Abstract
The aim of the study was to evaluate the possibility of using the process of dark fermentation to convert kitchen waste into valuable volatile fatty acids in a semi-continuous process at different values of the organic loading rate (2.5 and 5.0 gVS/(L × d)) and hydraulic retention time (5 and 10 d) using anaerobic mixed microbial consortia. The experiments were performed in a bioreactor of working volume 8L with pH control. The maximum volatile fatty acids yield in a steady state (22.3 g/L) was achieved at the organic loading rate of 5.0 gVS/(L × d) and HRT of 10 days. The main products of dark fermentation were acetic and butyric acids, constituting, respectively, 35.2–47.7% and 24.1–30.0% of all identified volatile fatty acids. Additionally, at the beginning of the fermentation and in a steady-state condition, the microbial population analysis (16S rDNA) of the fermentation mixture with the most effective volatile fatty acids generation has been performed to monitor the DF microflora development. The dominant microorganisms at a phylum level in a steady state were Firmicutes (44.9%) and Bacteroidetes (30.1%), which indicate the main role of those phyla in the volatile fatty acids synthesis.
1. Introduction
The 21st century consumer-oriented lifestyle coupled with the constant growth of the human population has caused a vast increase in municipal solid waste (MSW) production globally. According to the World Bank data, MSW generation worldwide is estimated to be about 1.3 billion tonnes per year, and it is expected to reach 2.2 billion tonnes/year by year 2025 (World Bank, 2012). Typically, MSW contains approximately 30% w/w (weight/weight) food waste (FW) [1]. The FW is a fraction of the bio-waste, which contains left-over meals and discarded foods from the stages of production, processing, retailing, and consumption. The amount of FW is continuously increasing. In Europe, it was expected to increase from 89 million tonnes in 2006 to 126 million tonnes in 2020 [2]. The traditional disposal and treatment techniques for FW are landfilling and incineration, though these methods have remarkable disadvantages. Landfilling leads to various environmental concerns, such as leaching, greenhouse gas emission (i.e., methane), and odor release [3]. Incineration is energy demanding and unstable due to the high moisture content of the FW [4]. Proper waste management is crucial to minimize further environmental pollution. According to the European Union Directive 75/442/EEC, re-use and recycling are the most favored options of waste management after the up-front incentives for waste generation. The equivalent of FW in a household environment is kitchen waste (KW) [5], which may include vegetables, fruits, as well as cooked and processed food, and is characterized by a high moisture content and low pH [6]. KW contains large amounts of easily available organic matter such as carbohydrates, proteins, and lipids, which form a valuable substrate for fermentation processes.
Among the various options for the valorization and management of organic matter, such as KW, methanogenic fermentation is the most preferable utilization method, due to the high moisture content in the substrate [7]. One of the variants of this process is dark fermentation (DF), a low-cost technology that can efficiently treat FW, producing a variety of value-added products, such as volatile fatty acids (VFAs) [5]. VFAs are important intermediates produced in the acidogenesis stage when organic materials are processed by anaerobic digestion. VFAs have various applications; they are used in the production of biodiesel fuel [8], the synthesis of complex biopolymers—polihydroxyalkanoates (PHAs) [9], as well as the generation of electricity through microbial fuel cells [10]. Besides VFAs, in the DF process, hydrogen is also produced [11], which is recognized as an ideal, clean, and renewable energy, due to mainly water generated after its oxidative combustion [12].
There are various natural sources of mixed microbial communities that can serve as the inoculum for DF, such as compost, soil, animal manure, or leachate. However, a growing interest in anaerobic digested sludge (DS) is recently noted [13,14]. Using the mixed-culture microorganisms as the inoculum has numerous advantages, that is, it reduces the production costs because of operating in non-sterile conditions, enables continuous generation of products, and allows for a variety of carbonaceous substrates due to high microbial diversity [15]. To enhance DF and inhibit competition from methanogenic bacteria in the anaerobic bioreactor, the most common solution is a heat-shock treatment of microbial consortia [16].
So far, numerous efforts had been addressed to optimize the DF process for FW and to maximize the production of VFAs by controlling the common operating conditions in the batch anaerobic bioreactor, such as the pH, temperature, organic load, different pre-treatment methods, and so on [13,17,18]. However, in the semi-continuous and continuous DF processes, where the VFAs production yields are higher, the organic loading rate (OLR) and hydraulic retention time (HRT) are crucial operational parameters that influence the VFAs generation [19]. To ensure an efficient VFAs production, the OLR should be abundant enough to provide an adequate amount of daily carbon source to fermentative microorganisms. It seems that a high ORL should produce higher concentration of VFAs, but the operation of the bioreactor at too high an OLR is unstable [20]. The HRT has to be long enough to allow hydrolysis of the complex organic matter. On the other hand, an HRT that is too long reduces the quantity of manageable substrate per day [21].
The values of different OLRs and HRTs used in different DF processes are presented in Table 1. However, a wide range of examined OLRs and HRTs precludes quantitative comparison with literature data, but only makes it possible to analyze the trends in changing values. Lim et al. [20] studied the effect of the OLR on acidic fermentation of FW and DS in a semi-continuous system at an HRT of 8 d and pH 5.5. They observed that VFAs synthesis increased at higher OLR values. Gou et al. [22] searched for the possible relationship between the OLR and temperature by co-fermenting activated sludge and FW in a semi-continuous system. At higher temperature and at higher OLR values, these researchers achieved the highest VFAs production [22]. Scoma et al. [23] have observed that shorter HRTs turned into a lower accumulation of VFAs when fermenting in continuous mode dephenolized olive mill wastewater with acidogenic inoculum. The positive impact of increasing HRT on the DF process was also reported by Lim et al. [20], who studied the effect of different HRT values on DF using FW as a substrate and DS as an inoculum.

Table 1.
Values of OLRs and HRTs used in different DF processes.
Among a few studies on the effects of the OLR and HRT on the VFAs production in continuous or semi-continuous DF processes, only two research groups used FW together with DS as inoculum—namely, Jiang et al. [29], who fermented four-compound simulated FW in a batch and semi-continuous mode; and Lim et al. [20], who performed pre-fermentation using glucose to reach a steady state faster, and then fermented the KW and DS. Using, from the beginning, the KW as a substrate together with DS as inoculum and processing DF in a semi-continuous mode at various values of HRT and OLR has not been described before. Therefore, the main objective of this study was to determine the impact of the OLR and HRT on VFAs generation in the process of DF using a semi-continuous type anaerobic bioreactor fermenting KW and DS.
Dark fermentation, as a complex biochemical and biotechnological process, is highly dependent on the concerted action of many species of microorganisms. The dynamic metabolic balance between hydrolytic and acidogenic bacteria determines the stability of the process and affects the products of the synthesis process. The control of fermentation process parameters, including the OLR and HRT in semi-continuous processes, is a key to maintaining this balance. Therefore, an additional aim of this work was the characterization of the structure and evolution of the microbial community during the DF process performed in a semi-continuous system.
2. Materials and Methods
2.1. Substrate and Inoculum
KW from local households (Lodz, Poland) was used as a substrate in this study for the production of VFAs. This organic substrate was composed of vegetable waste—approx. 44% w/w, fruit waste—approx. 30% w/w, and other bio-waste (i.e., bread, coffee and tea grounds, cooked pasta)—approx. 25% w/w [30]. About 200 kg of fresh KW was collected from 70 households within 2–3 days and kept in the cold room at 4 °C. These wastes were than shredded in a grinder to get a particle size of less than 3 mm. Thereafter, the thoroughly mixed substrate was stored at −20 °C.
As the inoculum, DS from the Wastewater Treatment Plant in Lodz (Poland) was used in our research. Following the treatment technology in this plant, the thickened sludge is stabilized through methane fermentation under mesophilic conditions (35–38 °C), in four anaerobic digesters (10,000 m3, each) at an HRT of 20–26 days. Before initializing the DF process, the 4 L sample of DS brought from the treatment plant was heated at 70 °C for 30 min in order to deactivate non-speculating forms of bacteria, as it was performed in our previous study [31].
The characteristics of the KW and DS are presented in Table 2, where the first number in each column signifies an arithmetic mean from 3 independent measurements, whereas the second number quantifies the standard deviation.

Table 2.
Physicochemical parameters of DS (inoculum) and KW (substrate) used in the experiments.
2.2. Experimental Setup
The semi-continuous fermentation was carried out in a bioreactor with 8.0 L working volume. The bioreactor was equipped with a stirrer and a heating jacket. After adding KW, the bioreactor was purged with high purity nitrogen for 8–10 min to remove oxygen. The temperature and stirrer speed were kept at 37.0 ± 0.1 °C and 100 rpm, respectively. The process was carried out at a constant pH of 7.0 (±0.3), controlled with aqueous 10% w/w NaOH solution. During the fermentation process, the measurements of the pH were performed continuously using a pH electrode (InPro 3250/225/PT1000, Mettler Toledo, Switzerland). Based on the changing pH values during the fermentation process, NaOH was dosed automatically via a peristaltic pump (101 U/R, Watson Marlow, Falmouth, UK) connected to a pH regulator (KD7, Lumel, Poland) to keep a constant pH.
The volumetric ratio of DS/KW at the beginning of the process was 1:1; however, the amount of organic matter added during the fermentation was dependent on the OLR. Once a day, the appropriate amount of the processed medium was removed, and the same volume of fresh medium with KW was added to the bioreactor. Fermentation processes were carried out in 4 different configurations at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and HRTs of 5 and 10 days. The duration of the carried-out processes was 15 or 30 days, depending on the HRT. Table 3 lists the parameters of the performed processes.

Table 3.
Conditions of the performed DF processes.
