Syngas Production via CO2 Reforming of Methane over SrNiO3 and CeNiO3 Perovskites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SrNiO3 and CeNiO3 Nanocrystals
2.2. Catalyst Analysis
2.3. Catalyst Activity Measurements
3. Results and Discussion
3.1. Characterization of As-Synthesized Catalysts
3.1.1. Thermal Decomposition of Precursors
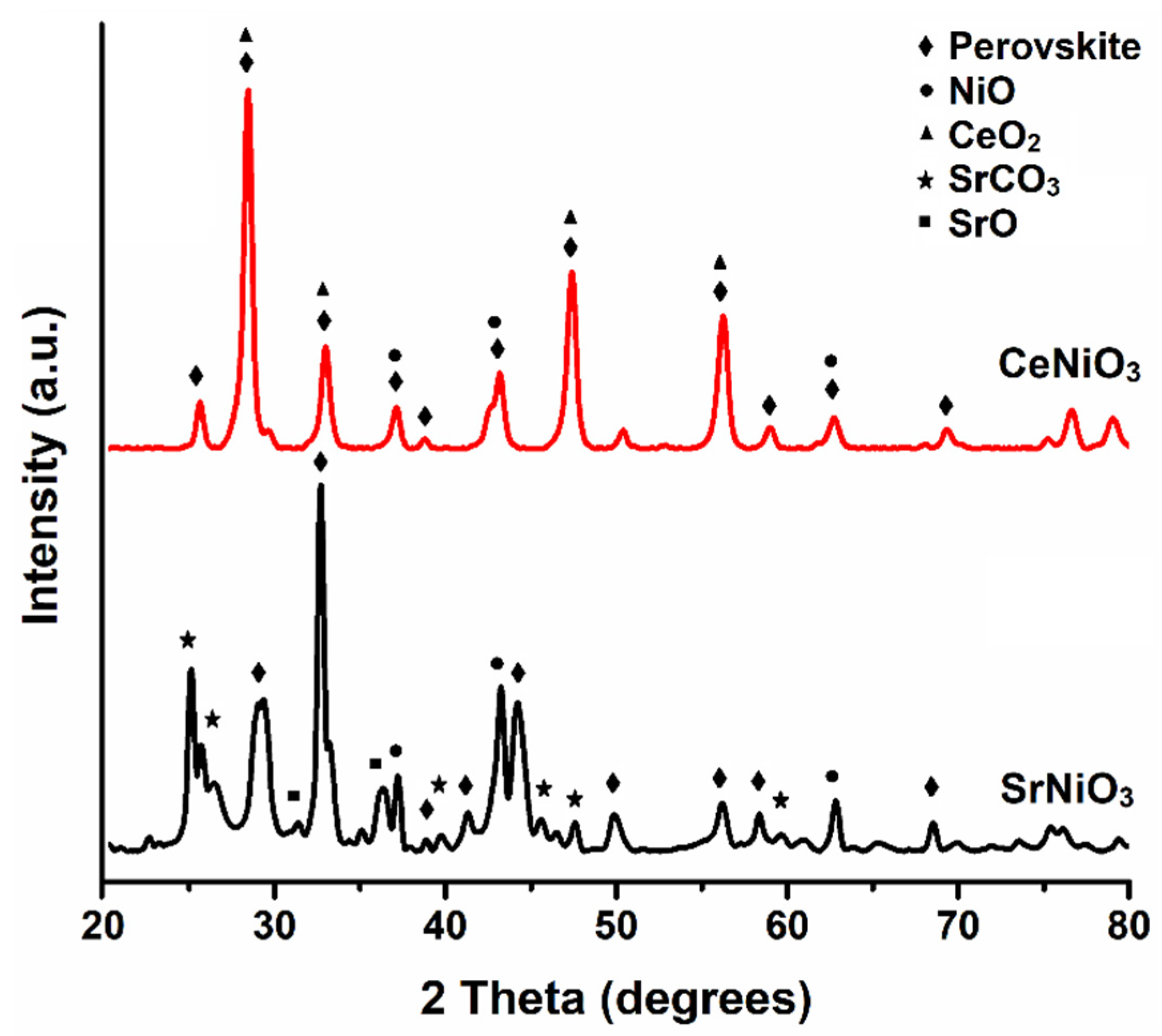
3.1.2. X-ray Diffraction (XRD)
3.1.3. Textural Properties
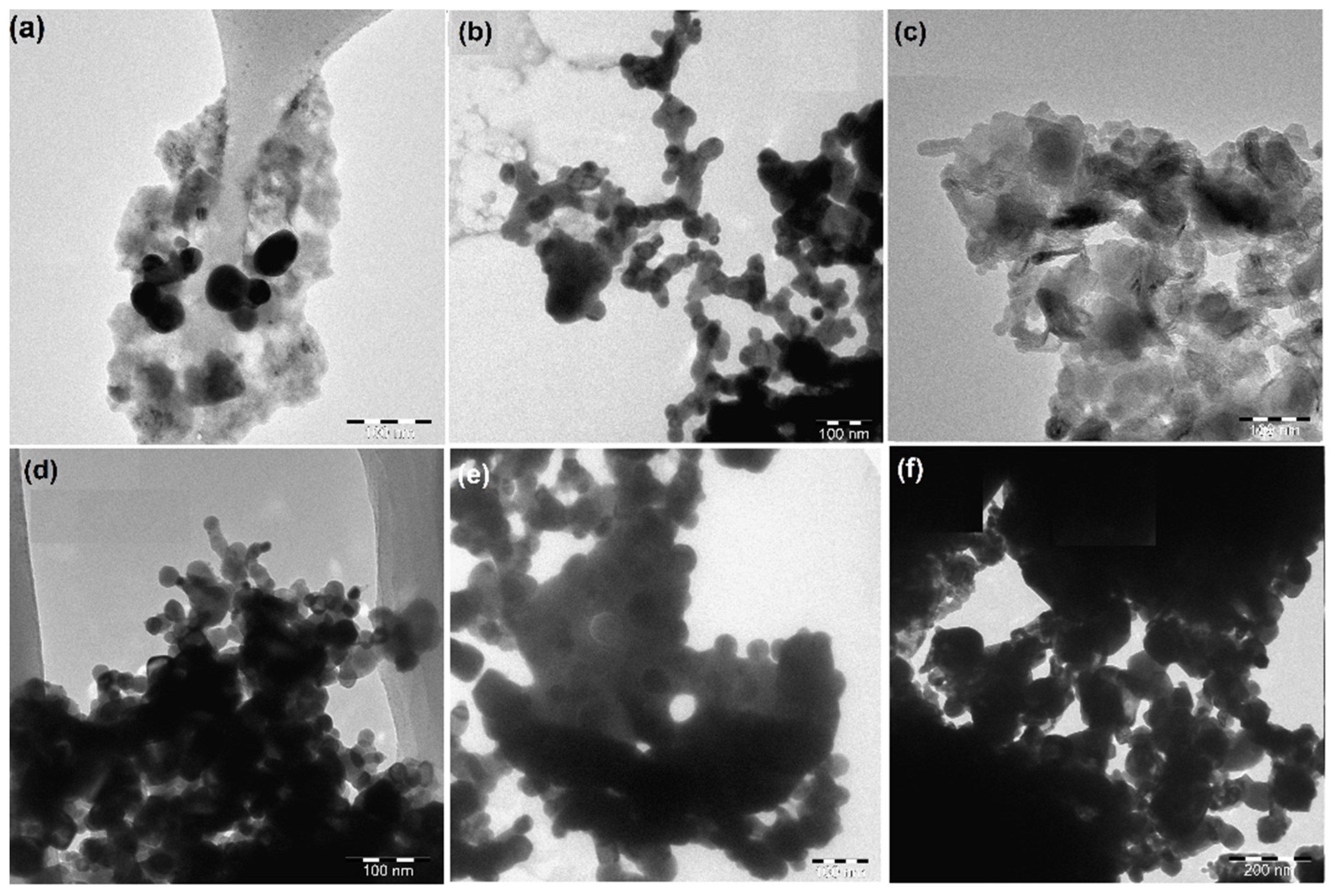
3.1.4. Morphological Study (TEM) of Fresh Perovskites
3.1.5. Temperature-Programmed Reduction (TPR)
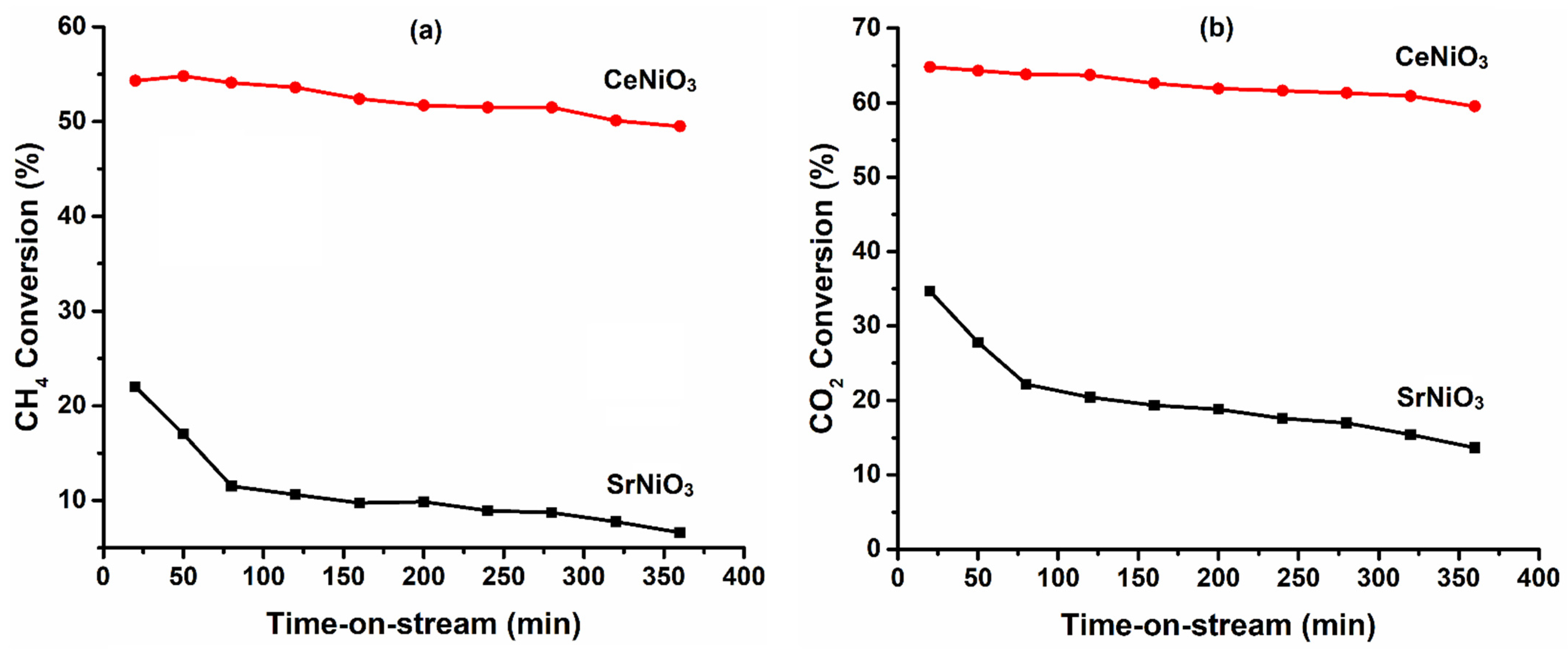
3.2. Catalytic Performances
3.3. Characterization of Spent Perovskites
3.3.1. Temperature-Programmed Oxidation (TPO)
3.3.2. Transition Electron Microscopy (TEM)
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Shen, D.; Cheng, S.; Wang, J.; Liu, H.; Xing, C.; Shan, S.; Lu, C.; Yang, R. Design an in-situ reduction of Ni/C-SiO2 catalyst and new insight into pretreatment effect for CH4–CO2 reforming reaction. Int. J. Hydrogen Energy 2017, 42, 10844–10853. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S. Enhancing hydrogen production by dry reforming process with strontium promoter. Int. J. Hydrogen Energy 2014, 39, 1680–1687. [Google Scholar] [CrossRef]
