Application of Phase Change Material and Artificial Neural Networks for Smoothing of Heat Flux Fluctuations
Abstract
:1. Introduction
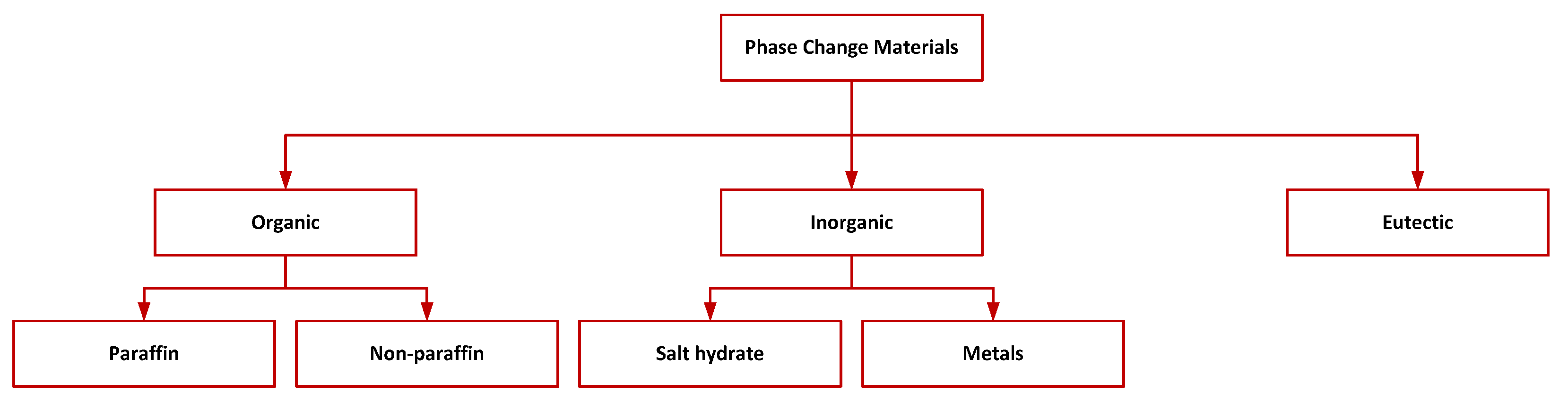
1.1. Classification and Properties of PCMs
- Thermal properties:
- −
- Matching phase transformation temperature of PCM with the temperature of the process in which it is to be used.
- −
- Large specific heat determining the amount of stored sensible heat at the same temperature difference.
- −
- Large phase transition enthalpy determining the amount of stored latent heat at the same phase transition temperature. Sensible and specific heat affects the density of heat stored in PCM.
- −
- Large heat transfer coefficient between PCM and the heat transfer fluid. This coefficient depends on thermal conductivities of PCM, the fluid and the structural elements of the TES Unit. Its high value influences the charging and discharging rate of the TES Unit.
- −
- Congruent melting means that PCM must have a consistent chemical composition in different phase states. This prevents phase separation phenomena caused by density differences between solid and liquid states.
- Physical Properties:
- −
- Phase transformation equilibrium, causing the phase transformation process to be de-reversible and only temperature dependent.
- −
- Low vapor pressure, meaning that PCM will not completely evaporate at operating temperature.
- −
- High density of PCM determines high ratio of stored heat per unit volume of PCM.
- −
- Small volume change with phase transition. The reduction in volume when PCM solidifies can lead to a smaller heat transfer field, which in turn worsens heat transfer. In pressurized storage tanks, a large volume change of PCM leads to a pressure increase, which has to be considered in the design of a storage tank and increases its cost.
- Dynamics:
- −
- Possibly low overcooling phenomenon and no supersaturation phenomenon during melting,
- −
- Fast crystallization of PCM, which means fast exothermic reaction. This affects the dynamics of the charge/discharge process.
- Chemical properties:
- −
- Chemical stability during the repeated charging/discharging process; there should be no segregation, chemical decomposition of PCM and other phenomena causing a change of PCM properties. This affects the maintenance of the TES Unit performance over a long period of time and increases its durability and reliability.
- −
- Absence or low corrosivity means that PCM and TES Unit construction material do not enter into chemical reactions with each other, which leads to a lack of degradation of both PCM and the construction material. This has the effect of increasing the durability and reliability of the TES Unit.
- −
- PCM should be non-toxic, non-flammable, non-explosive and safe to use and have the least environmental impact.
- Economics:
- −
- PCM should be easily available and cheap.
- Technical requirements
- −
- PCM should be checked in industrial conditions, should guarantee high reliability and should provide the smallest possible TES Unit size.
