Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalysts Characterisation
2.3. Catalytic Tests
3. Results and Discussions
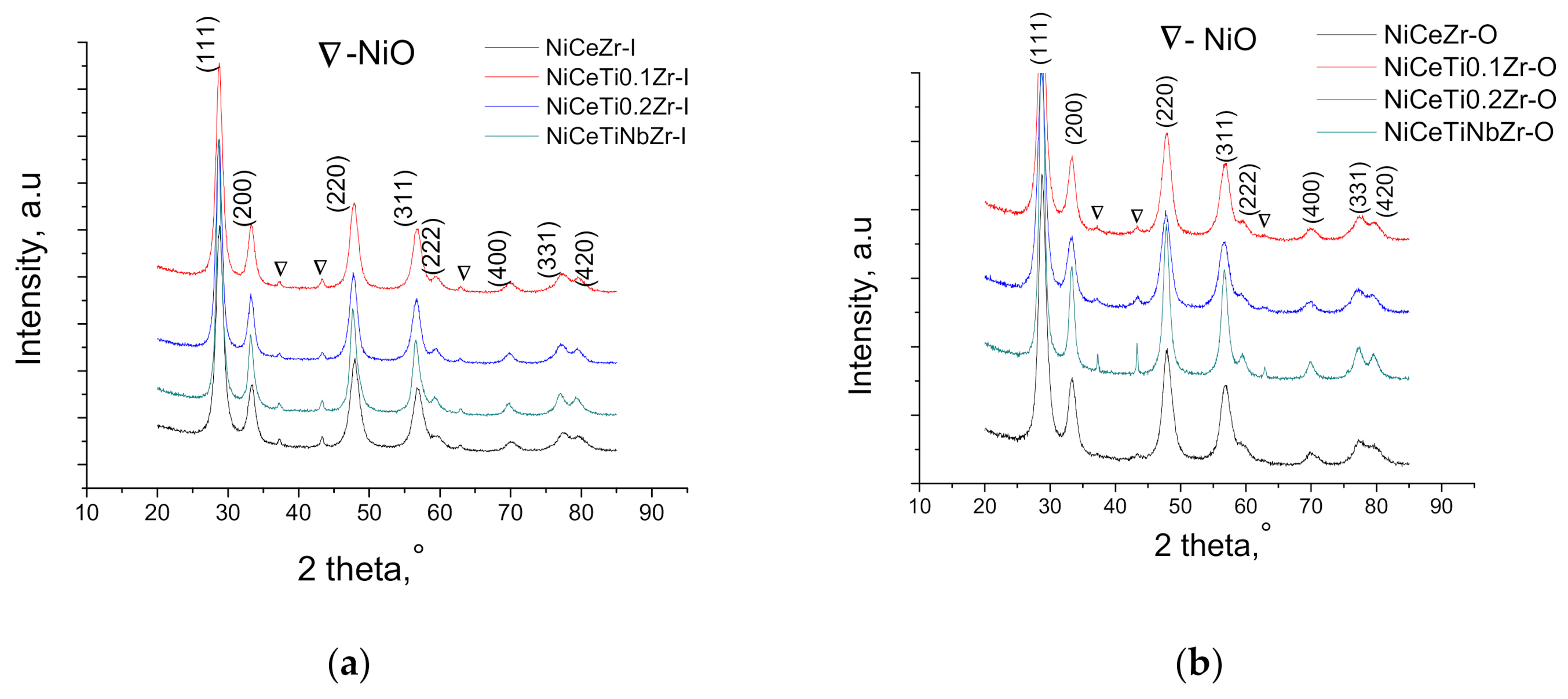
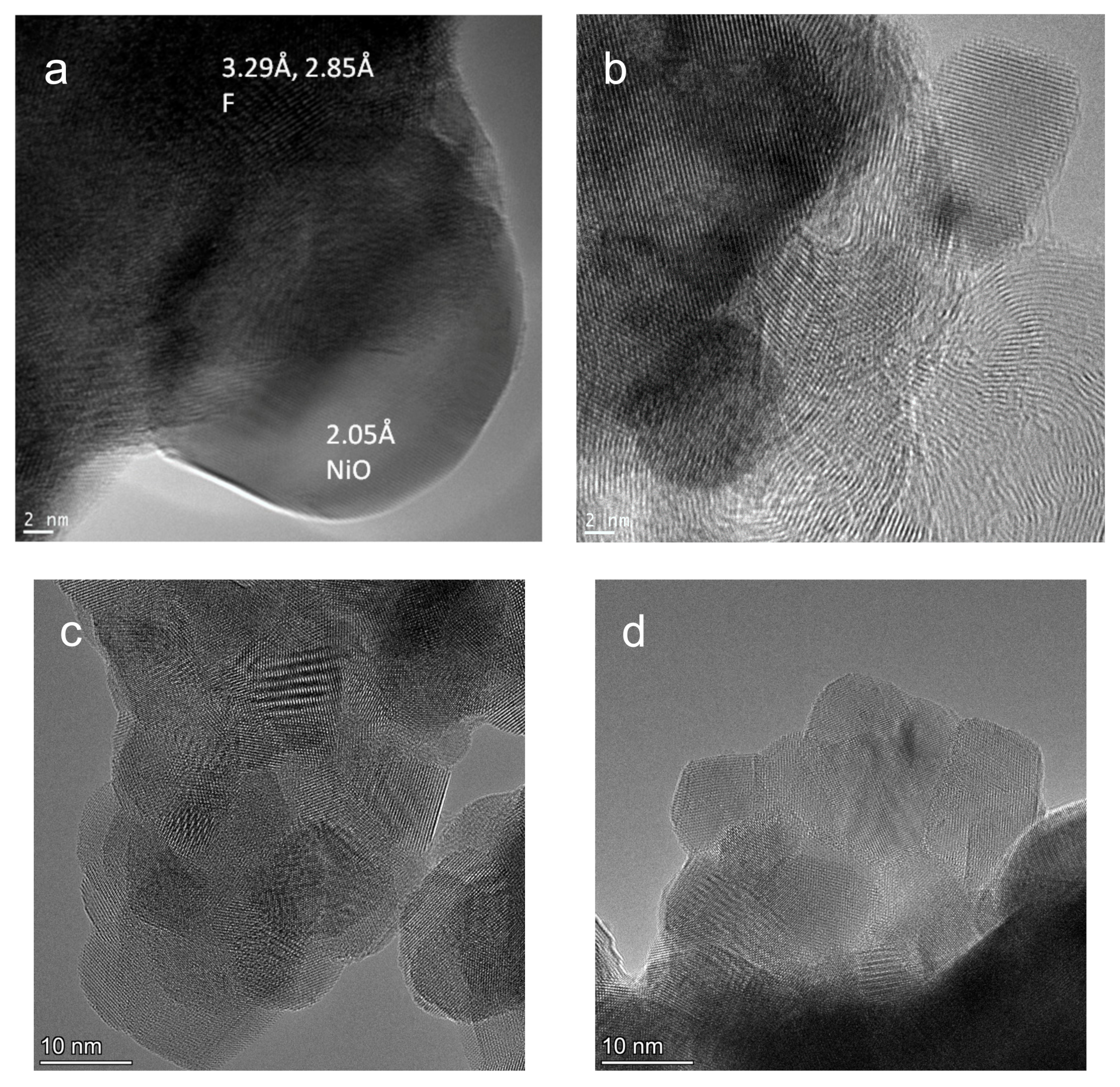
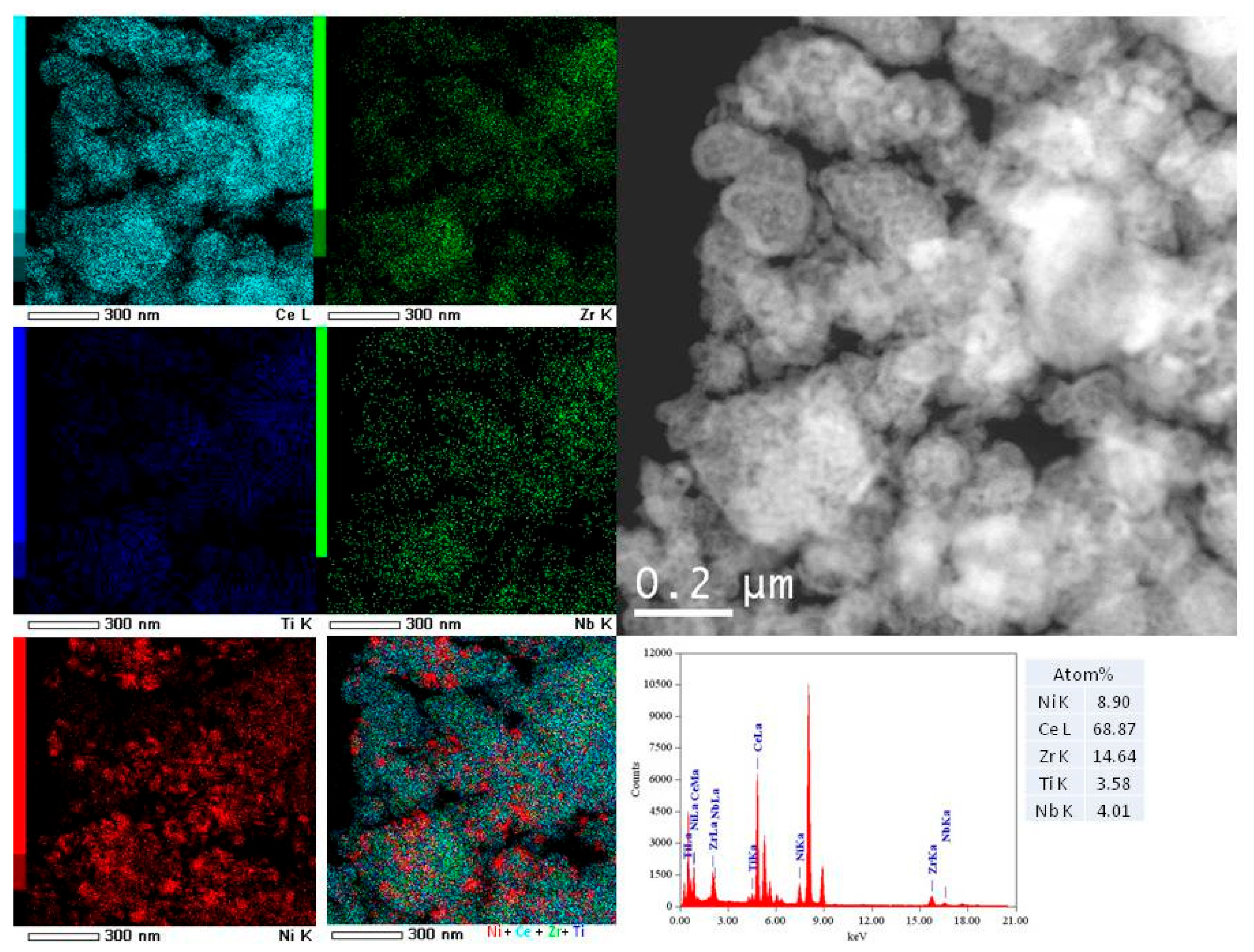
3.1. Textural and Structural Propeties of Catalysts
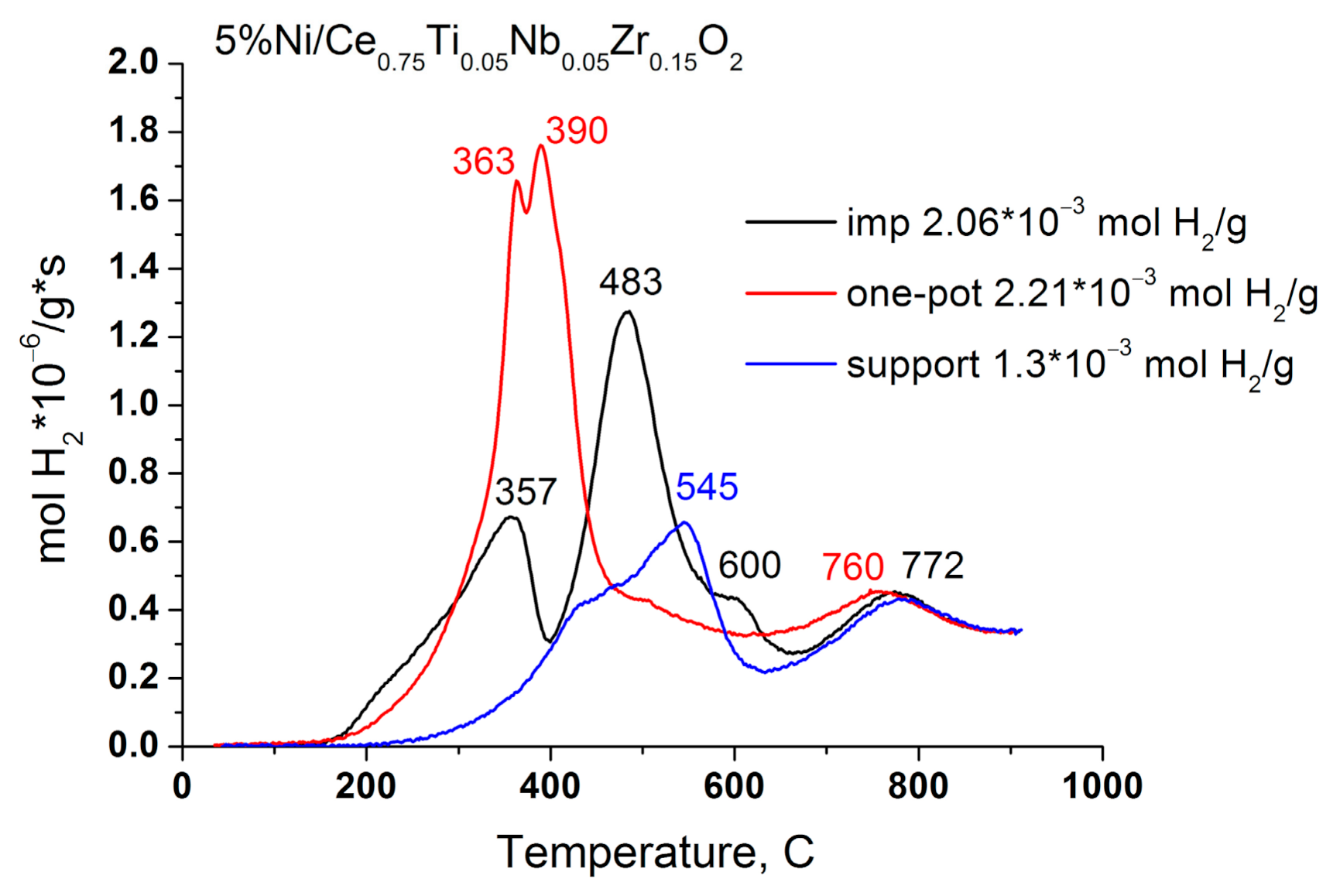
3.2. Catalyst Activation in Hydrogen
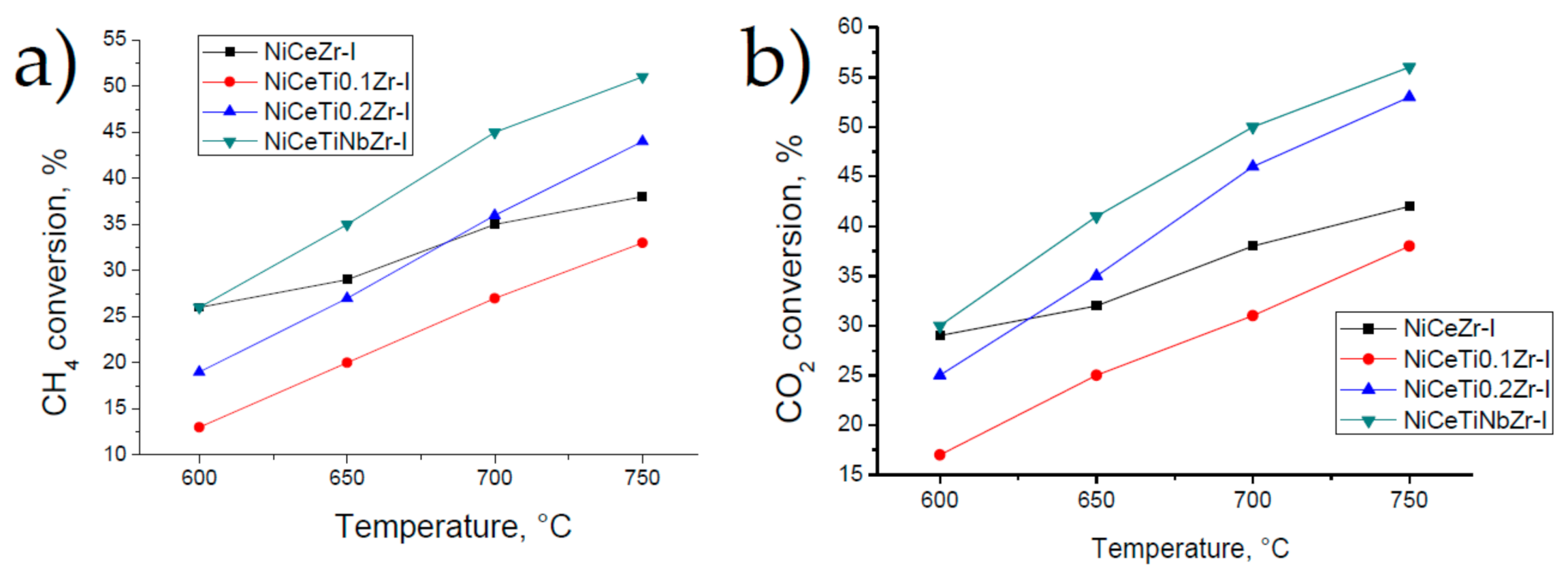
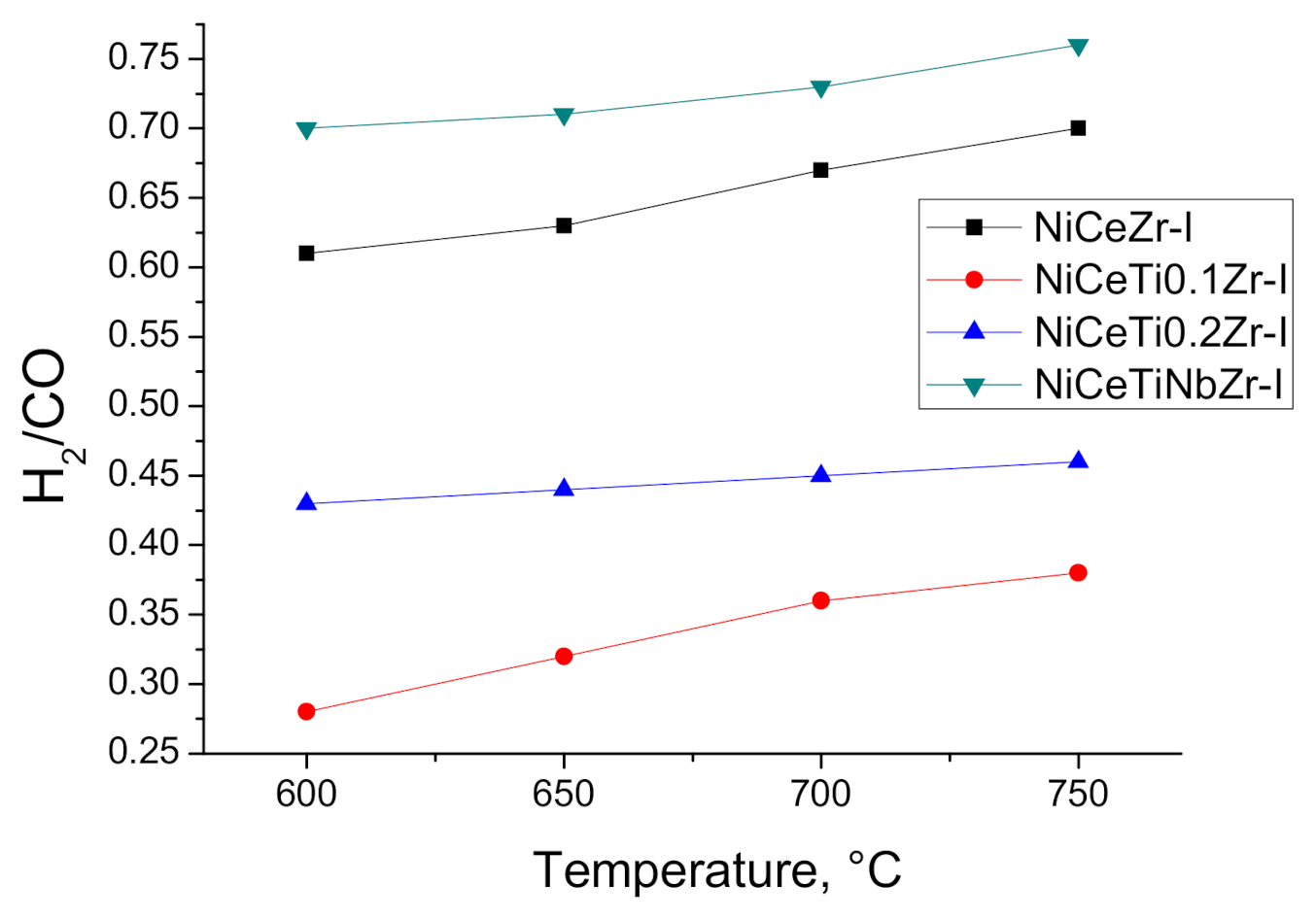
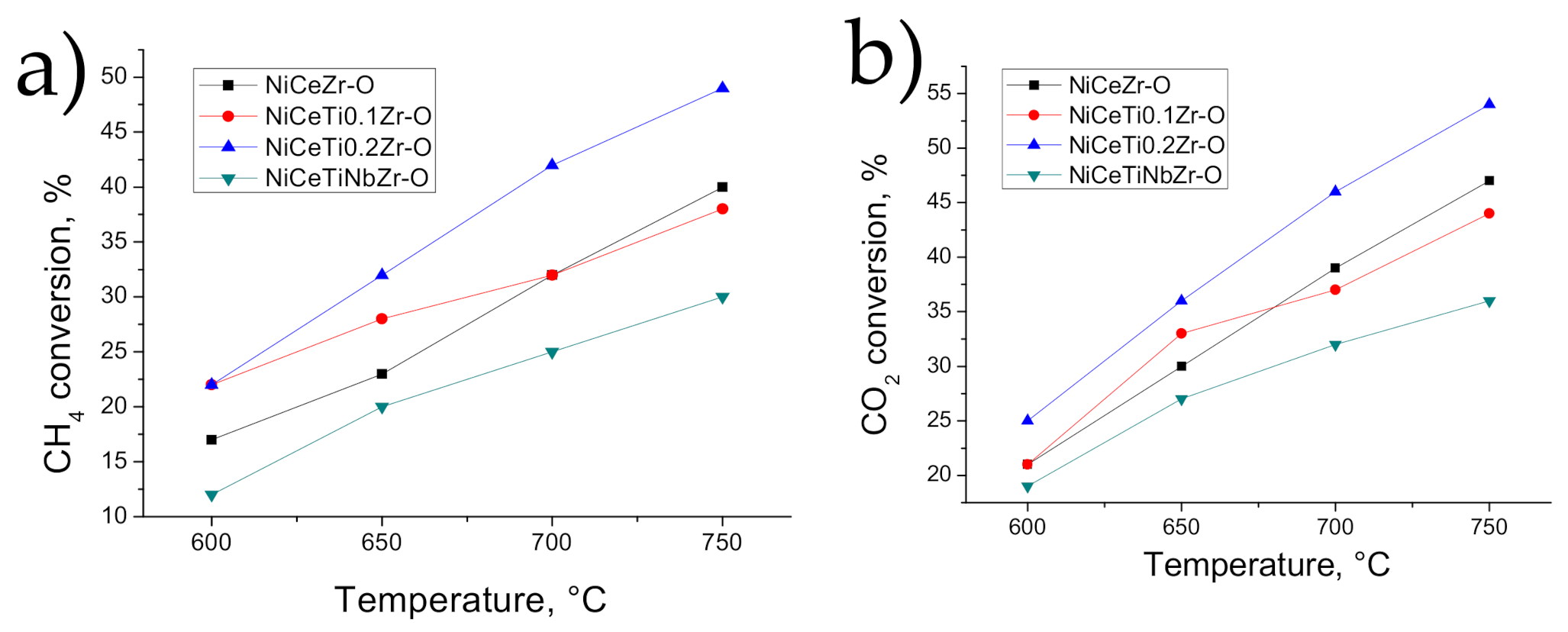
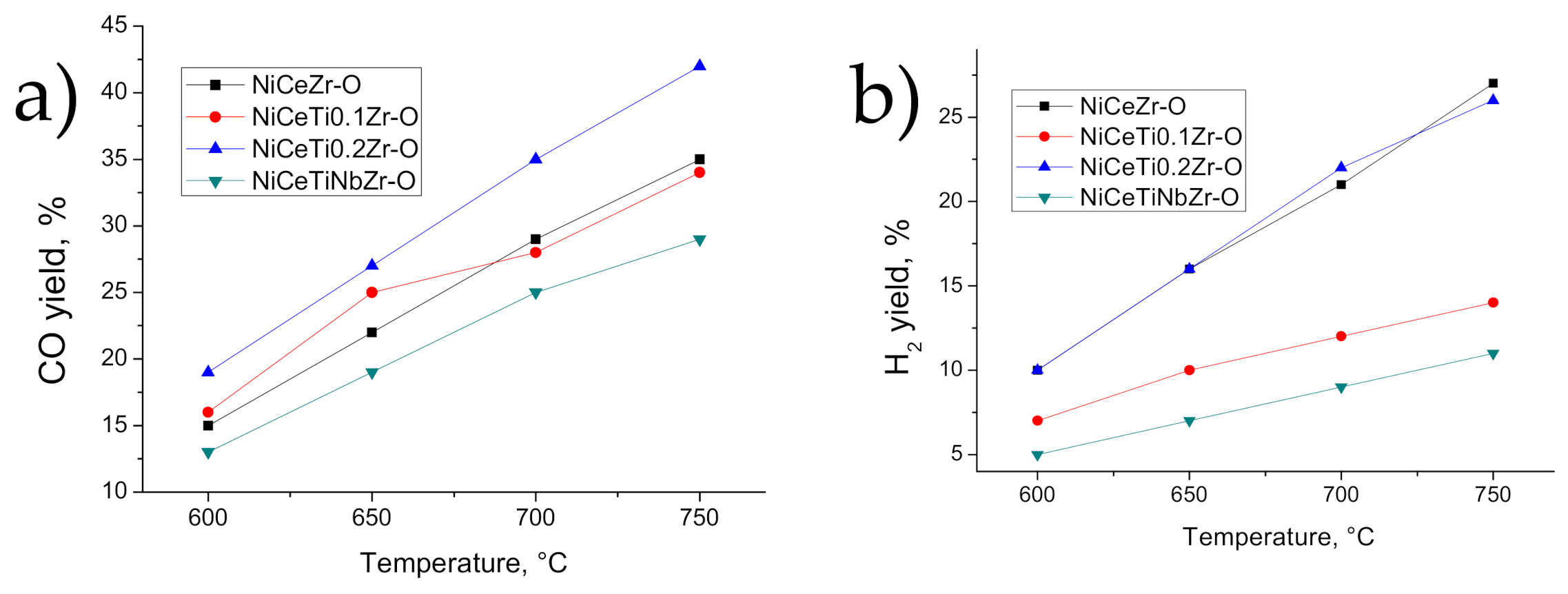
3.3. Catalysis Studies
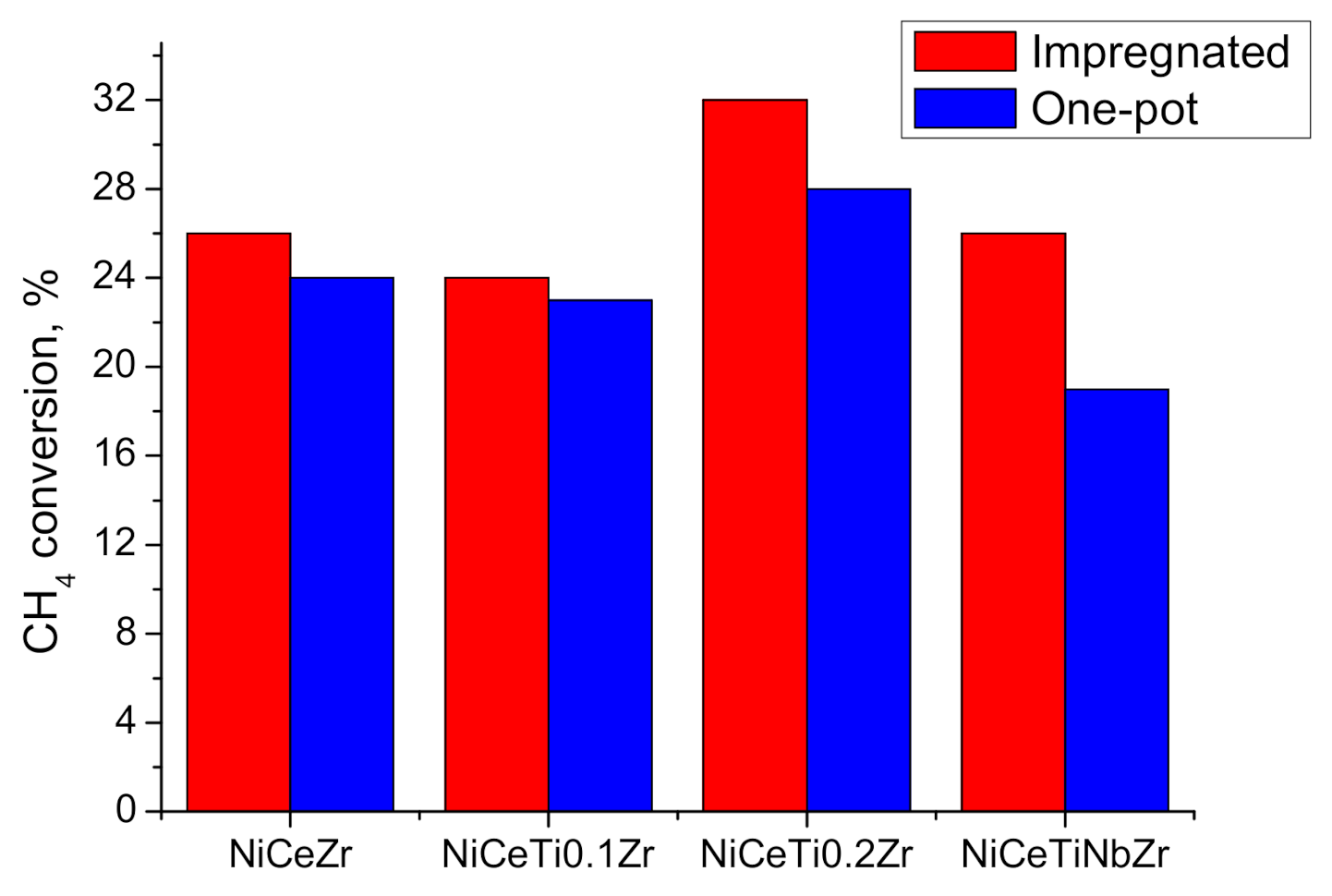
3.3.1. Influence of Reduction Conditions on Catalytic Properties
3.3.2. General Kinetic Patterns
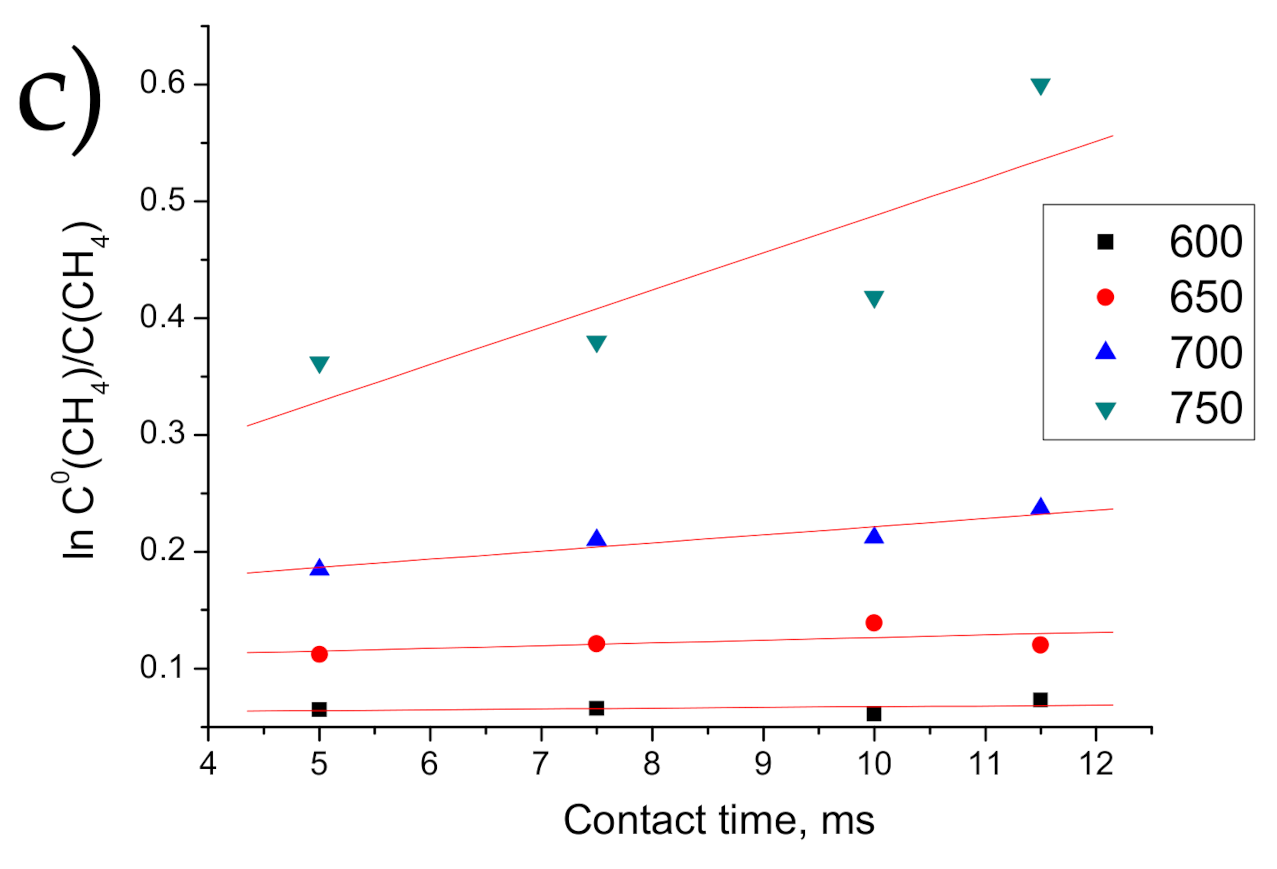
3.3.3. Calculations of Kinetic Characteristics of MDR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Shin, S.A.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Song, H.T.; Lee, K.-Y.; Moon, D.J. Dry reforming of methane over Ni/ZrO2-Al2O3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas production with dry reforming of methane over Ni/ZSM-5 catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrog. Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Singha, R.K.; Yadav, A.; Shukla, А.; Kumar, М.; Bal, R. Low temperature dry reforming of methane over Pd-CeO2 nanocatalyst. Catal. Commun. 2017, 92, 19–22. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Radlik, М.; Adamowska-Teyssier, М.; Krzton´, А.; Kozieł, К.; Krajewski, W.; Turek, W.; Costa, P.D. Dry Reforming of methane over Ni/Ce0.62Zr0.38O2 catalysts: Effect of Ni loading on the catalytic activity and on H2/CO production. C. R. Chim. 2015, 18, 1242–1249. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Zhang, J.; Zhang, J. Effect of preparation methods on structure and performance of Ni/Ce0.75Zr0.25O2 catalysts for CH4–CO2 reforming. Fuel 2008, 87, 2901–2907. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2–TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Yahya, E.A.; Zabidi, N.A.M.; Kait, C.F. A study on coke deposition in Ni-based catalysts for CO2 reforming of methane. AIP Conf. Proc. 2016, 1787, 030005. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Azapour, A.; Muhammad, A.F.S.; Abdullah, B. Dry reforming of methane for hydrogen production over Ni Co catalysts: Effect of Nb Zr promoters. Int. J. Hydrog. Energy 2019, 44, 20881–20888. [Google Scholar] [CrossRef]