- Li, D.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Jiang, L. Preparation of supported Co catalysts from Co-Mg-Al layered double hydroxides for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2017, 42, 5063–5071. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA–15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A Gen. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Djinovic, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst. Int. J. Hydrogen Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- El Hassan, N.; Kaydouh, M.N.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.U.; Li, X.; Baharudin, L.; Yip, A.C.K. Copper-promoted cobalt/titania nanorod catalyst for CO hydrogenation to hydrocarbons. Catal. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Khan, W.U.; Baharudin, L.; Choi, J.; Yip, A.C.K. Recent progress in CO hydrogenation over bimetallic catalysts for higher alcohol synthesis. ChemCatChem 2020, 13, 533–542. [Google Scholar] [CrossRef]
- Khan, W.U.; Chen, S.S.; Tsang, D.C.W.; Hu, X.; Lam, F.L.Y.; Yip, A.C.K. Catalytically active interfaces in titania nanorod-supported copper catalysts for CO oxidation. Nano Res. 2021, 13, 111–120. [Google Scholar] [CrossRef]
- Horn, R.; Schlögl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 2015, 45, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and synthesis gas by steam- and CO2 reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Kang, D.; Yu, J.; Ma, W.; Zheng, M.; He, Y.; Li, P. Synthesis of Cu/Ni-La0.7Sr0.3Cr0.5Mn0.5O3−δ and its catalytic performance on dry methane reforming. J. Rare Earth 2019, 37, 585–593. [Google Scholar] [CrossRef]