1.2. Applications of PCMs
2. Materials and Methods
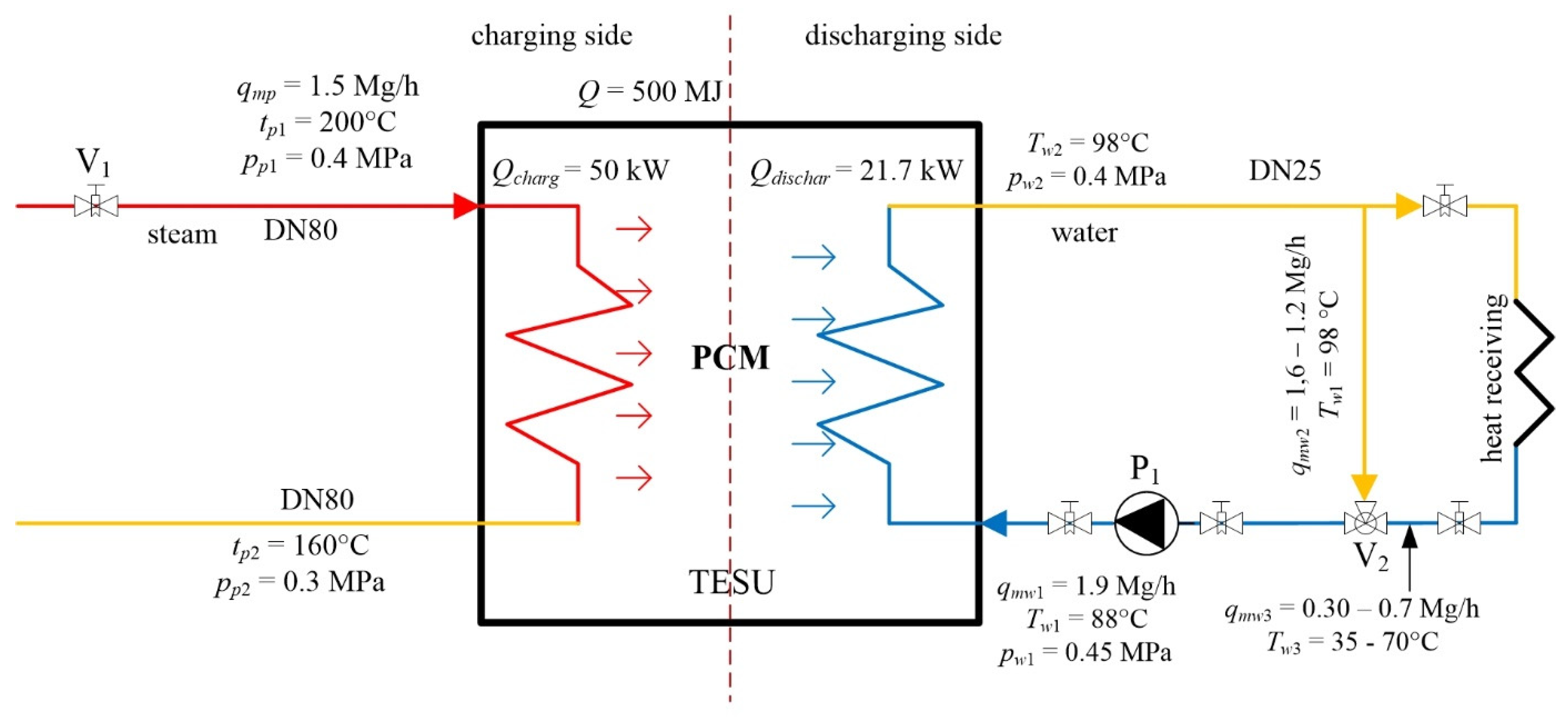
2.1. Description of TES Installation
2.2. Description of the TES Unit
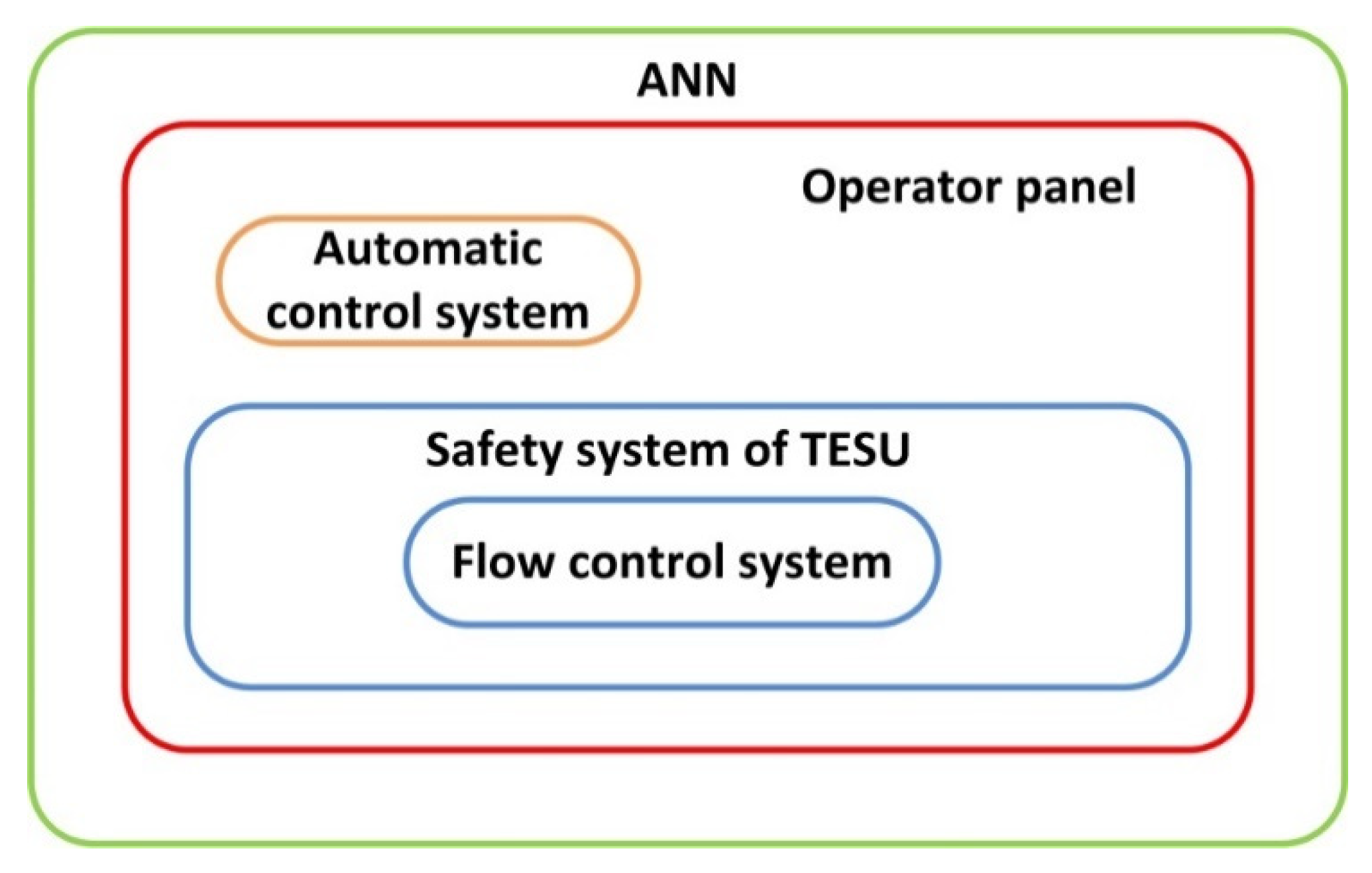
2.3. Description of the Control System with ANN
2.4. Implementation of ANN
2.5. Testing Methodology
3. Analysis of the results
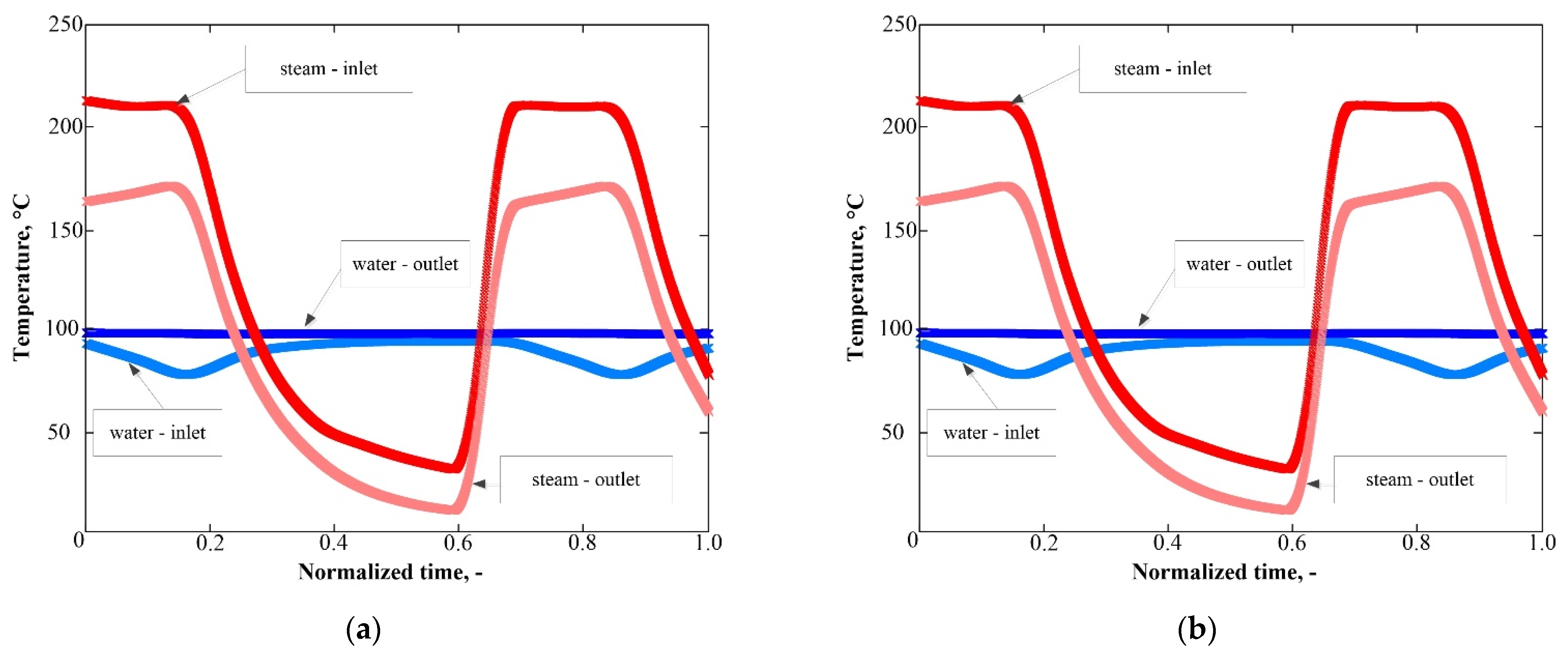
3.1. Plant Control using PID Controllers
3.2. Installation Control using ANN
3.3. Uncertainty Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paria, S.; Sarhan, A.A.D.; Goodarzi, M.S.; Baradaran, S.; Rahmanian, B.; Yarmand, H.; Alavi, M.A.; Kazi, S.N.; Metselaar, H.S.C. Indoor solar thermal energy saving time with phase change material in a horizontal shell and finned-tube heat exchanger. Sci. World J. 2015, 2015, 1. [Google Scholar] [CrossRef]
- Safaei, M.R.; Goshayeshi, H.R.; Chaer, I. Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM. Energies 2019, 12, 2002. [Google Scholar] [CrossRef] [Green Version]
- Sarafraz, M.M.; Safaei, M.R.; Leon, A.S.; Tlili, I.; Alkanhal, T.A.; Tian, Z.; Goodarzi, M.; Arjomandi, M. Experimental Investigation on Thermal Performance of a PV/T-PCM (Photovoltaic/Thermal) System Cooling with a PCM and Nanofluid. Energies 2019, 12, 2572. [Google Scholar] [CrossRef] [Green Version]
- Vivek, C.M.; Ramkumar, P.; Srividhya, P.K.; Sivasubramanian, M. Recent strategies and trends in implanting of renewable energy sources for sustainability—A review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771. [Google Scholar] [CrossRef]
- Bonamente, E.; Aquino, A. Environmental performance of innovative ground-source heat pumps with PCM Energy Storage. Energies 2020, 13, 117. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Elfeky, K.E.; Lin, L.; Wang, Q.W. Thermal performance analysis of thermocline combined sensible-latent heat storage system using cascaded-layered PCM designs for medium temperature applications. Renew. Energy 2020, 152, 684. [Google Scholar] [CrossRef]
- Liu, M.; Riahi, S.; Jacob, R.; Belusko, M.; Bruno, F. Design of sensible and latent heat thermal energy storage systems for concentrated solar power plants: Thermal performance analysis. Renew. Energy 2020, 151, 1286. [Google Scholar] [CrossRef]
- Gage, S.; Sharan, P.; Turchi, C.; Netter, J. Evaluation of formate salt PCM’s for latent heat thermal energy storage. Energies 2021, 14, 765. [Google Scholar] [CrossRef]