2.3. Analyses
Before adding KW to the bioreactor, the fermentation medium, consisting of solid and liquid fractions, was sampled and analyzed every 24 h during the fermentation. The descriptions of all the apparatuses, instruments, and methodology used in the experiment are listed in Table 4. Each experiment on the fermentation process was performed in triplicate, and the arithmetic average was taken for data interpretation. The error of the performed experiments was less than 10%. The separation of liquid and solid fractions was carried out in a centrifuge. The concentration and the composition of VFAs in the liquid fraction were measured with a gas chromatograph. For the pH measurement, a pH electrode was used. The contents of carbon (C) and nitrogen (N) were determined in the solid fraction by the elemental analyzer. The total solids (TS) and the volatile solids (VS) in the solid fraction were quantified by a gravimetric method.

Table 4.
Types of analysis which undergo liquid and solid fractions of the fermentation mixture, together with instruments and methodology used.
In order to identify the groups of microorganisms responsible for the production of VFAs in the semi-continuous process, metagenomic analysis has been performed on the samples taken from a bioreactor at the beginning of the experiment and in a steady-state condition with the OLR of 5.0 gVS/(L × d) and HRT of 10 d. Microbial characteristics were determined using the Illumina platform described in an earlier publication [32]. The total genomic DNA was extracted from the liquid fraction of a fermentation mixture. Amplification of conserved bacterial 16SrRNA gene with the fragment covering V3 and V4 regions was done in triplicate. Obtained amplicons were purified, pooled in equimolar ratio, and indexed according to the Nextera indexing strategy by PCR (Illumina). Sample indexing allowed pooling of amplicons for the sequencing run and further extraction of the sample sequence reads from the large batch of sequencing results data. Sequences were grouped based on their taxonomic classification, and highly similar sequences were clustered into operational taxonomic units.
3. Results and Discussion
3.1. An Impact of OLR and HRT on VFAs Synthesis
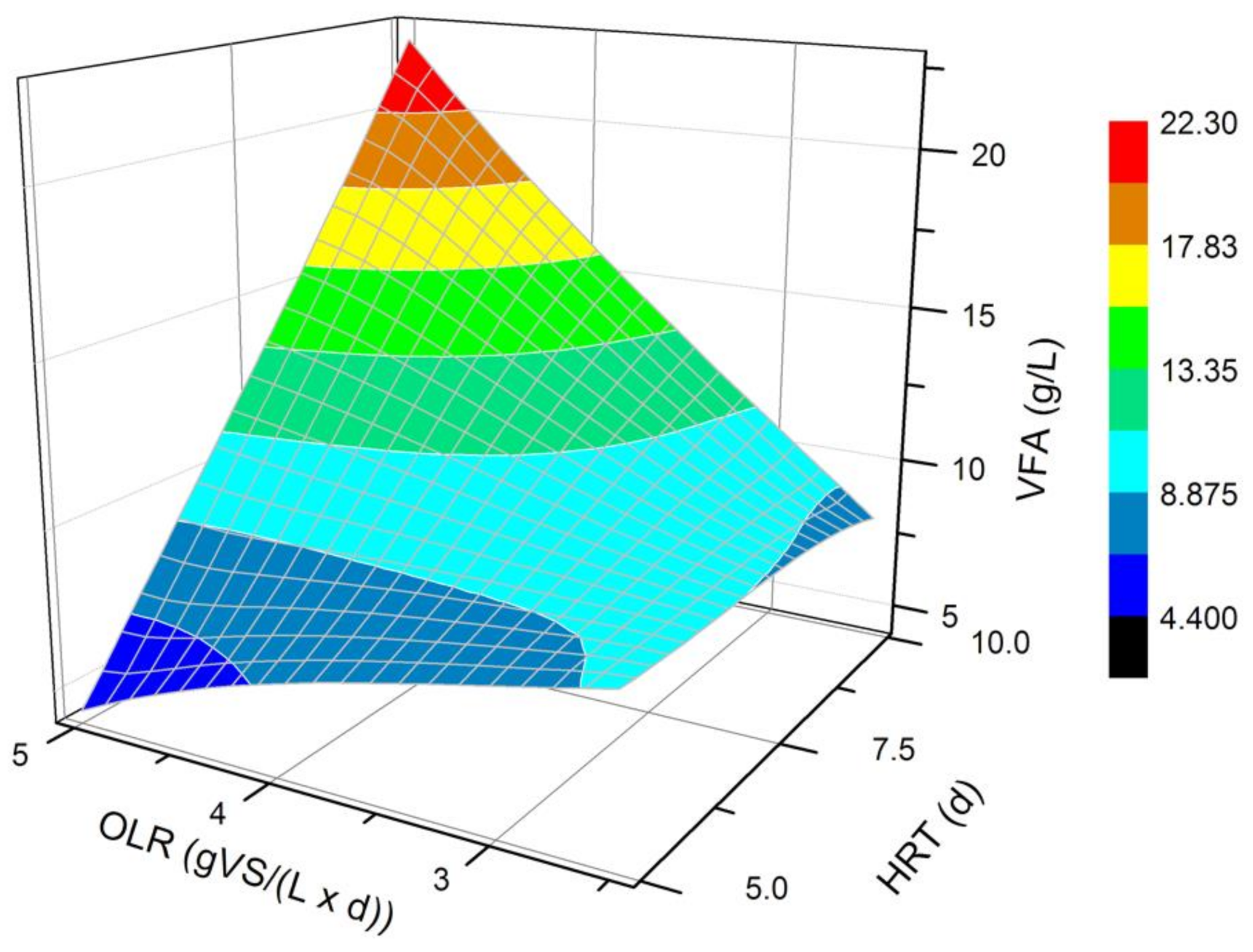
The impact of different OLRs (2.5 and 5.0 gVS/(L × d)) and HRTs (5 and 10 days) on the quantity and composition of the produced VFAs has been analyzed in each of the performed processes. The bioreactor pH was maintained at 7.0 ± 0.1 in all experimental runs. This pH value has been selected based on our own research [33] and available topical literature [27,34] confirming the highest VFAs yields in neutral pH. An increased production of VFAs in each of the processes was observed at the initial days of transient state. After that period, the fermentation turned into a steady state in all trials. The biological process was considered to be at a steady-state condition when the VFAs were detected at a similar level, and the bioreactor was operated during 3 HRTs. In the processes carried out at the HRT of 5 days, the change from transient to steady-state processing had already taken place on the 4th day of the process, regardless of the OLR value. The amount of VFAs in those bioreactors operated in the steady-state conditions varied between 8.2 and 10.1 g/L and between 3.9 and 4.7 g/L for the OLRs of 2.5 and 5.0 gVS/(L × d), respectively. In the processes run at 10 days HRT, a steady state appeared after 12 days of fermentation, both at the OLR of 2.5 and 5.0 gVS/(L × d). The amount of VFAs in those bioreactors in a steady state varied between 7.1 and 9.0 g/L and between 19.6 and 24.8 g/L for the OLRs of 2.5 and 5.0 gVS/(L × d), respectively. The subsequent discussion focuses on the results obtained from the production of VFAs during a steady-state condition.
At the OLR of 5.0 gVS/(L × d) and the HRT of 10 d, the VFAs concentration was nearly three times higher than at the OLR of 2.5 gVS/(L × d) and at the same HRT. In the processes where HRT was 5 d, the opposite relationship was noticed: at a lower OLR (2.5 gVS/(L × d)), two times more VFAs were formed than at the OLR of 5.0 gVS/(L × d). By comparing the effects of fermentation carried out at the same OLR of 5.0 gVS/(L × d), it was observed that longer retention time of the inoculum and substrate in the bioreactor resulted in higher production of VFAs. Characteristically, a five-fold increase was noticed in the amount of VFAs produced at the HRT of 10 days compared to the fermentation carried out at the HRT of 5 days.
According to the obtained data, the OLR of 5.0 gVS/(L × d) and the HRT of 10 d seem to be the most favorable for the production of VFAs in a semi-continuous system (Figure 1). This finding suggests that the daily dose of the substrate in the amount of 5.0 gVS/(L × d) corresponded with the inoculum volume in the bioreactor, which over 10 days was able to convert the supplied organic matter into high concentrations of VFAs. The OLR of 5.0 gVS/(L × d) and the HRT of 5 d was confirmed to be the least favorable for VFAs production. The reason for such an effect was probably that the HRT of the fermentation mixture in the bioreactor was too short in relation to the amount of delivered organic matter. Slowly reproducing microorganisms could have been washed away, leaving microbes with a higher growth rate in the fermentation medium.
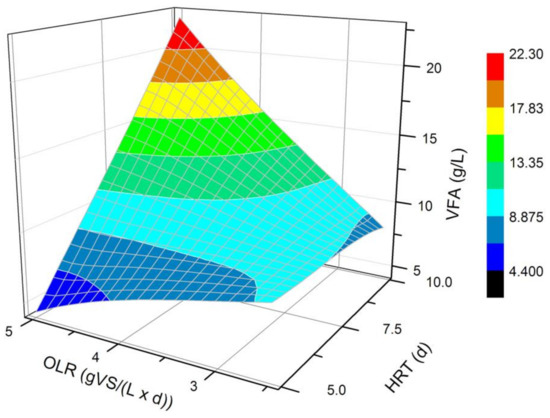
Figure 1.
Mean VFA concentration achieved in a steady state of the DF processes carried out at different OLRs (2.5 and 5 gVS/(L × d)) and HRTs (5 and 10 d).