- Chein, R.Y.; Fung, W.Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, L.; Fang, Y.; Li, H.; Zhang, J.; Zhao, T.S.; Yang, G.; Yoneyam, Y.; Tsubaki, N. Combined methane dry reforming and methane partial oxidization for syngas production over high dispersion Ni based mesoporous catalyst. Fuel Process. Technol. 2019, 188, 98–104. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Shen, J.; Reule, A.A.C.; Semagina, N. Ni/MgAl2O4 catalyst for low-temperature oxidative dry methane reforming with CO2. Int. J. Hydrogen Energy 2019, 44, 4616–4629. [Google Scholar] [CrossRef]
- Wang, F.; Han, K.; Yu, W.; Zhao, L.; Wang, Y.; Wang, X.; Yu, H.; Shi, W. Low temperature CO2 reforming with methane reaction over CeO2-modified Ni@SiO2 catalysts. ACS Appl. Mater. Interf. 2020, 12, 35022–35034. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Yu, W.; Xu, L.; Deng, Z.; Yu, H.; Wang, F. Reducing carbon deposition and enhancing reaction stability by ceria for methane dry reforming over Ni@SiO2@CeO2 catalyst. Fuel 2021, 291, 120182. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas. Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Gedamu, D.; Fourmont, P.; Rekola, H.; Hiltunen, A.; Cloutier, S.G.; Nechache, R.; Priimagi, A.; Vivo, P. Halide perovskite nanocrystals for next-generation optoelectronics. Small 2019, 15, 1900801. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, R.; Wang, J.; Li, X.; Chen, Y.