lei, Z.; Han, M.F. Syngas production via coal char-CO2 fluidized bed gasification and the effect on the performance of LSCFN//LSGM//LSCFN solid oxide fuel cell. J. Mater. Sci. Technol. 2018, 34, 403–408. [Google Scholar] [CrossRef]
- Gur, T.M.; Homel, M.; Virkar, A.V. High performance solid oxide fuel cell operating on dry gasified coal. J. Power Sources 2010, 195, 1085–1090. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, J.; Zeng, X.Y.; Li, X.; Yang, J.; Zou, Y.L.; Liu, S.D.F.; Dong, P.; Zhang, Y.J.; Liu, J. A high performance direct carbon solid oxide fuel cell—A green pathway for brown coal utilization. Appl. Energy 2019, 248, 679–687. [Google Scholar] [CrossRef]
- Dudek, M.; Skrzypkiewicz, M.; Moskala, N.; Grzywacz, P.; Sitarz, M.; Lubarska-Radziejewska, I. The impact of physicochemical properties of coal on direct carbon solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 18872–18883. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, X.; Chang, G.; Guo, Q. Treatment of isopropanol wastewater in an anaerobic fluidized bed microbial fuel cell filled with macroporous adsorptive resin as multifunctional biocarrier. Sci. Total Environ. 2020, 719, 137495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, Y.; Wang, L.; Guo, Q. Treatment of m-Cresol wastewater in an anaerobic fluidized bed microbial fuel cell equipped with different modified carbon cloth cathodes. Energy Fuels 2020, 34, 10059–10066. [Google Scholar] [CrossRef]
- Pongratz, G.; Subotić, V.; Schroettner, H.; Stoeckl, B.; Hochenauer, C.; Anca-Couce, A.; Scharler, R. Investigation of solid oxide fuel cell operation with synthetic biomass gasification product gases as a basis for enhancing its performance. Biomass Convers. Biorefin. 2020, 11, 121–139. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.; Li, S.; Mitchell, R.E.; Guer, T.M. Conversion of solid carbonaceous fuels in a fluidized bed fuel cell. Electrochem. Solid State Lett. 2008, 11, B20–B23. [Google Scholar] [CrossRef]
- Cai, W.Z.; Liu, J.; Liu, P.P.; Liu, Z.J.; Xu, H.R.; Chen, B.; Li, Y.Z.; Zhou, Q.; Liu, M.L.; Ni, M. A direct carbon solid oxide fuel cell fueled with char from wheat straw. Int. J. Energy Res. 2019, 43, 2468–2477. [Google Scholar] [CrossRef]
- Li, J.W.; Wei, B.; Wang, C.Q.; Zhou, Z.Y.; Lu, Z. High-performance and stable La0.8Sr0.2Fe0.9Nb0.1O3-delta anode for direct carbon solid oxide fuel cells fueled by activated carbon and corn straw derived carbon. Int. J. Hydrog. Energy 2018, 43, 12358–12367. [Google Scholar] [CrossRef]
- Yu, F.Y.; Wang, Y.S.; Xie, Y.J.; Zhang, W.M.; Zhang, J.J.; Meng, X.X.; Xiao, J.; Yang, N.T. A microtubular direct carbon solid oxide fuel cell operated on the biochar derived from pepper straw. Energy Technol. 2020, 8, 1901077. [Google Scholar] [CrossRef]
- An, W.T.; Sun, X.J.; Jiao, Y.; Juliao, P.S.B.; Wang, W.; Wang, S.B.; Li, S.D.; Shuang, S.M. A solid oxide carbon fuel cell operating on pomelo peel char with high power output. Int. J. Energy Res. 2019, 43, 2514–2526. [Google Scholar] [CrossRef]
- Nakagawa, N.; Ishida, M. Performance of an internal direct-oxidation carbon fuel-cell and its evaluation by graphic exergy analysis. Ind. Eng. Chem. Res. 1988, 27, 1181–1185. [Google Scholar] [CrossRef]
- Gur, T.M.; Huggins, R.A. Direct electrochemical conversion of carbon to electrical energy in a high-temperature fuel-cell. J. Electrochem. Soc. 1992, 139, L95–L97. [Google Scholar] [CrossRef]