Observing the effect of different OLR values analyzed in this study on DF carried out at a constant HRT of 10 d, it can be concluded that a higher concentration of the substrate led to a larger amount of produced VFAs. Lim et al. [20], who studied the effect of an OLR on acidic fermentation of FW and DS, have also observed that VFAs synthesis increased at higher OLR values, reaching a maximum concentration of approximately 14, 24, and 30 g/L at OLRs of 4.8, 8.6, and 12.5 gVS/(L × d), respectively. However, in their research, before initializing the fermentation process, the glucose-based synthetic medium was placed in the bioreactor with an OLR of 0.5 g glucose/(L × d) during the first 5 days, and then the FW was brought in with 5 g TS/(L × d). In the work of Jiang et al. [29], a similar result to our findings is described, but they used four-compound synthetic FW, fermented in a semi-continuous process at pH 6.0, 35 °C, and an HRT of 5 days [29]. An increase of the OLR from 4.9 to 10.7 gVS/(L × d) resulted in an increase of VFAs from approximately 13.0 to 21.0 g/L [29]. Further increase of the OLR from 10.7 to 15.5 gVS/(L × d) resulted in bioreactor instability. This fact was probably due to the high viscosity of the medium at high OLR values, which negatively affected the fermentation efficiency [35]. Gou et al. [22] co-fermented an activated sludge and FW mixed in a 2:1 mass ratio (in terms of TS) in a semi-continuous system at the OLR range 1–8.0 gVS/(L × d) and temperatures of 35 °C, 45 °C, and 55 °C. At higher temperature and at the OLR of 7–8.0 gVS/(L × d) they achieved VFAs production of 4.0 g/L, whereas during mesophilic fermentation, OLR had to be below 5.0 gVS/(L × d) to obtain VFAs production of 3–3.5 g/L [22]. To ensure the best conditions for VFAs synthesis in the bioreactor, an OLR should be high enough to provide an accurate amount of carbon source for efficient microbial metabolism. During mesophilic fermentation, an OLR higher than 5.0 gVS/(L × d) may, however, cause a slow increase in substrate viscosity, thus reducing mass and heat transfer, and consequently lowering the substrate conversion to VFAs. At the same moment, the HRT should be adequate to the amount of supplied organic matter, so that the DF process is maximally effective. Gou et al. [22] reached the best VFAs synthesis in a steady state at an HRT of 6.7 d.
Analyzing the impact of HRT on the carried out processes, it was observed in this study that longer HRT had a positive effect on the VFAs production at an OLR of 5.0 gVS/(L × d). For fermentations conducted at the OLR of 2.5 gVS/(L × d), the increase in the HRT did not affect the amount of formed VFAs. When performing the continuous fermentation process of dephenolized olive mill wastewater with acidogenic inoculum, Scoma et al. [23] observed that shorter HRTs (1 and 3 days) caused a lower accumulation of VFAs. The highest amounts of VFAs were found with HRTs of 7 d and 5 d (about 18.4 and 19.7 g COD/L, respectively). This finding is in line with our result. An intensification of the DF process and VFAs generation together with increasing HRT was also reported by Lim et al. [20]. These researchers studied the effect of different HRT values (4, 8, and 12 d) on DF using FW as a substrate and DS as an inoculum. The fermentation at mesophilic conditions was performed at pH 5.5 and OLR of 4.8 gVS/(L × d). Total VFAs concentration increased from 5.5 g/L at HRT of 4 d to 13 g/L at HRT of 8 d, and finally to 22 g/L at HRT of 12 d [20]. The conclusions of Lim et al. [20] are similar to those described in this paper, where the fermentation process conducted at HRT of 10 d and OLR of 5.0 gVS/(L × d) turned out to be the best for the synthesis of VFAs from KW. Dinsdale et al. [36] studied the effect of different HRT values on the efficiency of acidogenic (HRTs: 1, 2, 3, and 4 d) and methanogenic stages (HRTs: 10 and 13 d) of the anaerobic fermentation of fruit and vegetables waste and activated sludge without pH regulation. These authors noticed that in their acidogenic bioreactor, the VFAs concentration increased from 4.4 g/L to 6.1 g/L when HRT increased from 1 to 2 days. Low concentration of VFAs synthesized at a 1 d HRT could be due to the fact that at those parameters, the rate of microbial growth in the bioreactor was high, which caused elucidation of the slow-growing biomass. In addition, the high OLR of the bioreactor resulted in substrate inhibition. A similar relationship was observed in the process performed in the presented work at an OLR of 5.0 gVS/(L × d) and HRT of 5 d. The HRT should be long enough to allow decomposition of the complex organic matter, thus promoting subsequent acidogenesis of the resulting simple organic compounds. At the same time, longer HRT (10 d or more) may promote the development of methanogens at a certain pH range from 6.5 to 7.5 [17]. Dinsdale et al. [36] did not observe a visible improvement in VFAs concentration at the HRTs of 2 d (VFAs = 6.1 g/L) and 3 d (VFAs = 6.2 g/L). This observation could have been due to the fact that an HRT of 2 d and an OLR of 39 kgVS/(m3 × d) were the most favorable conditions for the VFAs synthesis in this set-up. The feeding regime of the bioreactor could also affect the acidogenesis, as feeding two times per day at a 2 d HRT (OLR of 39 kgVS/(m3 × d)) led to more VFAs than feeding once per day at an HRT of 3 d (OLR of 26 kgVS/(m3 × d)). To obtain optimal conditions for VFAs synthesis, the feeding regime should be tightened when increasing the HRT in order to minimize microorganism washing-out at the short HRTs [26].
3.2. Impact of OLR and HRT on VFAs Composition
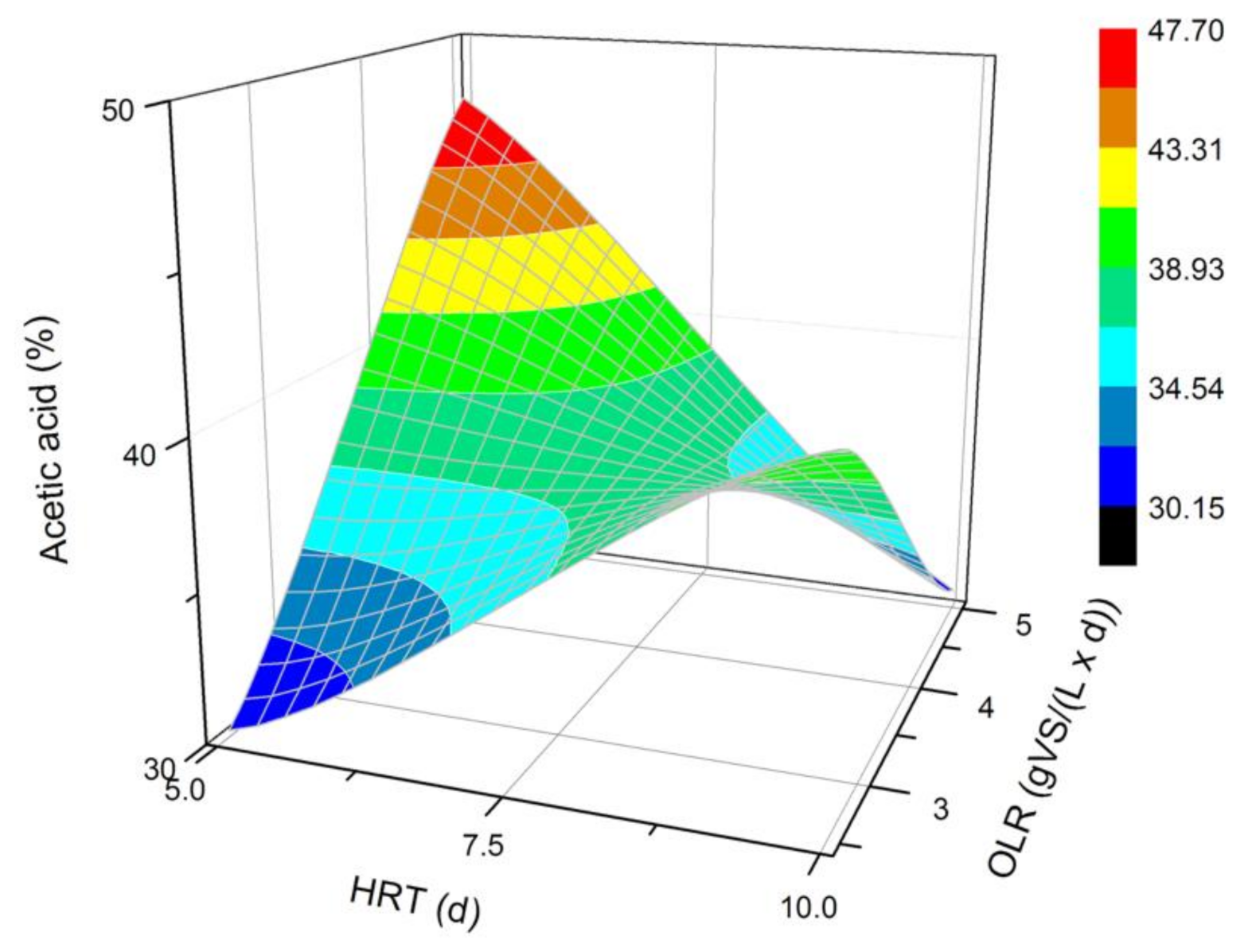
The VFAs composition analysis was performed for each fermentation process. Among all identified VFAs, the dominant ones were the acetic, butyric, propionic, caproic, and valeric acids. Additionally, isovaleric and isobutyric acids were produced, but their concentration did not exceed 2%. The average concentration of each VFA in a steady state is presented in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. Based on that data (Figure 2), it can be concluded that a two-fold increase in the HRT at the OLR of 2.5 gVS/(L × d) significantly improved the production of acetic acid. However, the increase in HRT at the OLR of 5.0 gVS/(L × d) caused a weaker synthesis of the acetate. For this reason, it is difficult to spot clear tendencies dependent on the HRT or OLR. It seems that for the examined type of DF, more acetic acid was produced at a higher OLR value for the shorter 5 d HRT. A longer HRT (10 d) shifted the microbial activity toward generating more acetate at a lower OLR. The biggest difference between acetic acid content (47.7% vs. 30.2%) was noticed at the HRT of 5 d and different OLRs.
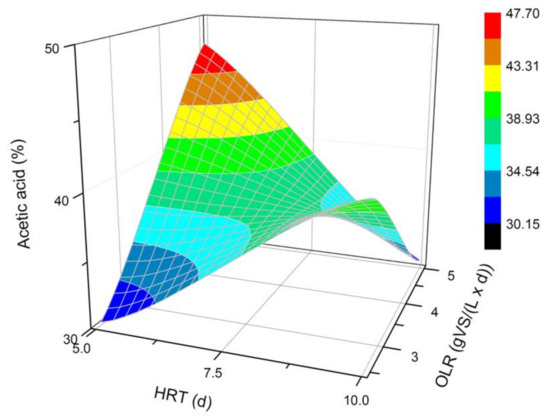
Figure 2.
