Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed
Abstract
1. Introduction
- -
- Water pollution: soil overfertilization and outflow from fields to groundwaters and surface waters;
- -
- Eutrophication: overfertilization of inland and sea waters (algal blooms, reduction of biodiversity and modification of aquatic ecosystems, loss of benthic fauna and the lack of oxygen);
- -
- Microbiological contamination: pathogenic microorganisms contained in slurry pose a serious sanitary threat; Staphylococcus sp., faecal streptococci, Escherichia coli, rhusiopathia suum, tuberculosis mycobacteria, pathogenic streptococci, foot-and-mouth disease virus, fungi and larvae, and eggs of parasitic worms (e.g., tapeworms) are considered the most significant;
- -
- Indirect and secondary impact on the formation of acid rain (emission of nitrogen oxides and sulfur oxides) and increase of the greenhouse effect (emission of greenhouse gases damaging the ozone layer) [3].
1.1. Properties of Pig Slurry Used in the Fermentation Process
1.1.1. Types of Slurry
1.1.2. Physical and Chemical Aspects of Slurry
1.2. Slurry Microflora
- -
- Immobilization, which allows to increase the active surface for the flora of fermenting bacteria;
- -
- Polydisperse substrate mixing system.
- -
- Biogas composition;
- -
- Course of changes in the average daily gas stream;
- -
- Gas permeability characteristics resulting from the pressure forcing this flow;
- -
- The dependence of the Reynolds number on the gas permeability coefficient.
2. Materials and Methods
2.1. Pig Slurry
2.2. Model of a Monosubstrate, Flow Biogas Reactor-Research Position
2.3. Agricultural Biogas Production Installation Using a Polydisperse Substrate (Pig Manure)-Research Position
2.4. Scope and Research Methodology
- -
- Rheology; pig slurry rheological tests were performed using the U-VIsc kinematic viscometer and Ubbelohde-modified viscometer, manufactured by Omnitek B.V., authorized distributor of EKMA, Poland;
- -
- The influence of flow resistance in relation to the Reynolds number was described;
- -
- The permeability of the adhesive bed was determined experimentally;
- -
- An attempt was made to compare the dependence of the Reynolds number on the gas permeability coefficient.
- -
- Pig slurry was classified as a polydisperse substrate;
- -
- Several leak tests were carried out for the biogas plant;
- -
- The installation was started up on a liquid inoculum (carried out for 10 days) until stable conditions for biogas production were obtained, while analyzing the process conditions necessary for mesophilic fermentation;
- -
- Biogas was produced using an adhesive bed in the immobilization system;
- -
- Cyclic hydrodynamic mixing of the polydisperse substance was used;
- -
- The mixing of the polydisperse substrate in the entire circuit of the installation was optimized;
- -
- Biogas production was optimized by analyzing the biogas composition;
- -
- Criteria for the evaluation of biogas production were developed depending on gas flow in time, gas pressure in time, and temperature in time;
- -
- The correlation of Reynolds number from the equivalent flow resistance coefficient, flow resistance from the gas permeability coefficient and Reynolds number on the gas permeability coefficient was indicated.
3. Results and Discussion
3.1. Rheological Research
3.2. Installation Tightness Tests
- -
- Biogas installation, type of pneumatic test, pressure 0.5 bar, test duration 1 h;
- -
- Heat recovery installation, type of hydraulic test, pressure 5 bar, test duration 1 h;
- -
- Mixing installation in the fermenter Figure 6, type of hydraulic test, pressure 3 bar, test duration 1 h;
- -
- Digestate installation, type of hydraulic test, pressure 3 bar, test duration 1 h.
3.3. Technological Start-Up
- -
- Heating the fermentation chamber circulation liquid;
- -
- Pumping the substrate into the pre-tank;
- -
- Gas pressure regulation;
- -
- Dosing of additives stabilizing the biogas mass flow rate;
- -
- The level of the primary tank is full.
- -
- Circulation of fermentation liquid in the fermentation chamber;
- -
- Dosing the feeding substrate into the fermentation chamber;
- -
- Assessment of the level of fermentation chamber filling, biogas composition and analytical parameters of technological liquids.
3.4. Agricultural Biogas Production System
3.5. Proper Research
- -
- -
- -
- -
- Controlling ammonia inhibition [85].