- Elmaazouzi, Z.; El Alami, M.; Agalit, H.; Bennouna, E.G. Performance evaluation of latent heat TES system-case study: Dimensions improvements of annular fins exchanger. Energy Rep. 2020, 6, 294. [Google Scholar] [CrossRef]
- White, M.T.; Sayma, A.I. A new method to identify the optimal temperature of latent-heat thermal-energy storage systems for power generation from waste heat. Int. J. Heat Mass Transf. 2020, 149, 119. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67. [Google Scholar] [CrossRef]
- Drissia, S.; Linga, T.-C.; Hung Mo, K. Thermal efficiency and durability performances of paraffinic phase change materials with enhanced thermal conductivity—A review. Thermochim. Acta 2019, 673, 198. [Google Scholar] [CrossRef]
- Paroutoglou, E.; Fojan, P.; Gurevich, L.; Hultmark, G.; Afshari, A. Thermal Analysis of Organic and NanoencapsulatedElectrospun Phase Change Materials. Energies 2021, 14, 995. [Google Scholar] [CrossRef]
- Chung, O.; Jeong, S.-G.; Yu, S.; Kim, S. Thermal performance of organic PCMs/micronized silica composite for latent heat thermal energy storage. Energy Build. 2014, 70, 180. [Google Scholar] [CrossRef]
- Bhagwat, V.V.; Roy, S.; Das, B.; Shah, N.; Chowdhury, A. Performance of finned heat pipe assisted parabolic trough solar collector system under the climatic condition of North East India. Sustain. Energy Technol. Assess. 2021, 45, 101. [Google Scholar]
- Gorzin, M.; Hosseini, M.J.; Ranjbar, A.A.; Bahrampoury, R. Investigation of PCM loading for the energy saving of domestic hot water system. Appl. Therm. Eng. 2018, 137, 659. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Chi, M.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. A Novel Encapsulation Method for Phase Change Materials with a AgBr Shell as a Thermal Energy Storage Material. Energies 2019, 12, 717. [Google Scholar] [CrossRef] [Green Version]
- Jebasingh, E.; Arasu, V. Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. J. Energy Storage 2020, 31, 101708. [Google Scholar]
- Veerakumar, C.; Sreekumar, A. Thermo-physical investigation and experimental unloading characteristics of lauryl alcohol as a potential phase change material for thermal management in buildings. Renew. Energy 2020, 148, 492. [Google Scholar] [CrossRef]
- Hegner, L.; Krimmel, S.; Ravotti, R.; Festini, D.; Worlitschek, J.; Stamatiou, A. Experimental feasibility study of a direct contact latent heat storage using an ester as a bio-based storage material. Energies 2021, 14, 511. [Google Scholar] [CrossRef]