- Ramírez-Cabrera, E.; Laosiripojana, N.; Atkinson, A.; Chadwick, D. Methane conversion over Nb-doped ceria. Catal. Today 2003, 78, 433–438. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Faga, M.G.; Miletto, I.; Colucci, S.; Caputo, G.; Sau, S.; Giaconia, А.; Berlier, G. Unravelling the structure and reactivity of supported Ni particles in Ni-CeZrO2 catalysts. Appl. Catal. B Environ. 2013, 138–139, 353–361. [Google Scholar] [CrossRef]
- Inagaki, M.; Kato, E. Hydrothermal Synthesis and Sintering of Fine Powders in CeO2-ZrO2 System. J. Ceram. Soc. Jpn. 1996, 104, 958–962. [Google Scholar]
- Pietraszek, A.; Koubaissy, B.; Roger, A.-C.; Kiennemann, A. The influence of the support modification over Ni-based catalysts for dry reforming of methane reaction. Catal. Today 2011, 176, 267–271. [Google Scholar] [CrossRef]
- Horváth, A.; Stefler, G.; Geszti, O.; Kienneman, A.; Pietraszek, A.; Guczi, L. Methane dry reforming with CO2 on CeZr-oxide supported Ni, NiRh and NiCo catalysts prepared by sol–gel technique: Relationship between activity and coke formation. Catal. Today 2011, 169, 102–111. [Google Scholar] [CrossRef]