Changes in the content of acetic acid produced in a steady state during the DF process at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and HRTs of 5 d and 10 d.
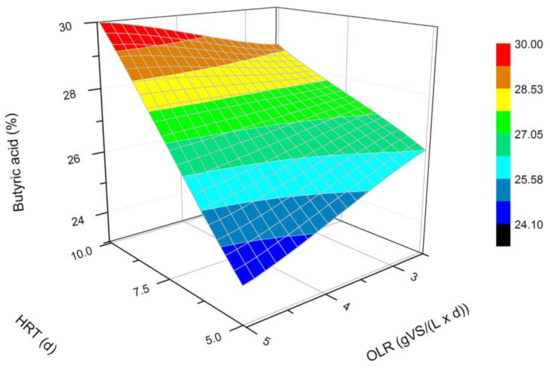
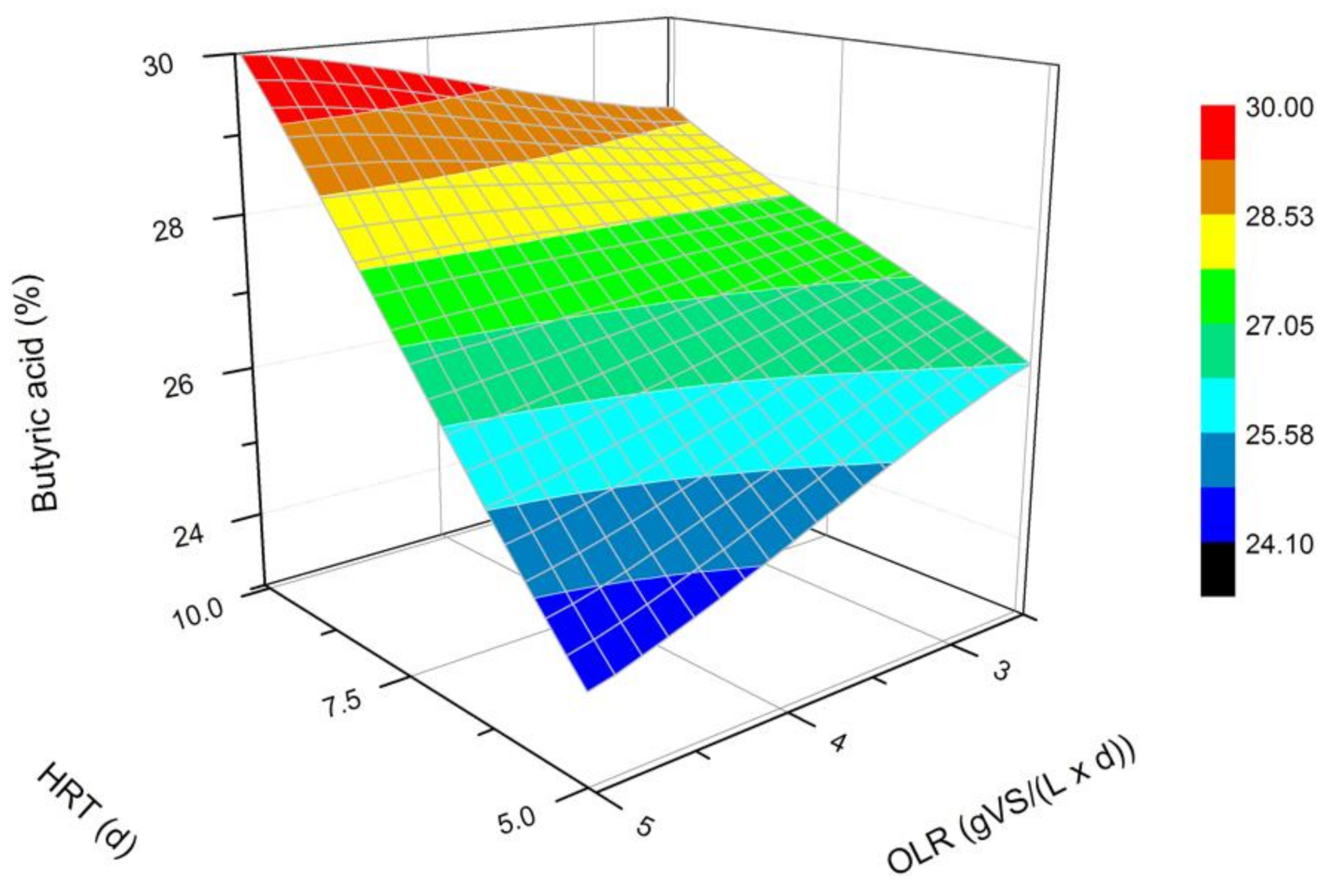
Figure 3.
Changes in the content of butyric acid produced in a steady state during the DF process at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and the HRTs of 5 d and 10 d.
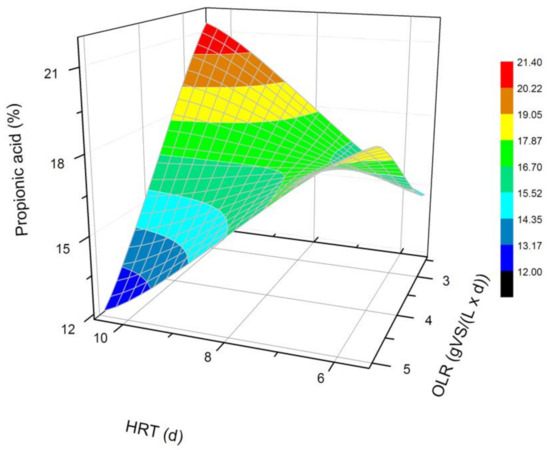
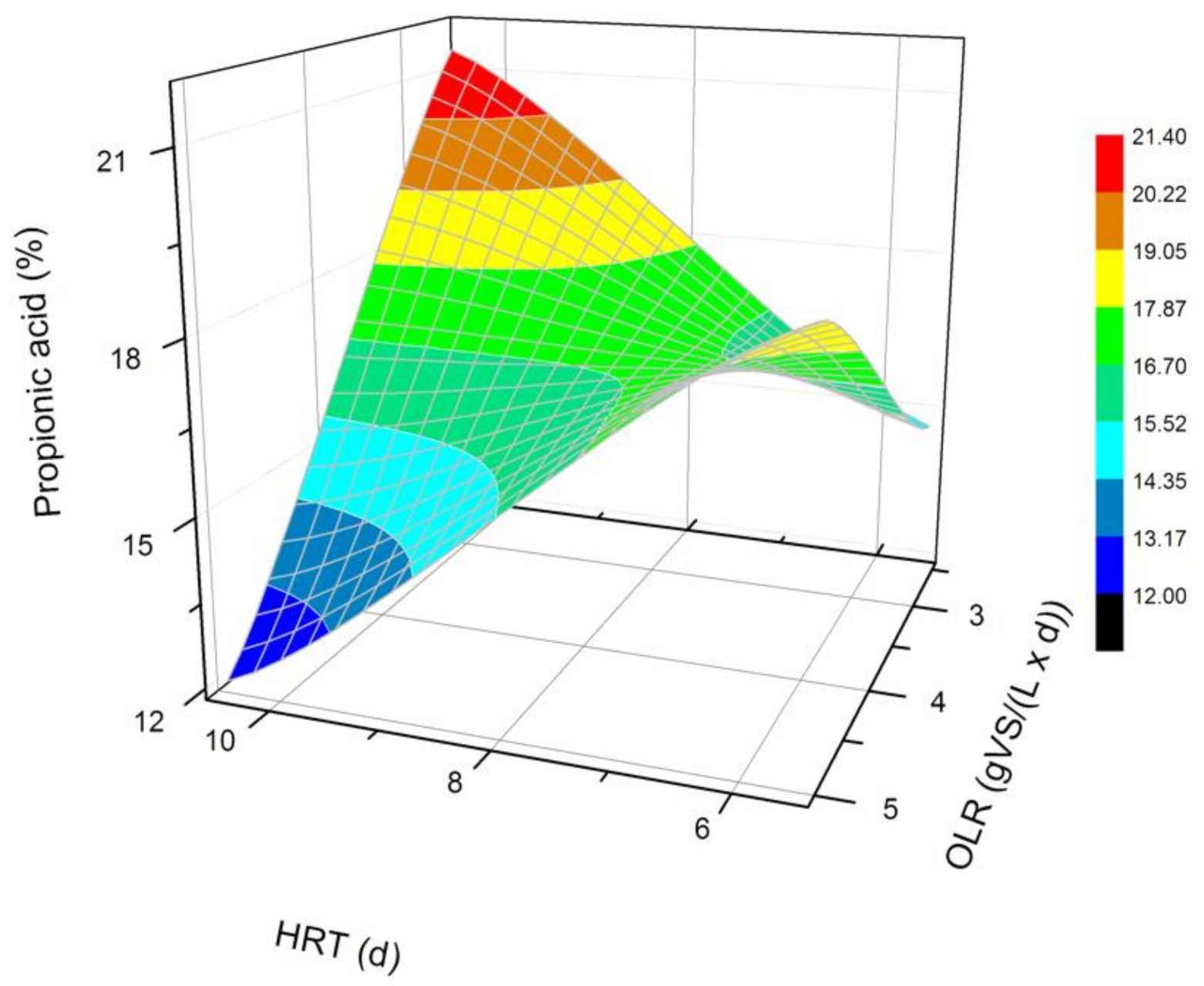
Figure 4.
Changes in the content of propionic acid produced in a steady state during the DF process at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and the HRTs of 5 d and 10 d.