- Stamatiou, A.; Obermeyer, M.; Fischer, L.J.; Schuetz, P.; Worlitschek, J. Investigation of unbranched, saturated, carboxylic esters as phase change materials. Renew. Energy 2017, 108, 401. [Google Scholar] [CrossRef]
- Yan, D.; Ming, W.; Liu, S.; Yin, G.; Zhang, Y.; Tang, B.; Zhang, S. Polyethylene glycol (PEG)/silicon dioxide grafted aminopropyl group and carboxylic multi-walled carbon nanopipes (SAM) composite as phase change material for light-to-heat energy conversion and storage. J. Energy Storage 2021, 36, 102428. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Yang, Y. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793. [Google Scholar] [CrossRef]
- Li, T.X.; Xu, J.X.; Wu, D.L.; He, F.; Wang, R.Z. High energy-density and power-density thermal storage prototype with hydrated salt for hot water and space heating. Appl. Energy 2019, 248, 406. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Wattsa, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Panesar, A.S.; Morgan, R. Molten salt selection methodology for medium temperature liquid air energy storage application. Appl. Energy 2019, 248, 500. [Google Scholar] [CrossRef]
- Liu, M.; Gomez, J.C.; Turchi, C.S.; Tay, N.H.S.; Samana, W.; Bruno, F. Determination of thermo-physical properties and stability testing of high-temperature phase-change materials for CSP applications. Sol. Energy Mater. Sol. Cells 2015, 139, 81. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, S.; Wang, Y.; Xie, L. Experimental investigation and thermodynamic modeling of an innovative molten salt for thermal energy storage (TES). Appl. Energy 2018, 212, 516. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.-D.; Eck, M.; Kaesche, S. High-Temperature Molten Salts for Solar Power Application. In Molten Salts Chemistry: From Lab to Applications; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 20; pp. 415–438. [Google Scholar]
- Kearney, D.; Herrmann, U.; Nava, P.; Kelly, B.; Mahoney, R.; Pacheco, J.; Cable, R.; Potrovitza, N.; Blake, D.; Price, H. Assessment of a molten salt heat transfer fluid in a parabolic trough solar field. J. Sol. Energy Eng. 2003, 125, 170. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.B.; Ding, J.; Yang, X.X. Heat accumulation technologies and systems for use in concentration type solar energy thermal power generation. J. Eng. Therm. Energy Power 2013, 1, 105. [Google Scholar]
- Herrmann, U.; Kearney, D.W. Survey of thermal energy storage for parabolic trough power plants. J. Sol. Energy Eng. 2002, 124, 145. [Google Scholar] [CrossRef] [Green Version]