-M.; Liu, K.; Taubert, C.J.; Cheng, S.Z.D.; Zhu, X. Crystalline organic pigment-based field-effect transistors. ACS Appl. Mater. Interf. 2017, 9, 21891–21899. [Google Scholar] [CrossRef]
- Khalesi, A.; Arandiyan, H.R.; Parvari, M. Production of Syngas by CO2 Reforming on MxLa1−xNi0.3Al0.7O3−d (M = Li, Na, K). Ind. Eng. Chem. Res. 2008, 47, 5892–5898. [Google Scholar] [CrossRef]
- Pérez-Camacho, M.N.; Abu-Dahrieh, J.; Goguet, A.; Sun, K.; Rooney, D. Self-cleaning perovskite-type catalysts for the dry reforming of methane. Chin. J. Catal. 2014, 35, 1337–1346. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H. 5—Perovskite-related oxides as oxidation—Reduction catalysts. In Advanced Materials in Catalysis; Burton, J.J., Garten, R.L., Eds.; Academic Press: New York, NY, USA, 1977; pp. 129–180. [Google Scholar]
- Ren, P.; Zhao, Z. Unexpected coke-resistant stability in steam-CO2 dual reforming of methane over the robust Mo2C-Ni/ZrO2 catalyst. Catal. Commun. 2019, 119, 71–75. [Google Scholar] [CrossRef]
- Ziaei-Azad, H.; Khodadadi, A.; Esmaeilnejad-Ahranjani, P.; Mortazavi, Y. Effects of Pd on enhancement of oxidation activity of LaBO3 (B = Mn, Fe, Co and Ni) pervoskite catalysts for pollution abatement from natural gas fueled vehicles. Appl. Catal. B Environ. 2011, 102, 62–70. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Barama, A. Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 2018, 24, 40–49. [Google Scholar] [CrossRef]