- Mastelaro, V.R.; Briois, V.; de Souza, D.P.; Silva, C.L. Structural studies of a ZrO2–CeO2 doped system. J. Eur. Ceram. Soc. 2003, 23, 273–282. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Near- and Supercritical Alcohols as Solvents and Surface Modifiers for the Continuous Synthesis of Cerium Oxide Nanoparticles. Langmuir 2012, 28, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Lee, K.-Y.; Suh, M.-J.; Ihm, S.-K. Ceria–zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Effect of Thermal Treatment on the Textural Properties of CeO2Powders Synthesized in Near- and Supercritical Alcohols. ChemPhysChem 2015, 16, 3493–3499. [Google Scholar] [CrossRef]
- Pavlova, S.; Smirnova, M.; Bobin, A.; Cherepanova, S.; Kaichev, V.; Ishchenko, A.; Selivanova, A.; Rogov, V.; Roger, A.-C.; Sadykov, V. Structural, Textural, and Catalytic Properties of Ni-CexZr1−xO2 Catalysts for Methane Dry Reforming Prepared by Continuous Synthesis in Supercritical Isopropanol. Energies 2020, 13, 3728. [Google Scholar] [CrossRef]
- Smirnova, M.; Pavlova, S.; Krieger, T.; Bespalko, Y.; Anikeev, V.; Chesalov, V.; Kaichev, V.; Mezentseva, N.; Sadykov, V. The Synthesis of Ce1−xZrxO2 Oxides in Supercritical Alcohols and Catalysts for Carbon Dioxide Reforming of Methane on Their Basis. Russ. J. Phys. Chem. B 2017, 11, 1312–1321. [Google Scholar]
- Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Smal, E.; Simonov, M.; Mezentseva, N.; Krieger, T.; Larina, T.; Saraev, A.; Glazneva, T.; Ishchenko, A.; Rogov, V.; Eremeev, N.; et al. Spinel-type MnxCr3-xO4-based catalysts for ethanol steam reforming. Appl. Catal. B Environ. 2021, 283, 119656. [Google Scholar] [CrossRef]
- Bespalko, Y.; Smal, E.; Simonov, M.; Valeev, K.; Fedorova, V.; Krieger, T.; Cherepanova, S.; Ishchenko, A.; Rogov, V.; Sadykov, V. Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Prepared in Supercritical Alcohol Media. Energies 2020, 13, 3365. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Simonov, M.N.; Mezentseva, N.V.; Pavlova, S.N.; Fedorova, Y.E.; Bobin, A.S.; Bespalko, Y.N.; Ishchenko, A.V.; Krieger, T.A.; Glazneva, T.S.; et al. Ni-loaded nanocrystalline ceria-zirconia solid solutions prepared via modified Pechini route as stable to coking catalysts of CH4 dry reforming. Open Chem. 2016, 14, 363–376. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Borchert, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P.; et al. Electronic and Chemical Properties of Nanostructured Cerium Dioxide Doped with Praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Akpan, E.; Sun, Y.; Kumar, P.; Ibrahim, H.; Aboudheir, А.; Idem, R. Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2–ZrO2 catalyst in a packed bed tubular reactor. Chem. Eng. Sci. 2007, 62, 4012–4024. [Google Scholar] [CrossRef]





| Composition | Method of Ni Addition | Code |
|---|---|---|
| 5 wt % Ni/Ce0.75Zr0.25O2 | Impregnation | NiCeZr-I |
| One-pot | NiCeZr-O | |
| 5 wt % Ni/Ce0.75Ti0.1Zr0.15O2 | Impregnation | NiCeTi0.1Zr-I |
| One-pot | NiCeTi0.1Zr-O | |
| 5 wt % Ni/Ce0.75Ti0.2Zr0.05O2 | Impregnation | NiCeTi0.2Zr-I |
| One-pot | NiCeTi0.2Zr-O | |
| 5 wt % Ni/Ce0.75Ti0.05Nb0.05Zr0.15O2 | Impregnation | NiCeTiNbZr-I |
| One-pot | NiCeTiNbZr-O |
| Code | SBET, m2g−1 | Vtotal, cm3g−1 | d pore 1, nm | Crystallite Size, nm | Lattice Parameter 2, Å | |
|---|---|---|---|---|---|---|
| Fluorite | NiO | |||||
| NiCeZr-I | 21 | 0.144 | 3.7 92.4 | 9.4 | 30 | 5.368 (1) |