- Dunn, R.I.; Hearps, P.J.; Wright, M.N. Molten-salt power towers: Newly commercial concentrating solar storage. Proc. IEEE 2012, 100, 504. [Google Scholar] [CrossRef]
- Relloso, S.; Delgado, E. Experience with molten salt thermal storage in a commercial parabolic trough plant. Andasol-1 commissioning and operation. In Proceedings of the 15th SolarPACES Conference, Berlin, Germany, 15–18 September 2009; p. 14. [Google Scholar]
- Moya, E.Z. 5—Innovative working fluids for parabolic trough collectors. In Advances in Concentrating Solar Thermal Research and Technology; Woodhead Publishing Series in Energy; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 5; pp. 75–106. [Google Scholar]
- Falchetta, M.; Mazzei, D.; Russo, V.; Campanella, V.A.; Floridia, V.; Schiavo, B.; Venezia, L.; Brunatto, C.; Orlando, R. The Partanna project: A first of a kind plant based on molten salts in LFR collectors. AIP Conf. Proc. 2020, 2303, 040001. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Du, Z.; Jiang, F.; Ding, Y. A review of performance investigation and enhancement of shell and tube thermal energy storage device containing molten salt based phase change materials for medium and high temperature applications. Appl. Energy 2019, 255, 113806. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.Y.; Markides, C.N.; Wang, H.; Li, W. Medium- and high-temperature latent and thermochemical heat storage using metals and metallic compounds as heat storage media: A technical review. Appl. Energy 2020, 280, 115950. [Google Scholar] [CrossRef]
- Fernández, A.I.; Barreneche, C.; Belusko, M.; Segarra, M.; Bruno, F.; Cabeza, L.F. Considerations for the use of metal alloys as phase change materials for high temperature applications. Sol. Energy Mater. Sol. Cells 2017, 171, 275. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rodríguez, P.; Rodríguez-Aseguinolaza, J.; Gil, A.; Risueño, E.; D’Aguanno, B.; Loroño, I.; Martín, L. Experiments on a lab scale TES unit using eutectic metal alloy as PCM. Energy Procedia 2015, 69, 769. [Google Scholar] [CrossRef] [Green Version]
- Buffa, S.; Soppelsa, A.; Pipiciello, M.; Henze, G.; Fedrizzi, R. Fifth-generation district heating and cooling substations: Demand response with artificial neural network-based model predictive control. Energies 2020, 13, 4339. [Google Scholar] [CrossRef]
- Mohanraj, M.; Jayaraj, S.; Muraleedharan, C. Applications of artificial neural networks for refrigeration, air-conditioning and heat pump systems—A review. Renew. Sustain. Energy Rev. 2012, 16, 1340. [Google Scholar] [CrossRef]