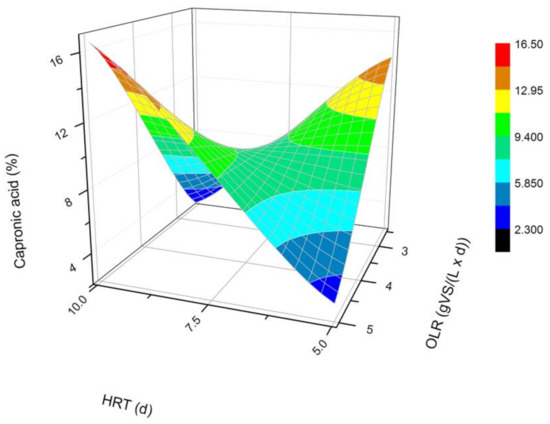
Figure 5.
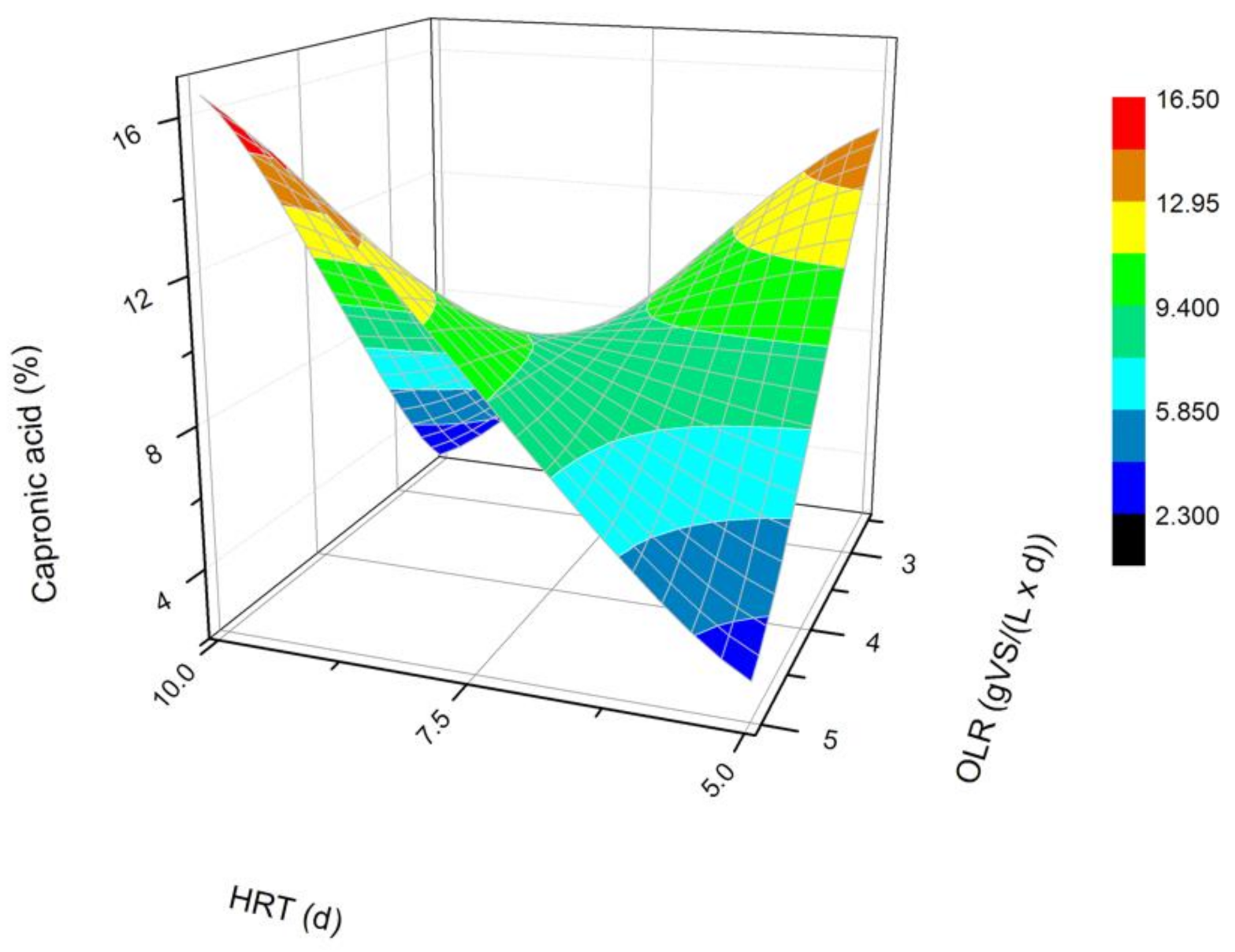
Changes in the content of caproic acid produced in a steady state during the DF process at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and the HRTs of 5 d and 10 d.
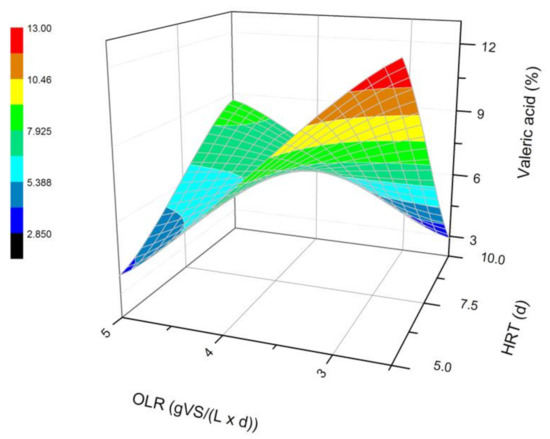
Figure 6.
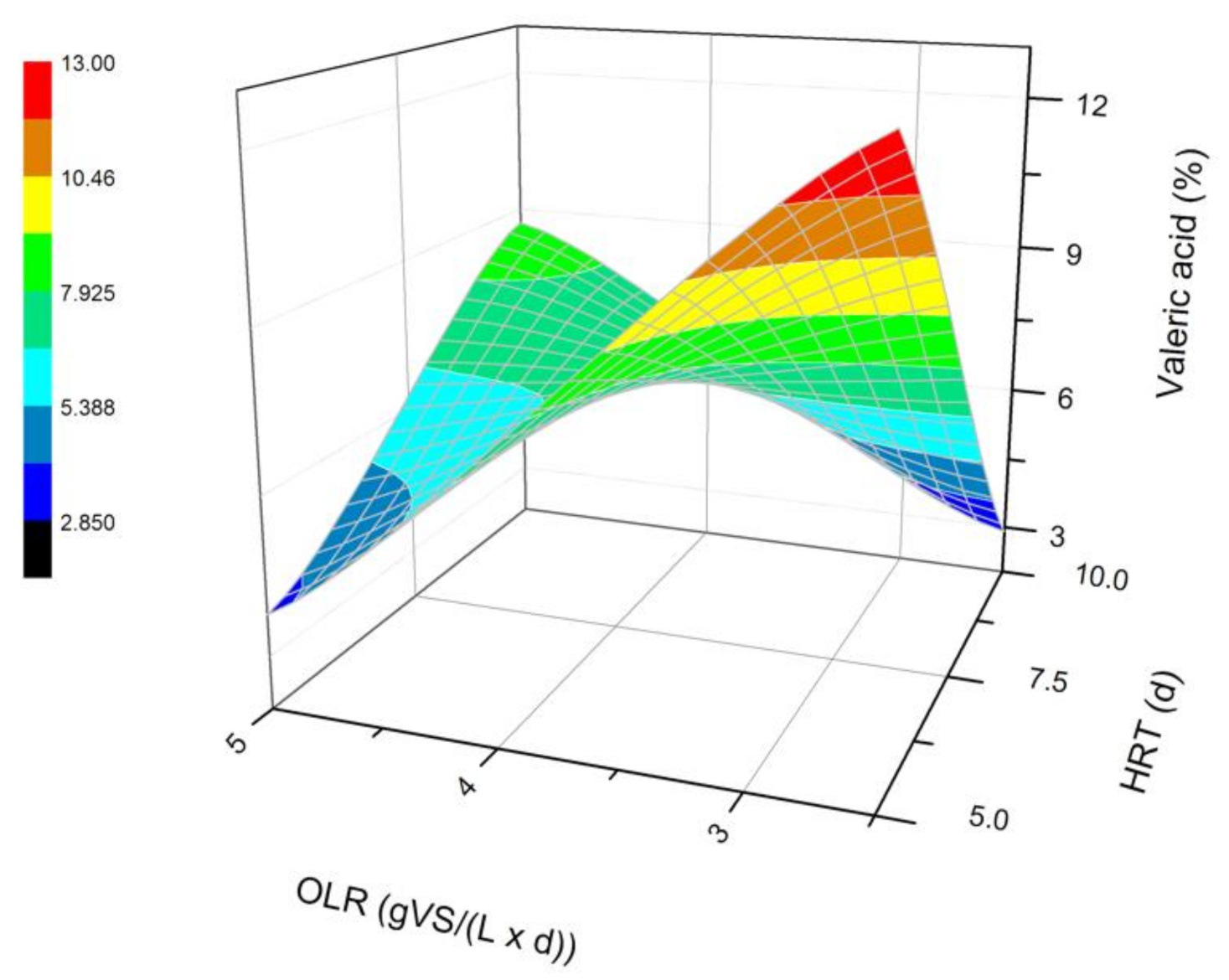
Changes in the content of valeric acid produced in a steady state during the DF process at the OLRs of 2.5 gVS/(L × d) and 5.0 gVS/(L × d) and the HRTs of 5 d and 10 d.
The next most common VFAs identified in the bioreactors were butyric and propionic acids (Figure 3 and Figure 4). In each of the processes, it was observed that the average steady-state butyric acid concentration was higher than that of the propionic acid. The obtained results suggest also that higher OLR and HRT values were favorable for butyric acid production during DF. Additionally, the OLR of 5 gVS/(L × d) and the HRT of 5 d seem to be optimal for the synthesis of both acetic and butyric acid. In the process performed at the OLR of 2.5 gVS/(L × d) and the HRT of 10 d, the content of propionic acid compared to all VFAs was the highest, and reached approximately 21.4%. The lowest concentration of propionate appeared at the same HRT but at a higher OLR value. The caproic acid and valeric acid were identified in all fermentation processes (Figure 5 and Figure 6). The average amount of both acids in a steady-state condition was the highest at the OLR of 2.5 gVS/(L × d) and the HRT of 5 d, as well as at the OLR of 5 gVS/(L × d) and the HRT of 10 d, counting, respectively, for caproic acid of 14% and 16.5%, and for valeric acid of 13% and 8.7%. The lowest content of both acids appeared at the OLR of 2.5 gVS/(L × d) and the HRT of 10 d: approximately 2.3% for caproic acid and approximately 2.9% for valeric acid. Based on that observation, it can be assumed that a two-fold increase in the OLR and HRT did not cause a significant increase in the caproic acid concentration, whereas it contributed to a decrease in the valeric acid content. Performing the DF process at the OLR of 2.5 gVS/(L × d) and the HRT of 10 d was not beneficial for the production of either caproic or valeric acid.