| NiCeTi0.1Zr-I | 23 | 0.184 | 4.3 123.8 | 10.4 | 20 | 5.379 (1) |
| NiCeTi0.2Zr-I | 16 | 0.186 | 9.9 92.3 | 11.5 | 18 | 5.392 (1) |
| NiCeTiNbZr-I | 26 | 0.184 | 8.5 74 | 14.5 | 24 | 5.392 (1) |
| NiCeZr-O | 13 | 0.126 | 59.2 | 8.9 | 19 | 5.371 (1) |
| NiCeTi0.1Zr-O | 16 | 0.143 | 3.9 59.3 | 8.5 | 13 | 5.376 (1) |
| NiCeTi0.2Zr-O | 11 | 0.102 | 3.9 92.6 | 8.5 | 15 | 5.393 (1) |
| NiCeTiNbZr-O | 11 | 0.157 | 92.6 | 13 | 90 | 5.385 (1) |
| Sample | H2 Treatment | [Ce]/[Zr] | [Ni] * | [O] * | Ce4+, % | Ce3+, % |
|---|---|---|---|---|---|---|
| NiCeZr-I | No | 2.70 | 0.35 | 2.94 | 80 | 20 |
| Yes | 4.18 | 0.15 | 1.63 | 50 | 50 | |
| NiCeZr-O | No | 3.85 | 0.15 | 2.86 | 80 | 20 |
| Yes | 6.56 | 0.08 | 1.74 | 60 | 40 |
| Code | keff (700 °С), s−1 | W0, mol L−1 s−1 | Ea, kJ/mol | TOF, s−1 [27] | Do, 10−15 cm2 s−1 | R, 10−3 s−1 |
|---|---|---|---|---|---|---|
| NiCeZr-I | 46 | 0.30 | 54 ± 7 | 2.9 | 7.1 | - |
| NiCeZr-O | 51 | 0.32 | 79 ± 20 | 2.4 | 13 | 27 |
| NiCeTi0.1Zr-I | 38 | 0.24 | - | 3.4 | 3.2 | - |
| NiCeTiNbZr-I | 64 | 0.41 | 78 ± 4 | 9.0 | 1.7 | - |