4. Conclusions
- (1)
- The mixing system used in the fermentor ensures the uniformity of the composition of the fermentation mass and provides qualitative ingredients supporting the fermentation process;
- (2)
- The method of using the installation significantly improves the process of converting liquid biomass, especially animal slurry, into high-calorific biogas and in cogeneration into electricity and heat;
- (3)
- Biogas can be produced easily and reliably near the livestock building.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smurzyńska, A.; Czekała, W.; Kupryaniuk, K.; Cieślik, M.; Kwiatkowska, A. Types and properties of slurry and the possibilities of its management. Probl. Agric. Eng. 2016, 4, 117–127. [Google Scholar]
- Kwiecińska, A. Ecological Management of the Slurry with the Use of Membrane Techniques. Ph.D. Thesis, Department of Environmental Chemistry and Membrane Processes, Faculty of Environmental and Power Engineering, The Silesian Technical University, Gliwice, Poland, 2016. [Google Scholar]
- Baltic Green Belt. Available online: http://balticgreenbelt.org.pl/ (accessed on 30 September 2012).
- Burton, C.H. Manure management–treatment strategies for sustainable agriculture, second edition. Livest. Sci. 2006, 102, 256–257. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C.; Janzen, H.H.; Hill, B.R.; Ormann, T. Potential nitrogen enrichment of soil and surface water by atmospheric ammonia sorption in intensive livestock production areas. Agric. Ecosyst. Environ. 2005, 110, 185–194. [Google Scholar] [CrossRef]
- Mantovi, P.; Fumagalli, L.; Beretta, G.P.; Guermandi, M. Nitrate leaching through the unsaturated zone following pig slurry applications. J. Hydrol. 2006, 316, 195–212. [Google Scholar] [CrossRef]
- Redding, M.R.A. Pig effluent—P application can increase the risk of P transport: Two case studies. Australian. J. Soil Res. 2001, 39, 161–174. [Google Scholar] [CrossRef][Green Version]
- Berenguer, P.; Cala, S.; Santiveri, F.; Boixadera, J.; Lloveras, J. Copper and zinc soil accumulation and plant concentration in irrigated maize fertilized with liquid swine manure. Agron. J. 2008, 100, 1056–1061. [Google Scholar] [CrossRef]
- Bicudo, J.R.; Schmidt, D.R.; Gay, S.W.; Gates, R.S.; Jacobson, L.D.; Hoff, S.J. Air quality and emissions from livestock and poultry production/waste management systems. In Prepared as a White Paper for National Center for Manure and Animal Waste Management; North Carolina University: Chapel Hill, NC, USA, 2002; p. 157. [Google Scholar]
- Smurzyńska, A.; Dach, J.; Dworecki, Z.; Czekała, W. Emisje gazowe podczas gospodarki gnojowicą [Gas emissions during slurry management]. Eng. Environ. Prot. 2016, 19, 109–125. [Google Scholar] [CrossRef]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Martinez, J.; Guiziou, F.; Peu, P.; Gueutier, V. Influence of treatment techniques for pig slurry on methane emissions during subsequent storage. Biosyst. Eng. 2003, 85, 347–354. [Google Scholar] [CrossRef]
- Adamowicz, M. The importance of agrarian policy in sustainable development of rural areas. Rocz. Nauk. Seria 2000, 2, 69–72. [Google Scholar]
- Smurzyńska, A.; Dach, J.; Czekała, W. Technologies to reduce emissions of noxious gases resulting from livestock farming. Inżynieria Ekol. 2016, 47, 189–198. [Google Scholar] [CrossRef]
- Boldrin, A.; Baral, K.R.; Fitamo, T.; Vazifehkhoran, A.H.; Jensen, I.G.; Kjaergaard, I.; Lyng, K.-A.; Nguyen, Q.V.; Nielsen, L.S.; Triolo, J.M. Optimised biogas production from the codigestion of sugar beet with pig slurry: Integrating energy, GHG and economic accounting. Energy 2016, 112, 606–617. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation, How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- Clemens, J.; Trinborn, M.; Weiland, P.; Amon, B. Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric. Ecosyst. Environ. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Fischer, T. Biogas aus Gras Monofermentation von Energiepflanzen [Make Biogas Out of Grass Silage–Mono Fermentation of Energy Crop]. Krieg & Fischer Ingenieure GmbH. 2005. Available online: http://www.kriegfischer.de/texte/Bremen_050414.pdf (accessed on 10 January 2021).