- Duan, J.; Li, F. Transient heat transfer analysis of phase change material melting in metal foam by experimental study and artificial neural network. J. Energy Storage 2021, 33, 102160. [Google Scholar] [CrossRef]
- Motahar, S.; Jahangiri, M. Transient heat transfer analysis of a phase change material heat sink using experimental data and artificial neural network. Appl. Therm. Eng. 2020, 167, 114817. [Google Scholar] [CrossRef]
- Yaïci, W.; Entchev, E. Performance prediction of a solar thermal energy system using artificial neural networks. Appl. Therm. Eng. 2014, 73, 1348. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Sopian, K.; Yousif, J.H.; Kazem, H.A.; Boland, J.; Chaichan, M.T. Artificial neural network modeling and analysis of photovoltaic/thermal system based on the experimental study. Energy Convers. Manag. 2019, 186, 368. [Google Scholar] [CrossRef]
- Wu, H.; Bagherzadeh, S.A.; D’Orazi, A.; Habibollahi, N.; Karimipour, A.; Goodarzi, M.; Bach, Q.-V. Present a new multi objective optimization statistical Pareto frontier method composed of artificial neural network and multi objective genetic algorithm to improve the pipe flow hydrodynamic and thermal properties such as pressure drop and heat transfer coefficient for non-Newtonian binary fluids. Phys. A Stat. Mech. Its Appl. 2019, 535, 122409. [Google Scholar]
- Fausett, L. Fundamentals of Neural Networks: Architectures, Algorithms, and Applications; Prentice Hall: Englewood Cliffs, NJ, USA, 1994. [Google Scholar]
- Villarrubia, G.; De Paz, J.F.; Chamoso, P.; De la Prieta, F. Artificial neural networks used in optimization problems. Neurocomputing 2018, 272, 10. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fleetwood, A.; Galarza, A.; Fontán, L. Predicting solar energy generation through artificial neural networks using weather forecasts for microgrid control. Renew. Energy 2018, 126, 855. [Google Scholar] [CrossRef]
- Kalani, H.; Sardarabadi, M.; Passandideh-Fard, M. Using artificial neural network models and particle swarm optimization for manner prediction of a photovoltaic thermal nanofluid based collector. Appl. Therm. Eng. 2017, 113, 1170. [Google Scholar] [CrossRef]
- Yousif, J.H.; Kazem, H.A.; Boland, J. Predictive models for photovoltaic electricity production in hot weather conditions. Energies 2017, 10, 971. [Google Scholar] [CrossRef]