The conclusions presented above are consistent with the results of qualitative analysis obtained by Lim et al. [20], who showed that acetic acid was dominant in the dark fermentation mixture of FW, and its concentration increased with an increasing OLR at a constant HRT value (8 d). On the contrary, the remaining VFAs (propionic, butyric, and valeric acids) concentrations decreased with an increasing OLR at the same HRT value. Jiang et al. [29] described as well that the acetic acid dominated at a higher OLR. Wijekoon et al. [37] examined the impact of three different OLR values (5, 8, and 12 COD/m3/d) on the hydrolysis and acidogenesis of molasses synthetic sewage. They observed that while increasing the OLR, the intensification of VFAs synthesis occurred. Among the identified VFAs, the butyric and acetic acids dominated in all the bioreactors. Moreover, for all the imposed OLR values, it was found that the concentration of propionic acid was the lowest, compared to acetic and butyric acids [37]. These results are similar to the ones obtained in our experiments, where the most common VFAs were in the following order: acetate > butyrate > propionate.
The aspect of biochemical pathways used by microorganisms is important in the synthesis of acetic, butyric, and propionic acids. The pathway for butyric acid fermentation is characterized by an increased synthesis of acetic and butyric acids, while acetic and propionic acids are produced in large quantities in the propionic acid fermentation pathway. It is possible that the synthesis pathways of both of these acids compete with each other [38]. In the processes where the substrate was simple sugar (glucose), for example, the butyric acid was found at high concentrations under uncontrolled pH [39,40]. This could imply that in the case of easily digestible substrates, fermentation did not require multi-stage hydrolysis processes, and the metabolism was directed to the butyric acid pathway. However, when the reaction was conducted using a substrate that was a mixture of complex organic compounds such as KW, the microorganisms preferred to change their metabolism to, for example, the propionic acid pathway. This observation can be confirmed by, among other things, the high concentrations of propionic and acetic acids identified in processes without pH regulation carried out using FW and activated sludge [41,42]. The type of metabolic pathway can be additionally dependent on the amount of a substrate supplied to the bioreactor. The results of Khan et al. [34] support this hypothesis, as they showed that the isobutyric acid concentration was the highest at maximum OLR load (715 mg CODfeed), indicating that the butyric-acid-type fermentation takes place at higher OLRs.
The effect of the HRT on the VFAs profile has also been depicted by Cavinato et al. [28], who processed manure and corn silage during mesophilic fermentation at three different HRT values, namely 2, 4, and 6 d. Acetic, butyric, and propionic acids were the most common VFAs in all performed fermentations, regardless of the HRT value. In addition, Cavinato et al. [28] recognized valeric and caproic acids in significant amounts in all processes. The highest concentration of valeric acid was generated at an HRT of 4 d and caproic acid at the HRT of 2 d [28]. A further increase in the HRT caused a decrease in the content of both acids [28]. Similar conclusions can be formed based on the data from experiments where a doubled increase of the HRT at OLR of 2.5 gVS/(L × d) contributed to a decrease in the amount of both acids in the mixture. On the contrary, in fermentations carried out at the OLR of 5 gVS/(L × d), changing the HRT from 5 d to 10 d consequently showed an increased content of valeric and caproic acids. Cavinato et al. [28] did not observe this phenomenon. The difference may be due to the higher range of OLR values at which Cavinato et al. [28] conducted their processes, namely 11.9–35.4 gVS/(L × d).
3.3. Metagenomic Analysis
The metagenomic analyses of samples taken at the beginning and in a steady state of the DF process at the OLR of 5.0 gVS/(L × d) and the HRT of 10 d were performed for identification of the main microbial communities responsible for the process of fermentation. The process performed at these OLR and HRT values was selected based on the most effective VFAs production of all examined trials (see Section 3.1). The results of the microbial analysis are presented in Figure 7, which illustrates the most abundant phyla of microorganisms found in both samples.
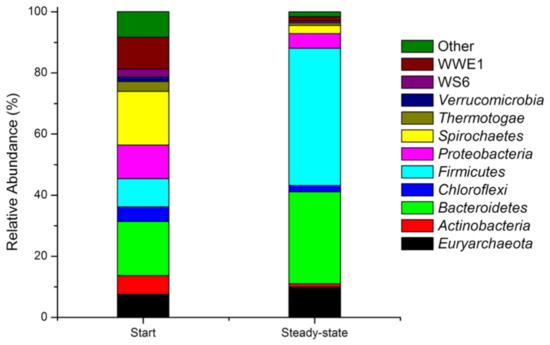
Figure 7.
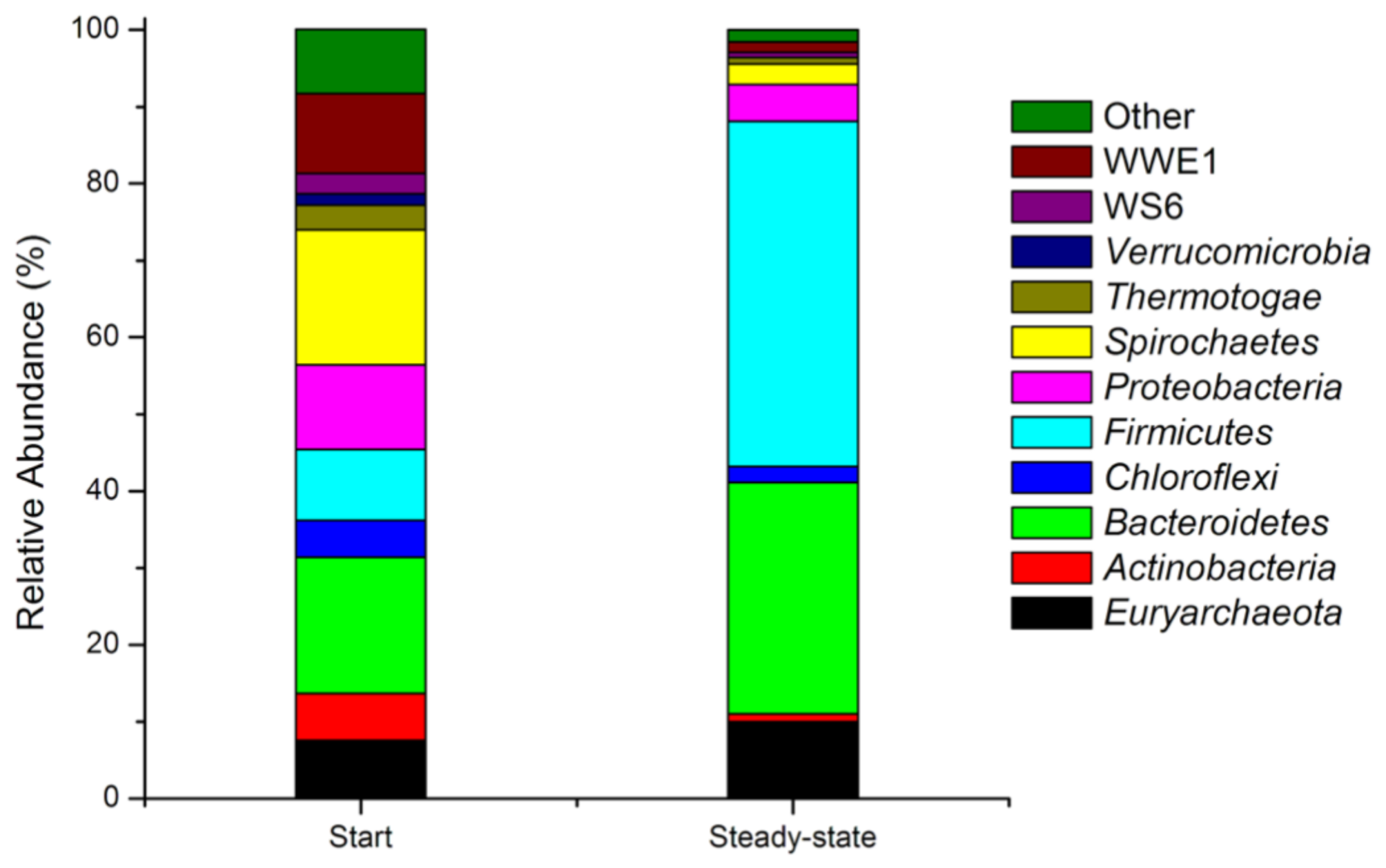
Phyla of microorganisms identified at the beginning and in a steady state of the dark fermentation conducted in a semi-continuous system at the OLR of 5 gVS/(L × d) and the HRT of 10 d.
The most common phyla identified at the beginning of the performed fermentation were Bacteroidetes (17.7%), Spirochaetes (17.6%), Proteobacteria (11.0%), WWE1 (10.4%), and Firmicutes (9.2%). In addition, the following phyla were present in the fermentation mixture: Euryarcheota (7.6%), Actinobacteria (6.1%), Chloroflexi (4.8%), Thermotogae (3.2%), Verrucomicrobia (1.5%), and WS6 (2.6%). The remaining phyla forming the group “Other” (8.4%) cluster these microorganisms that are present at abundancy lower than 1%. In a steady state, the profile of microorganisms has changed significantly. The number of microorganisms belonging to Firmicutes, Bacteroidetes, and Euryarcheota increased noticeably, constituting 44.9%, 30.1%, and 10.0%, respectively. The loads of other phyla decreased, including Actinobacteria (1.0%), Chloroflexi (2.1%), Proteobacteria (4.8%), Spirochaetes (2.7%), WWE1 (1.3%), WS6 (0.7%), and Thermotogae (0.8%). Phylum Verrucomicrobia was not identified in a steady state, and the group “Other” accounted for 1.6%.