- Hill, D.T.; Bolte, J.P. Methane production from low solid concentration liquid swine waste using conventional anaerobic fermentation. Bioresour. Technol. 2000, 74, 241–247. [Google Scholar] [CrossRef]
- Asam, Z.U.Z.; Poulsen, T.G.; Nizamiego, A.S.; Rafique, R.; Kiely, G.; Mmurphy, J.D. How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2011, 88, 2013–2018. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Chen, Z.; Liu, G.; Yang, H. Separation of swine slurry into different concentration fractions and its influence on biogas fermentation. Appl. Energy 2014, 114, 504–511. [Google Scholar] [CrossRef]
- Deng, L.; Chen, Z.; Yang, H.; Zhu, J.; Liu, Y.; Długi, Y.; Zheng, D. Biogas fermentation of swine slurry based on the separation of concentrated liquid and low content liquid. Biomass Bioenergy 2012, 45, 187–194. [Google Scholar] [CrossRef]
- Gabryszewska, M.; Rogulska, M. Agricultural biogas plants. Bariery rozwoju. Przemysł Chem. 2009, 3, 248–251. [Google Scholar]
- Bohodziewicz, J.; Kuglarz, M.; Mrowiec, B. Intensification of pig manure digestion by co-substrate addition in the form of municipal biowaste. Nauka Przyr. Technol. 2011, 5, 1–8. [Google Scholar]
- Rodhe, L.K.K.; Ascue, J.; Willén, A.; Vegerfors Persson, B.; Nordberg, Å. Greenhouse gas emissions from storage and field application of anaerobically digested and non-digested cattle slurry. Agric. Ecosyst. Environ. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Czekała, W.; Pilarski, K.; Dach, J.; Janczak, D.; Szymańska, M. Analysis of management possibilities for digestate from biogas plant. Tech. Rol. Ogrod. Leśna 2012, 4, 13–15. [Google Scholar]
- Kutera, J. Manure Management; Agricultural University Publishing House: Wroclaw, Poland, 1994. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Sung, S.; Liu, T. Ammonia inhibition on thermophilic aceticlastic methanogens. Water Sci. Technol. 2002, 10, 113–120. [Google Scholar]
- Sung, S.; Liu, T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 53, 43–52. [Google Scholar] [CrossRef]
- Hus, S. Water, Sewage and Slurry Chemistry; Agricultural University Publishing House: Wroclaw, Poland, 1995. [Google Scholar]
- Landry, H.; Lague, C.; Roberge, M. Physical and rheological properties of manure products. Appl. Eng. Agric. 2004, 20, 277–288. [Google Scholar] [CrossRef]
- Böhm, R. Epidemiological Risks Related to Chicken Manure and Strategies for the Validation of Treatment Methods under the Aspekt of Hygienic Safety; Universität Hohenheim Publishing House: Stuttgart, Germany, 2005. [Google Scholar]
- Paluszak, Z. Studies on the Behavior and Survival of Selected Faecal Microorganisms in the Soil Fertilized with Slurry; University ATR Publishing House: Bydgoszcz, Poland, 1998. [Google Scholar]
- Strauch, D. Survival of pathogenic micro-organisms and parasites in excreta, manure and sewage sludge. Rev. Sci. Tech. Off. Int. Epiz. 1991, 10, 813–846. [Google Scholar] [CrossRef]
- Olszewska, H.; Paluszak, Z.; Szejniuk, B. Survey of Salmonella Enteritidis Microorganisms in Slurry, Domestic Sewage and Water in Laboratory Conditions, Materials for the Symposium: Hygiene Problems in Agricultural Greening. SGGW; Warsaw University of Life Sciences Publishing House: Warsaw, Poland, 1997; pp. 208–213. [Google Scholar]
- Pesaro, F.; Sorg, I.; Metzler, A. In situ inactivation of animal viruses and a coliphage in nonaerated liquide and semiliquide animal wastes. Appl. Environ. Microbiol. 1995, 61, 92–97. [Google Scholar] [CrossRef]
- Guan, T.Y.; Holley, R.A. Pathogen survival in swine manure environments and transmission of human enteric illness—A review. J. Environ. Qual. 2003, 32, 383–392. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kaczmarek, A.; Budzyńska, A.; Gospodarek, E. Microbiological aspects of slurry management. Post. Mikrobiol. 2015, 54, 235–249. Available online: http://www.pm.microbiology.pl (accessed on 10 January 2021).