- Xie, Y.M.; Tang, Y.B.; Liu, J. A verification of the reaction mechanism of direct carbon solid oxide fuel cells. J. Solid State Electrochem. 2013, 17, 121–127. [Google Scholar] [CrossRef]
- Cai, W.Z.; Liu, J.; Xie, Y.M.; Xiao, J.; Liu, M.L. An investigation on the kinetics of direct carbon solid oxide fuel cells. J. Solid State Electrochem. 2016, 20, 2207–2216. [Google Scholar] [CrossRef]
- Jiang, Y.; Virkar, A.V. Fuel composition and diluent effect on gas transport and performance of anode-supported SOFCs. J. Electrochem. Soc. 2003, 150, A942–A951. [Google Scholar] [CrossRef]
- Siengchum, T.; Guzman, F.; Chuang, S.S.C. Analysis of gas products from direct utilization of carbon in a solid oxide fuel cell. J. Power Sources 2012, 213, 375–381. [Google Scholar] [CrossRef]
- Cai, W.Z.; Cao, D.; Zhou, M.Y.; Yan, X.M.; Li, Y.Z.; Wu, Z.; Lu, S.P.; Mao, C.Y.; Xie, Y.M.; Zhao, C.W.; et al. Sulfur-tolerant Fe-doped La0.3Sr0.7TiO3 perovskite as anode of direct carbon solid oxide fuel cells. Energy 2020, 211, 118958. [Google Scholar] [CrossRef]
- Xiao, J.; Han, D.; Yu, F.Y.; Zhang, L.; Liu, J.; Zhan, Z.L.; Zhang, Y.J.; Dong, P. Characterization of symmetrical SrFe0.75Mo0.25O3-delta electrodes in direct carbon solid oxide fuel cells. J. Alloy Compd. 2016, 688, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Pu, J.G.; Chen, X.; Ma, Z.; Ding, X.; Zhou, J.; Wang, S.R. Influence of anode’s microstructure on electrochemical performance of solid oxide direct carbon fuel cells. Int. J. Hydrog. Energy 2020, 45, 11784–11790. [Google Scholar] [CrossRef]
- Liu, R.Z.; Zhao, C.H.; Li, J.L.; Zeng, F.R.; Wang, S.R.; Wen, T.L.; Wen, Z.Y. A novel direct carbon fuel cell by approach of tubular solid oxide fuel cells. J. Power Sources 2010, 195, 480–482. [Google Scholar] [CrossRef]
- Bai, Y.H.; Liu, Y.; Tang, Y.B.; Xie, Y.M.; Liu, J. Direct carbon solid oxide Fuel Cell—A potential high performance battery. Int. J. Hydrog. Energy 2011, 36, 9189–9194. [Google Scholar] [CrossRef]
- Jiao, Y.; Xue, X.T.; An, W.T.; Juliao, P.S.B.; Wang, W.; Yang, G.M.; Zhou, W.; Li, S.D. Purified high-sulfur coal as a fuel for direct carbon solid oxide fuel cells. Int. J. Energy Res. 2019, 43, 2501–2513. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Wang, X.Q.; Zhang, Y.P.; Qiu, Q.Y.; Liu, M.L.; Liu, J. Effect of counter diffusion of CO and CO2 between carbon and anode on the performance of direct carbon solid oxide fuel cells. Solid State Ion. 2019, 343, 115127. [Google Scholar] [CrossRef]
- Li, S.; Lee, A.C.; Mitchell, R.E.; Gur, T.M. Direct carbon conversion in a helium fluidized bed fuel cell. Solid State Ion. 2008, 179, 1549–1552. [Google Scholar] [CrossRef]
- Lee, A.C.; Mitchell, R.E.; Gur, T.M. Modeling of CO2 gasification of carbon for integration with solid oxide fuel cells. AIChE J. 2009, 55, 983–992. [Google Scholar] [CrossRef]
- Alexander, B.R.; Mitchell, R.E.; Gur, T.M. Modeling of experimental results for carbon utilization in a carbon fuel cell. J. Power Sources 2013, 228, 132–140. [Google Scholar] [CrossRef]
- Armstrong, G.J.; Alexander, B.R.; RMitchell, E.; Guer, T.M. Modeling heat transfer effects in a solid oxide carbon fuel cell. In Batteries and Energy Technology; Manivannan, A., Minakshi, M., Narayan, S.R., Mukerjee, S., Eds.; ECS Transactions: Pennington, NJ, USA, 2013; pp. 143–150. [Google Scholar]