| NiCeTiNbZr-O | 38 | 0.13 | 183 ± 16 | 2.6 | 7.8 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorova, V.; Simonov, M.; Valeev, K.; Bespalko, Y.; Smal, E.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Ishchenko, A.; Sadykov, V. Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia. Energies 2021, 14, 2973. https://doi.org/10.3390/en14102973
Fedorova V, Simonov M, Valeev K, Bespalko Y, Smal E, Eremeev N, Sadovskaya E, Krieger T, Ishchenko A, Sadykov V. Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia. Energies. 2021; 14(10):2973. https://doi.org/10.3390/en14102973
Chicago/Turabian StyleFedorova, Valeria, Mikhail Simonov, Konstantin Valeev, Yuliya Bespalko, Ekaterina Smal, Nikita Eremeev, Ekaterina Sadovskaya, Tamara Krieger, Arcady Ishchenko, and Vladislav Sadykov. 2021. "Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia" Energies 14, no. 10: 2973. https://doi.org/10.3390/en14102973
APA StyleFedorova, V., Simonov, M., Valeev, K., Bespalko, Y., Smal, E., Eremeev, N., Sadovskaya, E., Krieger, T., Ishchenko, A., & Sadykov, V. (2021). Kinetic Regularities of Methane Dry Reforming Reaction on Nickel-Containing Modified Ceria–Zirconia. Energies, 14(10), 2973. https://doi.org/10.3390/en14102973