- Maćkowiak, C. Rules for the Use of Slurry. Fertilization Recommendations, Part IV; IUNG Publishing House: Pulawy, Poland, 1994. [Google Scholar]
- Maćkowiak, C. Slurry, Its Properties and Application Rules, Taking into Account Environmental Protection. Training Materials 75/99; IUNG Publishing House: Pulawy, Poland, 1999. [Google Scholar]
- Kupryś-Caruk, M. The agri-food industry as a source of substrates for biogas production. Postępy Nauk. I Technol. Przemysłu Rolno-Spożywczego 2017, 72, 69–85. [Google Scholar]
- Marszałek, M.; Banach, M.; Kowalski, Z. Manure utilization by methane and aerobic fermentation–biogas and compost production. Czas. Tech. 2011, 10, 143–158. [Google Scholar]
- Dach, J.; Przybył, J.; Zbytek, Z.; Lewicki, A.; Janczak, D.; Cieślik, M. Methane emissions from livestock production in Poland: Scale and potential costs. J. Res. Appl. Agric. Eng. 2013, 58, 25–28. [Google Scholar]
- Podkówka, Z. Biogas from Slurry. In Engineering of Renewable Energy Sources; Mroziński, A., Ed.; IX EKO-€URO-ENERGIA: Bydgoszcz, Poland, 2016; pp. 147–165. ISBN 978-83-64423-33-8. [Google Scholar]
- Wałowski, G.; Borek, K.; Romaniuk, W.; Wardal, W.J.; Borusiewicz, A. Modern Systems of Obtaining Energy–Biogas; Romaniuk, W., Ed.; Publishing House of the Higher School of Agribusiness in Łomża: Warszawa, Poland, 2019; pp. 1–116. [Google Scholar]
- Myczko, A.; Myczko, R.; Szulc, R.; Tupalski, L. Reactor for Methane Slurry Fermentation. Poland Patent PL220,074, 4 July 2012. [Google Scholar]
- Implementation Agreement No. AT-23/2019; ITP: Falenty, Poland, 2019; Available online: www.itp.edu.pl (accessed on 20 May 2021).
- Danbred Genes for Global Pig Production. Available online: www.danbred.com (accessed on 20 May 2021).
- Tuczniki. Available online: http://neorol.eu/tuczniki/html (accessed on 20 May 2021).
- Myczko, A. A Scenario for the Development of a Network of Micro-Energy Producers; Czysta Energia Publishing House, 2012; pp. 26–27. [Google Scholar]
- Myczko, A.; Kołodziejczyk, T.; Aleszczyk, Ł.; Myczko, R.; Łaska-Zieja, B.; Jędrusiak-Wrzesińska, E.; Wałowski, G.; Sawiński, R. Monosubstrate Fermentor for Methane Slurry Fermentation. Poland Patent PL424,291, 22 January 2018. [Google Scholar]
- Kutera, J.; Hus, S. Agricultural Treatment and Use of Sewage and Slurry. Scientific Journals of the Agricultural University in Wroclaw; Agricultural University in Wroclaw Publishing House: Wroclaw, Poland, 1998. [Google Scholar]
- Kutera, J.; Hus, S. Principles of Liquid Manure Management in Agriculture in Mountain Areas, Taking into Account Environmental Protection Conditions, Agricultural University, Wroclaw; Agricultural University in Wroclaw Publishing House: Wroclaw, Poland, 1990. [Google Scholar]
- Makowiak, C. Fertilization Value of Sludge and Waste from the Food Industry. In Conference Materials Sewage Sludge-Processing and Use. Poznan University of Technology; Poznan University of Technology Publishing House: Poznan, Poland, 1997. [Google Scholar]
- Magrel, L.; Boruszko, D. Agricultural Use of Slurry on the Example of Selected Pig Fattening Farms. In Conference Materials Wastewater Treatment-New Trends, Modernization of Existing Treatment Plants and Sludge Management. Foundation of Environmental Economists and Natural Resources, Rajgrod; Foundation of Environmental Economists and Natural Resources Publishing House: Rajgrod, Poland, 1997; pp. 399–404. [Google Scholar]
- El-Mashad, H.M.; Van Loon, W.K.; Zeeman, G.; Bot, G.P. Rheological Properties of Dairy Cattle Manure. Available online: https://www.academia.edu/17788307/Rheological_properties_of_dairy_cattle_manure (accessed on 10 January 2021).