- Ma, L. Combustion and Gasification of Chars in Oxygen and Carbon Dioxide at Elevated Pressure; Mechnical Engieeering Department, Stanford University: Stanford, CA, USA, 2006. [Google Scholar]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Jiang, M.; Chen, X.; Zhou, Q. A gas pressure gradient-dependent subgrid drift velocity model for drag prediction in fluidized gas-particle flows. AIChE J. 2020, 66, e16884. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Wang, L.X.; Zhou, Q. Development of a filtered reaction rate model for reactive gas-solid flows based on fine-grid simulations. AIChE J. 2021, 67, e17185. [Google Scholar] [CrossRef]
- Li, T.W.; Rogers, W.A.; Syamlal, M.; Dietiker, J.F.; Musser, J.; Shahnam, M.; Rabha, S. The NETL MFiX Suite of multiphase flow models: A brief review and recent applications of MFiX-TFM to fossil energy technologies. Chem. Eng. Sci. 2017, 169, 259–272. [Google Scholar] [CrossRef]
- Holloway, W.; Sundaresan, S. Filtered models for reacting gas-particle flows. Chem. Eng. Sci. 2012, 82, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Ireland, E.; Pitt, K.; Smith, R. A review of pulsed flow fluidisation; the effects of intermittent gas flow on fluidised gas–solid bed behaviour. Powder Technol. 2016, 292, 108–121. [Google Scholar] [CrossRef]
- Grace, J.R.; Bi, X.; Ellis, N. Essentials of Fluidization Technology; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Li, G.D.; Gou, Y.J.; Qiao, J.S.; Sun, W.; Wang, Z.H.; Sun, K.N. Recent progress of tubular solid oxide fuel cell: From materials to applications. J. Power Sources 2020, 477, 228693. [Google Scholar] [CrossRef]
- Huang, K.; Singhal, S.C. Cathode-supported tubular solid oxide fuel cell technology: A critical review. J. Power Sources 2013, 237, 84–97. [Google Scholar] [CrossRef]
- Udomsilp, D.; Lenser, C.; Guillon, O.; Menzler, N.H. Performance Benchmark of Planar Solid Oxide Cells Based on Material Development and Designs. Energy Technol. 2021, 9, 2001062. [Google Scholar] [CrossRef]









| Pre-Exponent Constant | Value | Activation Energy (J/mol) | Value |
|---|---|---|---|
| 5.00 × 103 | 1.85 × 105 | ||
| 1.08 × 102 | 8.97 × 105 | ||
| 1.00 × 1013 | 3.75 × 105 | ||
| 1.00 × 10−4 | 5.80 × 104 | ||
| 0.877 | 1.48 × 105 | ||
| 1.00 × 1013 | 4.55 × 105 | ||
| 1.01 × 107 | 2.62 × 105 |
| Parameter | Value |
|---|---|
| 0.200 | |
| 0.100 | |
| 1173 | |
| 155 | |
| 1000 | |
| 0.450 | |
| 4.446 × 10−5 | |
| 4.443 × 10−5 | |
| 750 | |
| 9.807 | |
| ) | 96,485.3 |
| 8.314 | |
| 100% | |
| Gibbs free energy, G0 | 180.549 |
| 10−8~10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Min, T.; Jiang, M.; Yu, Y.; Zhou, Q. Numerical Simulation of Fluidized Bed Gasifier Coupled with Solid Oxide Fuel Cell Fed with Solid Carbon. Energies 2021, 14, 2800. https://doi.org/10.3390/en14102800
Zhang D, Min T, Jiang M, Yu Y, Zhou Q. Numerical Simulation of Fluidized Bed Gasifier Coupled with Solid Oxide Fuel Cell Fed with Solid Carbon. Energies. 2021; 14(10):2800. https://doi.org/10.3390/en14102800
Chicago/Turabian StyleZhang, Dongxu, Ting Min, Ming Jiang, Yaxiong Yu, and Qiang Zhou. 2021. "Numerical Simulation of Fluidized Bed Gasifier Coupled with Solid Oxide Fuel Cell Fed with Solid Carbon" Energies 14, no. 10: 2800. https://doi.org/10.3390/en14102800
APA StyleZhang, D., Min, T., Jiang, M., Yu, Y., & Zhou, Q. (2021). Numerical Simulation of Fluidized Bed Gasifier Coupled with Solid Oxide Fuel Cell Fed with Solid Carbon. Energies, 14(10), 2800. https://doi.org/10.3390/en14102800