Based on metagenomic analysis, it was also possible to determine the class, family, order, and in some cases the genus of microorganisms identified in both samples. A summary of the results is presented in Table 5, which portrays microorganisms identified in at least one of the samples at a minimum level of 1% abundance. The discussion below focuses, however, on those microbial groups for which the relative abundance increased in a steady state, as their presence seems to be crucial in the VFA generation process.

Table 5.
The main types of microorganisms identified in samples taken at the beginning and in a steady state of DF, carried out in a semi-continuous system at the OLR of 5 gVS/(L × d) and the HRT of 5 d.
The significant abundance of Bacteroidetes and Firmicutes types has already been reported in numerous publications with reference to fermentation processes of agricultural residues [43] or FW [25,44,45]. In experiments by Gaby et al. [45], when conducting mesophilic fermentations, the Firmicutes represented on average about 60% and Bacteroidetes about 20% of all identified bacteria in each bioreactor. Sundberg et al. [43] identified Firmicutes with the occurrence of relative sequences of approximately 70% and Bacteroidetes sequences of 14%, when co-digesting various combinations of wastes from slaughterhouses, restaurants, households, and so on. Magdalena et al. [46], when fermenting microalgae biomass with anaerobic sludge, noted that the relative abundance of two main phyla, Firmicutes (74%) and Bacteroidetes (20%), were found to be the best combination to promote organic matter conversion into VFAs. This confirms the observation from the present research, as the analyzed bioreactor working at the OLR of 5 gVS/(L × d) and the HRT of 10 d had the highest amount of detected VFAs.
In this research, the Bacteroidetes type was represented by the Bacteroidaceae, Porphyromonadaceae, and Prevotellaceae families in a steady state of the process. Among them, the largest group was the Bacteroides genus (15.8%), belonging to the Bacteroidaceae, which was the most commonly identified genus of all found in a steady-state process (Table 4). Microorganisms belonging to Bacteroides have been isolated from various environments, and most of them are able to produce acetic acid, propionic acid, formic acid, and butyric acid in the fermentation process [47]. In the study of Zhang et al. [48], the Bacteroides dominated the process of co-fermentation of FW and sewage sludge, constituting 32.7% of abundance. Mainly, this group was responsible for VFAs production [48]. The Porphyromonadaceae was the dominant bacteria family among the Bacteroidetes type in the continuous acid fermentation process described by Goux et al. [44]. In the presented study, this group was the second largest family in the Bacteroidia class (8.3% abundance). Bacteria from the Porphyromonadaceae family can produce VFAs from proteins [49] or carbohydrates, including acetate and propionate [50]. The Prevotella genus, which occurred in the performed DF with 3.4% abundance, are able to break down hemicellulose, starch, and pectin [51].
In the experiments carried out in this study, two main classes were identified within the type of Firmicutes: Bacilli and Clostridia. It is worth mentioning that at the beginning of DF, none of them accounted for a load of more than 1.0% abundance. The Bacilli class, represented by three types of bacteria (Enterococcus, Lactobacillus, Streptococcus) was less diverse than the Clostridia class, represented by 8 types (Blautia, Caprococcus, Lachnospira, Ruminococcus, Megamonas, and 3 unidentified) (Table 4). Among Bacilli, the most abundant genus was Enterococcus, which accounted for 13.8% of all identified microorganisms in a steady state. Other types, Streptococcus and Lactobacillus, constituted less, 2.0% and 0.1%, respectively. All of these types belong to lactic-acid-producing bacteria—LAB [52]. The lactate production is generally considered harmful to the DF process because it is a deviation from the main acetyl-CoA pathway. The LAB activity may shift the metabolism from the acetate-butyrate to the ethanol-lactate pathway [52]. Nevertheless, the role of LAB in VFAs generation is still not clear. For example, Yun et al. [53], when processing molasses wastewater, found that total VFAs and acetate, butyrate, and propionate concentrations were positively correlated with the LAB such as Bacillales, Sporolactobacillus, and Lactobacillus. A similar situation could have happened in the performed study, as the amount of LAB rose in a steady state when VFAs production was intensified. All bacteria representing the Clostridia class appeared during the fermentation, as at the starting point of the process, their abundance was approximately 2%. The most abundant steady-state bacterial families identified among the Clostridia class were Lachnospiraceae, Ruminococcaceae, and Veillonellaceae. Goux et al. [44], conducting mesophilic fermentation of sugar beet waste, described the replacement of several dominant types of Clostridia by Lachnospiraceae and Ruminococcaceae families along the process. The same situation as in the performed study, Lachnospiraceae were the most plentiful group among Clostridia found by Goux et al. [44]. Additionally, Palomo-Briones et al. [54] showed that low HRT values maintained in the DF favored the emergence of a. bacterial community dominated by the Clostridiaceae-Lachnospiraceae-Enterobacteriaceae families, forming a consortium of the so-called hydrogen-producing bacteria (HPB). Moreover, studies by the same authors have shown that maintaining short HRT can prevent the development of lactate-producing bacteria in the bioreactor [54]. The authors confirmed that the HRT was an effective factor that can shape DF towards two different pathways: the lactate-type fermentation or acetate-formate-ethanol-type fermentation. In the process analyzed in this paper, the HRT was 10 d, which may explain the presence of LAB in the bioreactor. The Veillonellaceae family was extensively described by Moreno-Andrade et al. [55], who studied the effect of HRT on the mesophilic fermentation of FW. They showed that for each tested HRT value, the Veillonellaceae was the dominant bacterial community. Microorganisms belonging to the Veillonellaceae family are absolute anaerobes and occur in habitats such as rivers, lakes, and intestines of vertebrates. The Veillonellaceae family can contribute to an increase in the production of acetic and propionic acids using readily biodegradable substrates (e.g., glucose) and/or lactic acid [56].
Among the phylum Euryarcheota, three genera were recognized: Methanosphaera, Methanosarcina, and Methanosaeta. At the beginning of the process, Methanosaeta was the only identified genus of archebacteria (5.8%), but in a steady state its number decreased in favor of Methanosphaera (7.2%) and Mehanosarcina (1.2%) representatives. The presence of archeons in the bioreactor could suggest that the DS heating at the beginning of the process was not effective enough. Nevertheless, the development of methanogenic bacteria could have been influenced as well by the high HRT of the process (10 d), which promotes the metabolic switch from acidogenesis to methanogenesis [57]. Archeons representing the Methanosarcina genus require higher acetic acid concentrations, and these are characterized by a higher substrate processing rate and lower sensitivity to reduced pH as compared to Methanosaeta. Such features may explain the presence of Methanosaeta at the beginning of the process, where the acetate content was low, and the predominance of Methanosarcina when the acetic acid concentration was higher. In addition, the members of the genus Methanosphaera have one of the most restricted energy metabolisms of all methanogens, as they are dependent on acetate as their main carbon source [48]. As the acetate was the most frequently generated VFA, it could cause higher amount of Methanosphaera in a steady-state condition.
Among the bacterial communities, whose presence was also quite strongly outlined in the DF of FW, the topical literature lists the following types: Spirochaetes, Chloroflexi [25], and WWE1 [45]. All three types also appeared in the samples analyzed in this study; however, their abundance was higher at the beginning of dark fermentation and decreased when a steady-state condition was attained. The reason for their presence at a start of the fermentation could be that all three types are detectable in wastewater treatment plants [58,59], which is where our inoculum come from.
4. Conclusions
In the presented experiments, the impact of the OLR (2.5 and 5.0 gVS/(L × d)) and the HRT (5 and 10 d) on the concentration and composition of VFAs produced in DF of KW in a semi-continuous system using anaerobic mixed microbial consortia has been investigated. The highest VFAs concentration (an average of 22.3 g/L) was observed in a steady-state condition at the OLR of 5.0 gVS/(L × d) and the HRT of 10 days. This suggests that the daily dose of the substrate in the amount of 5.0 gVS/(L × d) corresponded with the inoculum volume in the bioreactor, which over 10 days was able to effectively convert the supplied organic matter into VFAs. It was noticed that longer retention time of the inoculum and substrate in the bioreactor caused higher production of VFAs. The OLR of 5.0 gVS/(L × d) and the HRT of 5 d were confirmed to be the least favorable for the VFAs production. Additionally, higher concentration of the substrate led to larger amount of produced VFAs. The main products of DF were acetic (35.2–47.7%) and butyric acids (24.1–30.0%). The third most common VFA was the propionic acid (12.0–21.4%). Significant amounts of caproic acid (2.3–16.5%) and valeric acid (2.9–13%) were found as well in the product obtained in the steady-state conditions. It was found that at the OLR 5 gVS/(L × d) and HRT 10 d, the process of dark fermentation in this semi-continuous-operated bioreactor was highly intensive, which was confirmed by the dominant concentration of the Firmicutes and Bacteroidetes types in the steady-state conditions. Together, those two types of bacteria constituted 75% of all identified microorganisms in the steady state, while at the beginning of the fermentation process, the relative abundance of Firmicutes and Bacteroidetes attained 26.9%. Among the type of Firmicutes, two main classes were identified, which are the Bacilli and Clostridia. The Bacteroidetes were represented by one class, namely the Bacteroidia. The abundance of other phyla of anaerobic mixed microbial consortia originated from DS, including Actinobacteria, Chloroflexi, Proteobacteria, Spirochaetes, WWE1, WS6, and Thermotogae, and significantly decreased in the steady-state condition.