- Wang, H.; Aguirre-Villegas, H.A.; Larson, R.A.; Alkan-Ozkaynak, A. Physical Properties of Dairy Manure Pre- and Post-Anaerobic Digestion. Appl. Sci. 2019, 9, 2703. [Google Scholar] [CrossRef]
- Szpendowski, J.; Siemianowski, K. Nutritional and functional properties and the use of caseinates in food processing. Eng. Sci. Technol. 2013, 3, 122–138. [Google Scholar]
- Darewicz, M.; Dziuba, J. Structure and functional properties of milk proteins. Food Sci. Technol. Qual. 2005, 2, 47–60. [Google Scholar]
- Walstra, P.; de Roos, A.L. Proteins at air-water and oil-water interfaces: Static and dynamic aspects. Food Rev. Int. 1993, 9, 503–525. [Google Scholar] [CrossRef]
- The Problem with Foaming Manure. Available online: https://www.3trzy3.pl/artyku%C5%82y/problem-z-pieni%C4%85cym-si%C4%99-obornikiem_1091/ (accessed on 10 January 2021).
- Donham, K.J.; Thelin, A. Rural Occupational and Environmental Health, Safety, and Prevention; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 127–136, 534–539. ISBN 978-1-118-64720-2. [Google Scholar]
- Beware of Manure Pit Hazards. Available online: https://nasdonline.org/1292/d001097/beware-of-manure-pit-hazards.html (accessed on 10 January 2021).
- Gas Monitors For Consideration in Swine Barn Activities (High-Hazard H2S and Methane Operations). Available online: https://gpcah.public-health.uiowa.edu/wp-content/uploads/2016/11/Monitor-Information.pdf (accessed on 10 January 2021).
- Myczko, R. Protocol of transfer of the technological process. In Dokument Wydany Przez Laboratorium Badawcze Technologii i Biosystemów Rolniczych; Institute of Technology and Life Sciences Publishing House: Poznan, Poland, 2018; pp. 1–2. [Google Scholar]
- Strzelecki, T.; Kostecki, S.; Żak, S. Modeling of Flows through Porous Media; Lower Silesian Educational Publisher: Wroclaw, Poland, 2008. [Google Scholar]
- Waluk, J. Laboratorium z Mechaniki Gruntów. Politechnika Wrocławska [Laboratory of Soil Mechanics]; Wroclaw University of Science and Technology: Wroclaw, Poland, 1973. [Google Scholar]