- Lazić, V.; Arsić, D.; Nikolić, R.R.; Rakić, D.; Aleksandrović, S.; Djordjević, M.; Hadzima, B. Selection and analysis of material for boiler pipes in a steam plant. Procedia Eng. 2016, 149, 216. [Google Scholar] [CrossRef] [Green Version]
- Parmar, V.; Kumar, A.; Prakash, G.V.; Datta, S.; Kalyanasundaram, D. Investigation, modelling and validation of material separation mechanism during fiber laser machining of medical grade titanium alloy Ti6Al4V and stainless steel SS316L. Mech. Mater. 2019, 137, 103125. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, H.; Wen, D.; Zhao, X. Thermal performance analysis of a solar energy storage unit encapsulated with HITEC salt/copper foam/nanoparticles composite. Energy 2020, 192, 116593. [Google Scholar] [CrossRef]
- Lu, W.; Liu, G.; Xiong, Z.; Wu, Z.; Zhang, G. An experimental investigation of composite phase change materials of ternary nitrate and expanded graphite for medium-temperature thermal energy storage. Sol. Energy 2020, 195, 573. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Wang, X.; Zheng, S. Thermal analysis of melting and freezing processes of phase change materials (PCMs) based on dynamic DSC test. Energy Build. 2016, 130, 388. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17. [Google Scholar] [CrossRef]
- Mohseni, E.; Tang, W.; Khayat, K.H.; Cui, H. Thermal performance and corrosion resistance of structural-functional concrete made with inorganic PCM. Constr. Build. Mater. 2020, 249, 118768. [Google Scholar] [CrossRef]
- Mohseni, E.; Tang, W.; Wang, S. Development of thermal energy storage lightweight structural cementitious composites by means of macro-encapsulated PCM. Constr. Build. Mater. 2019, 225, 182. [Google Scholar] [CrossRef]
- Sun, G.; Liu, Y.; Dong, S.; Wang, J. Study on novel molten salt-ceramics composite as energy storage material. J. Energy Storage 2020, 28, 101237. [Google Scholar] [CrossRef]
- Park, J.; Yi, D.; Ji, S. Analysis of Recurrent Neural Network and Predictions. Symmetry 2020, 12, 615. [Google Scholar] [CrossRef]
- Pashaei, S.; Bagheri, P. Parallel cascade control of dead time processes via fractional order controllers based on Smith predictor. ISA Trans. 2020, 98, 186. [Google Scholar] [CrossRef]
- Nagarsheth, S.H.; Sharma, S.N. Smith predictor embedded analytical fractional-order controller design: A delayed Bode’s ideal transfer function approach. IFAC Pap. 2020, 53, 3749. [Google Scholar] [CrossRef]
- Tan, K.K.; Chua, K.Y.; Zhao, S.; Yang, S.; Tham, M.T. Repetitive control approach towards automatic tuning of Smith predictor controllers. ISA Trans. 2009, 48, 16. [Google Scholar] [CrossRef]