- Ruocco, C.; Caprariis, B.D.; Palma, V.; Petrullo, A.; Ricca, A.; Scarsella, M.; Filippis, P.D. Methane dry reforming on Ru perovskites, AZrRuO3: Influence of preparation method and substitution of A cation with alkaline earth metals. J. CO2 Util. 2019, 30, 222–231. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La (CoxNi1−x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2009, 245, 302–313. [Google Scholar] [CrossRef]
- Dehghani, F.; Ayatollahi, S.; Bahadorikhalili, S.; Esmaeilpour, M. Synthesis and characterization of mixed–metal oxide nanoparticles (CeNiO3, CeZrO4, CeCaO3) and application in adsorption and catalytic oxidation–decomposition of asphaltenes with different chemical structures. Pet. Chem. 2020, 60, 731–743. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Mary, A.J.C.; Bose, A.C. Electrochemical performance of ANiO3 (A = La, Ce) perovskite oxide material and its device performance for supercapattery application. Electrochim. Acta 2020, 362, 137095. [Google Scholar] [CrossRef]
- García de la Cruz, R.M.; Falcón, H.; Peña, M.A.; Fierro, J.L.G. Role of bulk and surface structures of La1−xSrxNiO3 perovskite-type oxides in methane combustion. Appl. Catal. B Environ. 2001, 33, 45–55. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Shu, Z.; Chen, H.; Shi, J. A simple co-nanocasting method to synthesize high surface area mesoporous LaCoO3 oxides for CO and NO oxidations. Microporous Mesoporous Mater. 