- Wałowski, G. Phenomenological approach to hydrodynamics of gas flow through the Porous structure. Chem. Ind. 2017, 96, 1171–1178. [Google Scholar] [CrossRef]
- Zamojska-Jaroszewicz, A.; Matuszewska, A.; Owczuk, M.; Wardzińska, D. Methanogenic potential of mixtures of agricultural substrates. Studia Ecol. Bioethicae 2013, 2, 147–159. [Google Scholar]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Oniszek-Popławska, A.; Zowsik, M.; Wiśniewski, G. Production and Use of Agricultural Biogas; EC BREC/IBMER: Gdańsk–Warszawa, Poland, 2003. [Google Scholar]
- Głodek, E.; Janecka, L.; Kalinowski, W.; Werszler, A.; Garus, T.; Kościanowski, J.; Siemiątkowski, G. Harvesting and Energetic Use of Agricultural Biogas: Technological Process, Part I; Institute of Mineral Building Materials: Opole, Poland, 2007. [Google Scholar]
- Budiyono; Widiasa, I.N.; Johari, S.; Sunarso. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–44. [Google Scholar]
- Krylova, N.I.; Khabiboulline, R.E.; Naumova, R.P.; Nagel, M.A. The influence of ammonium and methods for removal during the anaerobic treatment of poultry manure. J. Chem. Technol. Biotechnol. 1997, 70, 99–105. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Codigestion of waste organic solids-batch studies. Bioresour. Technol. 1999, 67, 117–122. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klassonb, K.T.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef]
- Lo, K.V.; Whitehead, A.J.; Liao, P.H.; Bulley, N.R. Methane production from screened dairy manure using a fixed-film reactor. Agric. Wastes 1984, 9, 175–188. [Google Scholar] [CrossRef]