Author Contributions
Conceptualization, J.S., R.S., L.K., and S.L.; methodology, J.S., R.S., L.K., and S.L; validation, J.S., R.S., L.K., and S.L; formal analysis, J.S. and R.S.; investigation, J.S. and R.S.; writing, review, and editing, J.S., R.S., L.K., and S.L; supervision, R.S., L.K., and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was done within the statutory fund of Lodz Technical University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| MSW | municipal solid waste |
| FW | food waste |
| KW | kitchen waste |
| DF | dark fermentation process |
| DS | digested sludge |
| VFAs | volatile fatty acids (g/L) |
| OLR | organic loading rate (gVS/(L × d)) |
| HRT | hydraulic retention time (d) |
| TS | total solids (gTS/L), |
| TVS/VSS/VS | total volatile solids/volatile suspended solids/volatile solids (gTVS/L or gVSS/L or gVS/L) |
| C | total carbon content (% w/w) |
| N | total nitrogen content (% w/w) |
References
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-economic analysis of a novel bioprocess combining solid state fermentation and dark fermentation for H2 production from food waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef]
- Monier, V.; Shailendra, M.; Escalon, V.; O’Connor, C.; Gibon, T.; Anderson, G.; Hortense, M.; Reisinger, H. Preparatory Study on Food Waste across EU 27. European Commission (DG ENV) Directorate C-Industry. Final Report. BIO Intelligence Service: Paris, France, 2010; Volume 33. [Google Scholar]
- Capson-Tojo, G.; Rouez, M.; Crest, M.; Steyer, J.P.; Delgenès, J.P.; Escudié, R. Food waste valorization via anaerobic processes: A review. Rev. Environ. Sci. Biotechnol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Bench, M.L.; Woodard, R.; Harder, M.K.; Stantzos, N. Waste minimisation: Home digestion trials of biodegradable waste. Resour. Conserv. Recycl. 2005, 45, 84–94. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.J.; Kang, J.W. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate production from fermented volatile fatty acids: Effect of pH and feeding regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Kim, H.W.; Kim, M.S.; Shin, H.S. Sewage sludge addition to food waste synergistically enhances hydrogen fermentation performance. Bioresour. Technol. 2011, 102, 8501–8506. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.M.; Zeng, G.M.; Zhou, Y. Effective hydrogen production using waste sludge and its filtrate. Energy 2010, 35, 3557–3562. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Principle and application of different pretreatment methods for enriching hydrogen-producing bacteria from mixed cultures. Int. J. Hydrogen Energy 2017, 42, 4804–4823. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation (review). Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Temudo, M.F.; Muyzer, G.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Diversity of microbial communities in open mixed culture fermentations: Impact of the pH and carbon source. Appl. Microbiol. Biotechnol. 2008, 80, 1121–1130. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. An experimental study on fermentative H2 production from food waste as affected by pH. Waste Manag. 2014, 34, 1510–1519. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Liberti, F.; Pistolesi, V.; Mouftahi, M.; Hidouri, N.; Bartocci, P.; Massoli, S.; Zampilli, M.; Fantozzi, F. An incubation system to enhance biogas and methane production: A case study of an existing biogas plant in Umbria, Italy. Processes 2019, 7, 925. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Yu, H.Q. Effect of HRT on mesophilic acidogenesis of dairy wastewater. J. Environ. Eng. 2000, 126, 1145–1149. [Google Scholar] [CrossRef]
- Gou, C.; Yang, Z.; Huang, J.; Wang, H.; Xu, H.; Wang, L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Scoma, A.; Bertin, L.; Fava, F. Effect of hydraulic retention time on biohydrogen and volatile fatty acids production during acidogenic digestion of dephenolized olive mill wastewaters. Biomass Bioenergy 2013, 48, 51–58. [Google Scholar] [CrossRef]
- Paudel, S.; Kang, Y.; Yoo, Y.S.; Seo, G.T. Effect of volumetric organic loading rate (OLR) on H2 and CH4 production by two-stage anaerobic co-digestion of food waste and brown water. Waste Manag. 2017, 61, 484–493. [Google Scholar] [CrossRef]
- Li, Q.; Yuwen, C.; Cheng, X.; Yang, X.; Chen, R.; Wang, X.C. Responses of microbial capacity and community on the performance of mesophilic co-digestion of food waste and waste activated sludge in a high-frequency feeding CSTR. Bioresour. Technol. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Romero Aguilar, M.A.; Fdez-Güelfo, L.A.; Álvarez-Gallego, C.J.; Romero García, L.I. Effect of HRT on hydrogen production and organic matter solubilization in acidogenic anaerobic digestion of OFMSW. Chem. Eng. J. 2013, 219, 443–449. [Google Scholar] [CrossRef]
- Hong, C.; Haiyun, W. Optimization of volatile fatty acid production with co-substrate of food wastes and dewatered excess sludge using response surface methodology. Bioresour. Technol. 2010, 101, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, J.; Krzystek, L. Changes in the morphological composition of municipal solid waste in the city of Łódź in 1995–2011, with particular emphasis on the organic fraction. Inżynieria I. Apar. Chem. 2012, 51, 128–130. (In Polish) [Google Scholar]
- Slezak, R.; Grzelak, J.; Krzystek, L.; Ledakowicz, S. Influence of initial pH on the production of volatile fatty acids and hydrogen during dark fermentation of kitchen waste. Environ. Technol. 2020, 1–10. (In press) [CrossRef]
- Slezak, R.; Grzelak, J.; Krzystek, L.; Ledakowicz, S. The effect of initial organic load of the kitchen waste on the production of VFA and H 2 in dark fermentation. Waste Manag. 2017, 68, 610–617. [Google Scholar] [CrossRef]
- Grzelak, J.; Ślęzak, R.; Krzystek, L.; Ledakowicz, S. Effect of pH on the production of volatile fatty acids in dark fermentation process of organic waste. Ecol. Chem. Eng. S 2018, 25, 295–306. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Gomez Almendros, M.; Rousset, R.; Boivineau, S.; Bouillon, P.A. Enzymatic hydrolysis at high dry matter content: The influence of the substrates’ physical properties and of loading strategies on mixing and energetic consumption. Bioresour. Technol. 2018, 250, 191–196. [Google Scholar] [CrossRef]
- Dinsdale, R.M.; Premier, G.C.; Hawkes, F.R.; Hawkes, D.L. Two-stage anaerobic co-digestion of waste activated sludge and fruit/vegetable waste using inclined tubular digesters. Bioresour. Technol. 2000, 72, 159–168. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Leite, J.A.C.; Fernandes, B.S.; Pozzi, E.; Barboza, M.; Zaiat, M. Application of an anaerobic packed-bed bioreactor for the production of hydrogen and organic acids. Int. J. Hydrogen Energy 2008, 33, 579–586. [Google Scholar] [CrossRef]
- Lu, Y.; Slater, F.R.; Mohd-Zaki, Z.; Pratt, S.; Batstone, D.J. Impact of operating history on mixed culture fermentation microbial ecology and product mixture. Water Sci. Technol. 2011, 64, 760–765. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Zhou, Q.; Song, Z. The effect of pH on anaerobic fermentation of primary sludge at room temperature. J. Hazard. Mater. 2009, 172, 196–201. [Google Scholar] [CrossRef]
- Sundberg, C.; Al-Soud, W.A.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.J.; Karlsson, A. 454 Pyrosequencing Analyses of Bacterial and Archaeal Richness in 21 Full-Scale Biogas Digesters. FEMS Microbiol. Ecol. 2013, 85, 612–626. [Google Scholar] [CrossRef]
- Goux, X.; Calusinska, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2015, 8, 1–18. [Google Scholar] [CrossRef]
- Gaby, J.C.; Zamanzadeh, M.; Horn, S.J. The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, M.; Kaneshige, M.; Nakamura, A.; Yamaguchi, T. Bacteroides luti sp. nov., an anaerobic, cellulolytic and xylanolytic bacterium isolated from methanogenic sludge. Int. J. Syst. Evol. Microbiol. 2014, 64, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef]
- Chen, S.; Dong, X. Proteiniphilum acetatigenes gen. nov., sp. nov., from a UASB reactor treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2005, 55, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef]
- Stolze, Y.; Zakrzewski, M.; Maus, I.; Eikmeyer, F.; Jaenicke, S.; Rottmann, N.; Siebner, C.; Pühler, A.; Schlüter, A. Comparative metagenomics of biogas-producing microbial communities from production-scale biogas plants operating under wet or dry fermentation conditions. Biotechnol. Biofuels 2015, 8, 1–18. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Yun, J.; Cho, K.S. Effects of organic loading rate on hydrogen and volatile fatty acid production and microbial community during acidogenic hydrogenesis in a continuous stirred tank reactor using molasses wastewater. J. Appl. Microbiol. 2016, 121, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Palomo-Briones, R.; Razo-Flores, E.; Bernet, N.; Trably, E. Dark-fermentative biohydrogen pathways and microbial networks in continuous stirred tank reactors: Novel insights on their control. Appl. Energy 2017, 198, 77–87. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Carrillo-Reyes, J.; Santiago, S.G.; Bujanos-Adame, M.C. Biohydrogen from food waste in a discontinuous process: Effect of HRT and microbial community analysis. Int. J. Hydrogen Energy 2015, 40, 17246–17252. [Google Scholar] [CrossRef]
- Shida, G.M.; Sader, L.T.; Cavalcante De Amorim, E.L.; Sakamoto, I.K.; Maintinguer, S.I.; Saavedra, N.K.; Amâncio Varesche, M.B.; Silva, E.L. Performance and composition of bacterial communities in anaerobic fluidized bed reactors for hydrogen production: Effects of organic loading rate and alkalinity. Int. J. Hydrogen Energy 2012, 37, 16925–16934. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Chiranjeevi, P.; Chandrasekhar, K.; Babu, P.S.; Sarkar, O. Acidogenic Biohydrogen Production From Wastewater. In Biohydrogen; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 279–320. ISBN 9780444642035. [Google Scholar]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Luo, F.; Zhang, D.; Dai, L.; Chen, Y.; Dong, B. Waste-activated sludge fermentation for polyacrylamide biodegradation improved by anaerobic hydrolysis and key microorganisms involved in biological polyacrylamide removal. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).