2013, 176, 8–15. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Jiao, J.; Liu, J.; Duan, A.; Jiang, G. Facile synthesis of three-dimensionally ordered macroporous LaFeO3-supported gold nanoparticle catalysts with high catalytic activity and stability for soot combustion. Catal. Today 2015, 245, 37–45. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Li, G.; An, T.; Shi, H.; Li, Y. Visible-light-enhanced photothermocatalytic activity of ABO3-type perovskites for the decontamination of gaseous styrene. Appl. Catal. B Environ. 2017, 209, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Moral, A.; Reyero, I.; Alfaro, C.; Bimbela, F.; Gandía, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]
- Verykios, X. Mechanistic aspects of the reaction of CO2 reforming of methane over Rh/Al2O3 catalyst. Appl. Catal. A. Gen. 2003, 255, 101–111. [Google Scholar] [CrossRef]
- Rynkowski, J.; Samulkiewicz, P.; Ladavos, A.K.; Pomonis, P.J. Catalytic performance of reduced La2−xSrxNiO4 perovskite-like oxides for CO2 reforming of CH4. Appl. Catal. A Gen. 2004, 263, 1–9. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Belhekar, A.A. Oxidative conversion of methane to syngas over LaNiO3 perovskite with or without simultaneous steam and CO2 reforming reactions: Influence of partial substitution of La and Ni. J. Catal. 1996, 163, 312–318. [Google Scholar] [CrossRef]
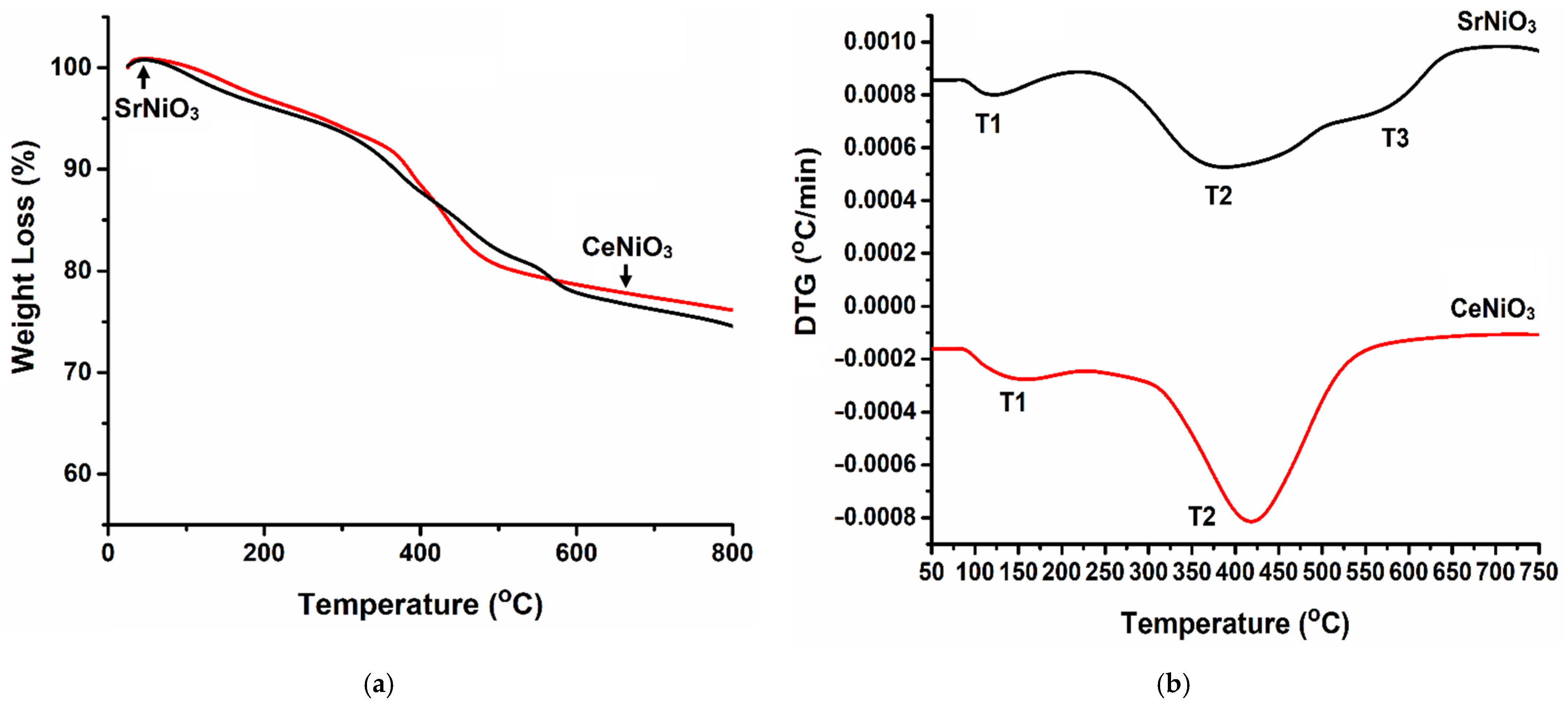