- Vartak, D.R.; Engler, C.R.; McFarland, M.J.; Ricke, S.C. Attachedfilm media performance in psychrophilic anaerobic treatment of dairy cattle wastewater. Bioresour. Technol. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Hamilton, D.W.; Lalman, J.A.; Cumba, H.J. Effects of cycle-frequency and temperature on the performance of anaerobic sequencing batch reactors (ASBRs) treating swine waste. Bioresour. Technol. 2008, 99, 1972–1980. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Lehtomaki, A.; Huttunen, S.; Rintala, J.A. Laboratory investigations on co-digestion of energy crops and crop residues with cattle manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Boe, K.; Angelidaki, I. Serial CSTR digester configuration for improving biogas production from manure. Water Res. 2009, 43, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kaparaju, P.; Ellegaard, L.; Angelidaki, I. Optimisation of biogas production from manure through serial digestion: Lab-scale and pilotscale studies. Bioresour. Technol. 2009, 100, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Kaparaju, P.; Buendía, I.; Ellegaard, L.; Angelidaki, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Labscale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef]
- Liao, P.H.; Lo, K.V.; Chieng, S.T. Effect of liquid-solids separation on biogas production from dairy manure. Energy Agric. 1984, 3, 61–69. [Google Scholar] [CrossRef]
- Kaparaju, P.; Angelidaki, I. Effect of temperature and microbial activity on passive separation of digested cattle manure. Bioresour. Technol. 2008, 99, 1345–1352. [Google Scholar] [CrossRef]
- Møller, H.B.; Nielsen, A.M.; Nakakubo, R.; Olsen, H.J. Process performance of biogas digesters incorporating pre-separated manure. Livest. Sci. 2008, 112, 217–223. [Google Scholar] [CrossRef]
- Kayhanian, M.; Rich, D. Pilot-scale high solids thermophilic anaerobic digestion of municipal solid waste with an emphasis on nutrient requirements. Biomass Bioenergv 1995, 8, 433–444. [Google Scholar] [CrossRef]
- Demirci, G.G.; Demirer, G.N. Effect of initial COD concentration, nutrient addition, temperature and microbial acclimation on anaerobic treatability of broiler and cattle manure. Bioresour. Technol. 2004, 93, 109–117. [Google Scholar] [CrossRef]