- Karan, S.; Dey, C.; Mukherjee, S. Simple internal model control based modified Smith predictor for integrating time delayed processes with real-time verification. ISA Trans. 2021. [Google Scholar] [CrossRef]
- Lin, X.; Xi, W.; Dai, J.; Wang, C.; Wang, Y. Prediction of Slag Characteristics Based on Artificial Neural Network for Molten Gasification of Hazardous Wastes. Energies 2020, 13, 5115. [Google Scholar] [CrossRef]
- Langer, S. Approximating smooth functions by deep neural networks with sigmoid activation function. J. Multivar. Anal. 2021, 182, 104696. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Development of Artificial Neural Network Models to Assess Beer Acceptability Based on Sensory Properties Using a Robotic Pourer: A Comparative Model Approach to Achieve an Artificial Intelligence System. Beverages 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]





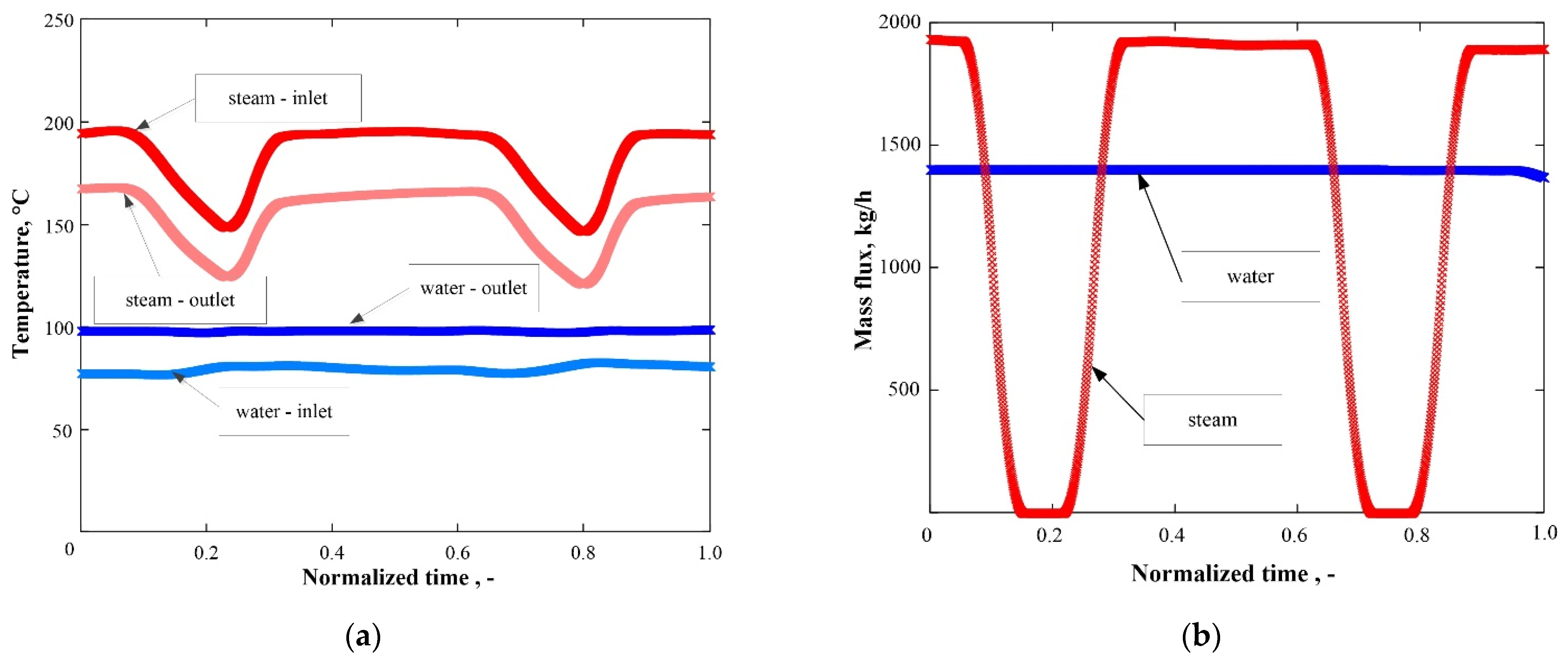


| No. | Factor | PID Only Control | ANN Enhanced Control | ||
|---|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | ||
| 1. | Charging heat flux (steam) | 22.011 kW | 25.462 kW | 27.549 kW | 17.669 kW |
| 2. | Discharging heat flux (water) | 20.032 kW | 11.206 kW | 30.004 kW | 2.793 kW |
| Parameters | Max Absolute Error | Max Relative Error |
|---|---|---|
| Steam mass flow rate | <10 kg/h | ±0.5% |
| Water mass flow rate | <19 kg/h | ±1% |
| Steam temperature | <3.0 °C | ±0.3% |
| Water temperature | <1.2 °C | ±0.3% |
| Steam specific heat | <25 kJ/kg K | ±0.6% |
| Water specific heat | <25 kJ/kg K | ±0.6% |
| Charging heat flux (steam) | <4.6 kW | ±7.5% |
| Discharging heat flux (water) | <3.2 kW | ±7.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tietze, T.; Szulc, P.; Smykowski, D.; Sitka, A.; Redzicki, R. Application of Phase Change Material and Artificial Neural Networks for Smoothing of Heat Flux Fluctuations. Energies 2021, 14, 3531. https://doi.org/10.3390/en14123531
Tietze T, Szulc P, Smykowski D, Sitka A, Redzicki R. Application of Phase Change Material and Artificial Neural Networks for Smoothing of Heat Flux Fluctuations. Energies. 2021; 14(12):3531. https://doi.org/10.3390/en14123531
Chicago/Turabian StyleTietze, Tomasz, Piotr Szulc, Daniel Smykowski, Andrzej Sitka, and Romuald Redzicki. 2021. "Application of Phase Change Material and Artificial Neural Networks for Smoothing of Heat Flux Fluctuations" Energies 14, no. 12: 3531. https://doi.org/10.3390/en14123531