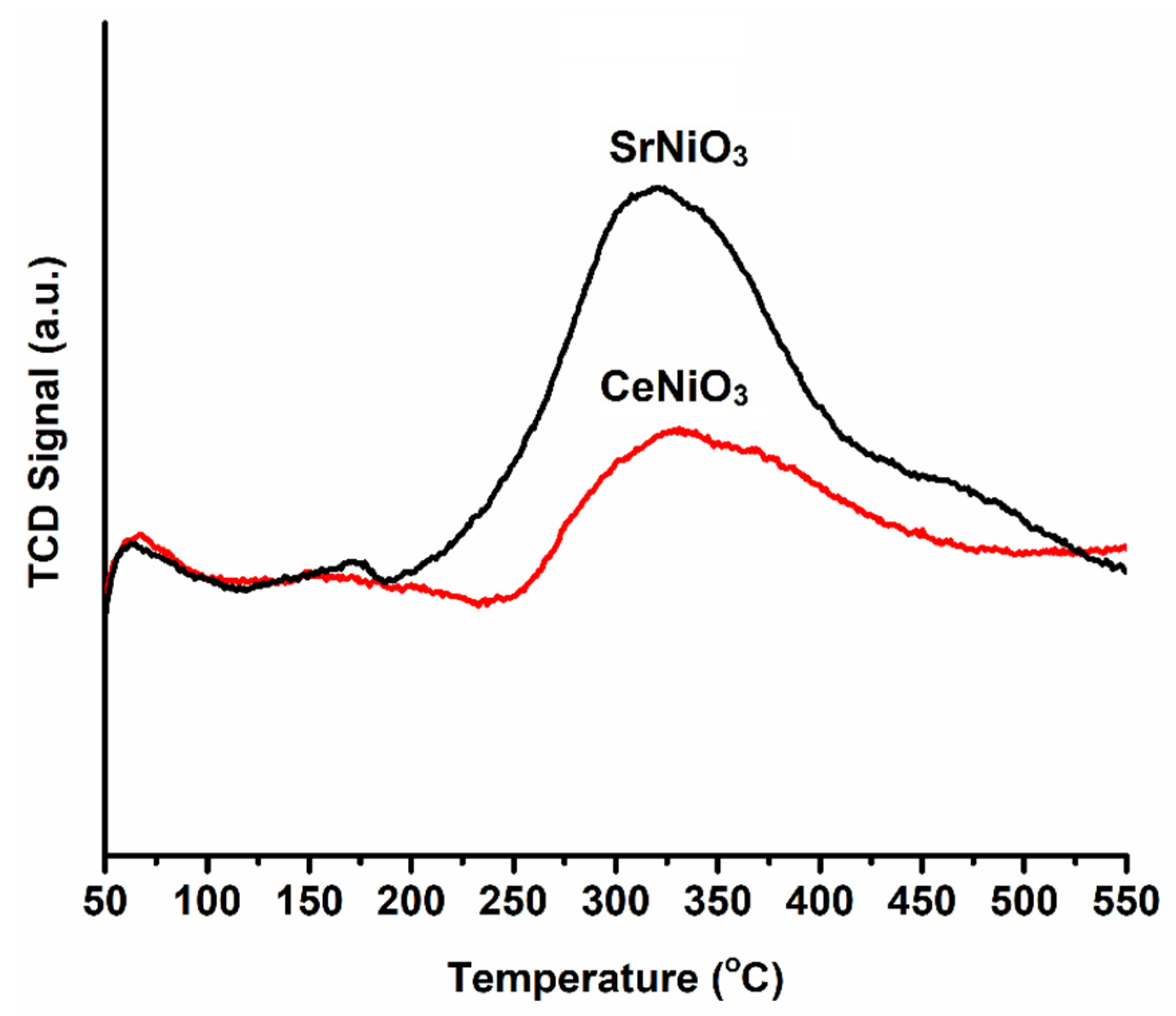
| Perovskite | T1 (°C) | T2 (°C) | T3 (°C) |
|---|---|---|---|
| CeNiO3 | 150 | 420 | - |
| SrNiO3 | 125 | 390 | 570 |
| Perovskite | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Deactivation Factor (%) a | Total Hydrogen Consumption (mmol/g) b | Degree of Reduction (%) c |
|---|---|---|---|---|---|---|
| CeNiO3 | 20.7 | 0.162 | 30.1 | 7.7 | 1.11 | 90.5 |
| SrNiO3 | 12.2 | 0.026 | 9.3 | 64.7 | 0.84 | 50.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Alharthi, F.; Alam, M.; Wahab, R.; Manoharadas, S.; Alrayes, B. Syngas Production via CO2 Reforming of Methane over SrNiO3 and CeNiO3 Perovskites. Energies 2021, 14, 2928. https://doi.org/10.3390/en14102928
Ahmad N, Alharthi F, Alam M, Wahab R, Manoharadas S, Alrayes B. Syngas Production via CO2 Reforming of Methane over SrNiO3 and CeNiO3 Perovskites. Energies. 2021; 14(10):2928. https://doi.org/10.3390/en14102928
Chicago/Turabian StyleAhmad, Naushad, Fahad Alharthi, Manawwer Alam, Rizwan Wahab, Salim Manoharadas, and Basel Alrayes. 2021. "Syngas Production via CO2 Reforming of Methane over SrNiO3 and CeNiO3 Perovskites" Energies 14, no. 10: 2928. https://doi.org/10.3390/en14102928
APA StyleAhmad, N., Alharthi, F., Alam, M., Wahab, R., Manoharadas, S., & Alrayes, B. (2021). Syngas Production via CO2 Reforming of Methane over SrNiO3 and CeNiO3 Perovskites. Energies, 14(10), 2928. https://doi.org/10.3390/en14102928