- Luengo, P.L.; Alvarez, J.M. Influence of temperature, buffer, composition and straw particle length on the anaerobic digestion of wheat straw-pig manure mixtures. Resour. Conserv. Recycl. 1988, 1, 27–37. [Google Scholar] [CrossRef]
- Castillo, R.T.; Luengo, P.L.; Alvarez, J.M. Temperature effect on anaerobic of bedding manure in a one phase system at different inoculums concentration. Agric. Ecosyst. Environ. 1995, 54, 55–66. [Google Scholar] [CrossRef]
- Sans, C.; Mata-Alvarez, J.; Cecchi, F.; Pavan, P.; Bassetti, A. Acidogenic fermentation of organic urban wastes in a plug-flow reactor under thermophilic conditions. Bioresour. Technol. 1995, 54, 105–110. [Google Scholar] [CrossRef]
- Lopes, W.S.; Leite, V.D.; Prasad, S. Influence of inoculum on performance of anaerobic reactors for treating municipal solid waste. Bioresour. Technol. 2004, 94, 261–266. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Pérez, M.; Romero, L.I. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour. Technol. 2008, 99, 6994–7002. [Google Scholar] [CrossRef]
- Magrel, L. Methodology for Assessing the Effectiveness of the Methane Fermentation Process of Selected Sewage Sludge; Scientific Dissertations No. 93; Białystok University of Technology: Białystok, Poland, 2020. [Google Scholar]
- Buraczewski, G. Methane Fermentation; PWN: Warsaw, Poland, 1993. [Google Scholar]
- Czekała, W.; Gawrych, K.; Smurzyńska, A.; Mazurkiewicz, J.; Pawlisiak, A.; Chełkowski, D.; Brzoski, M. The possibility of functioning microbiogas plant in selected farm. J. Water Land Dev. 2017, 35, 19–25. [Google Scholar] [CrossRef][Green Version]
- Voytovych, I.; Malovanyy, M.; Zhuk, V.; Mukha, O. Facilities and problems of processing organic wastes by family-type biogas plants in Ukraine. J. Water Land Dev. 2020, 45, 185–189. [Google Scholar] [CrossRef]

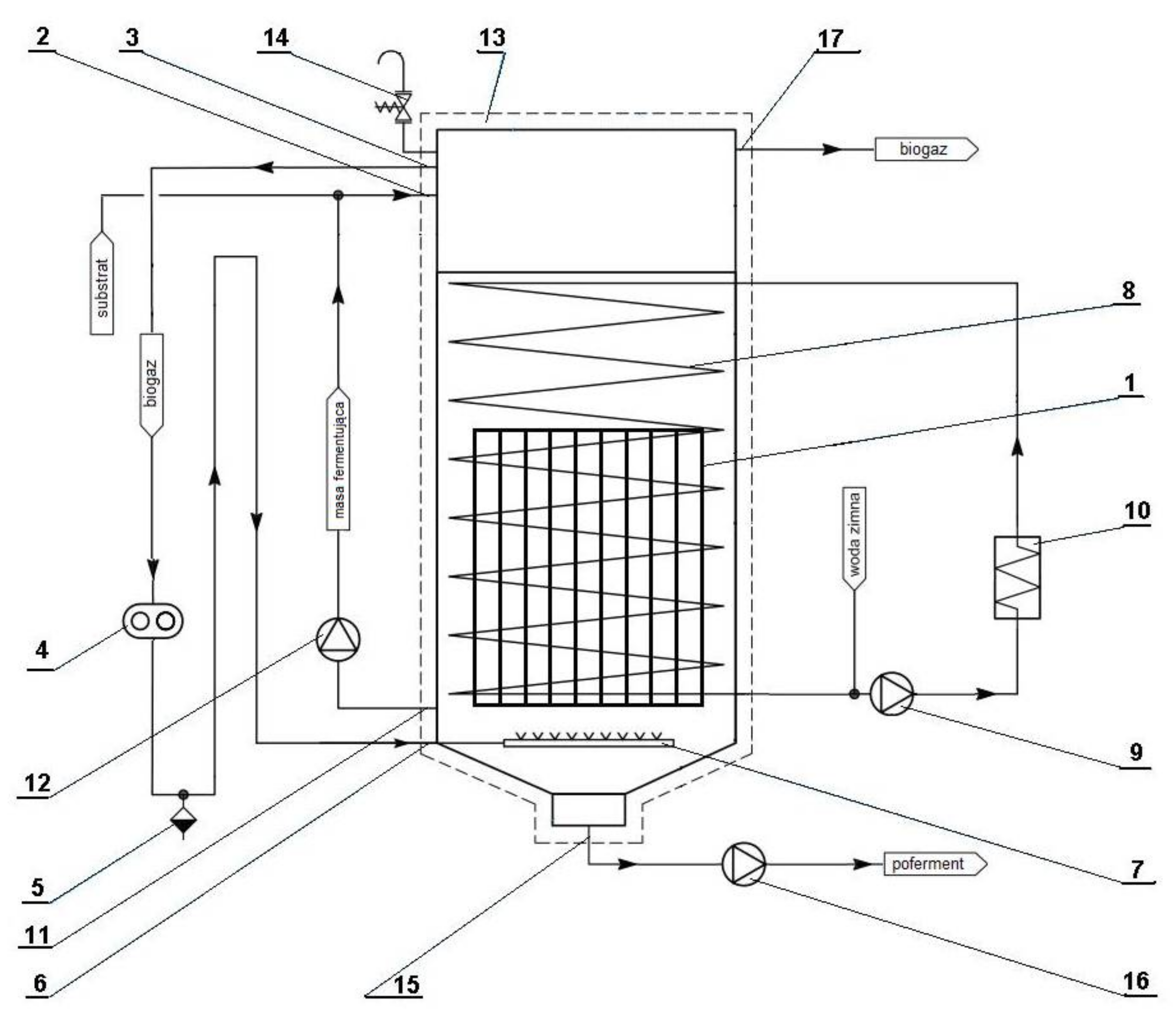

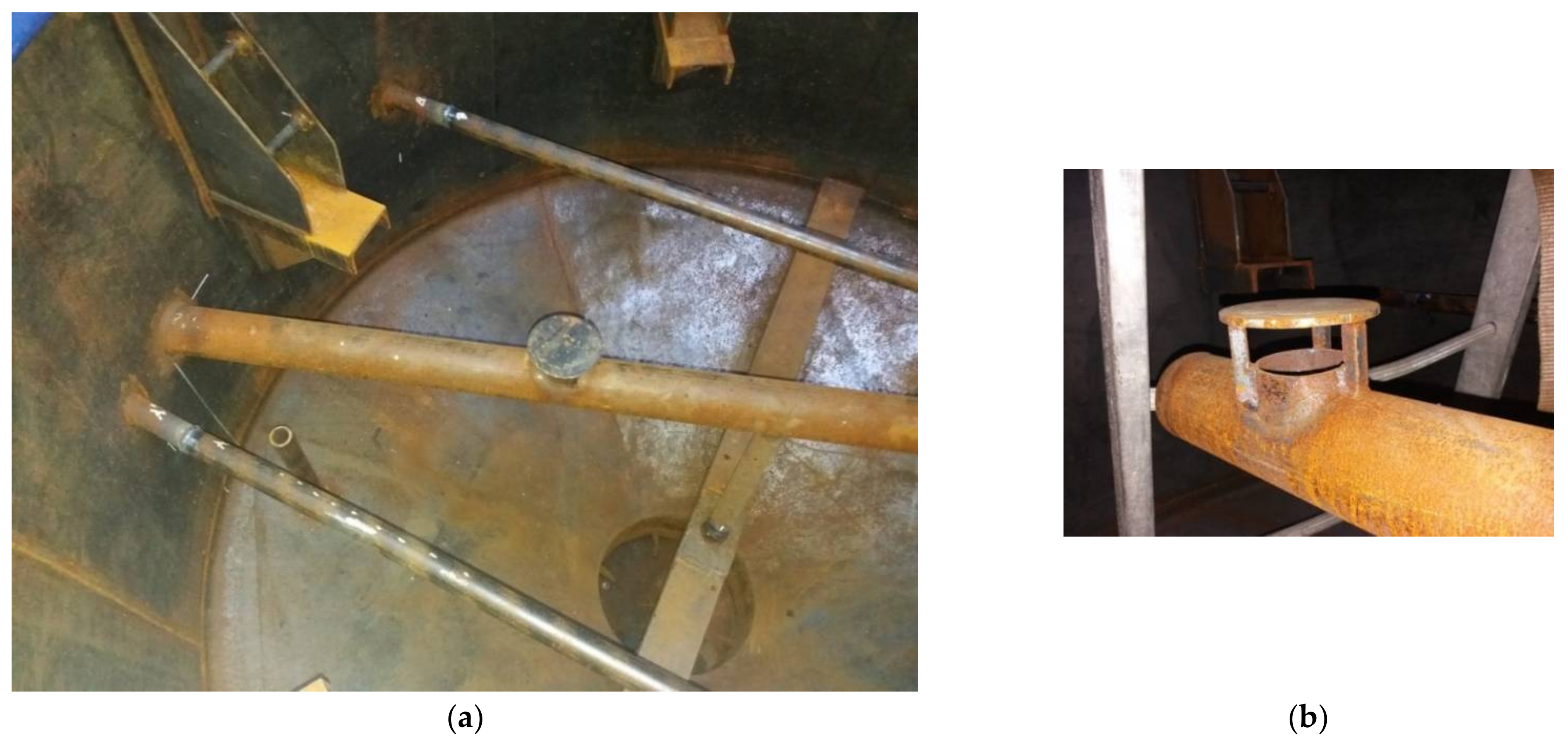
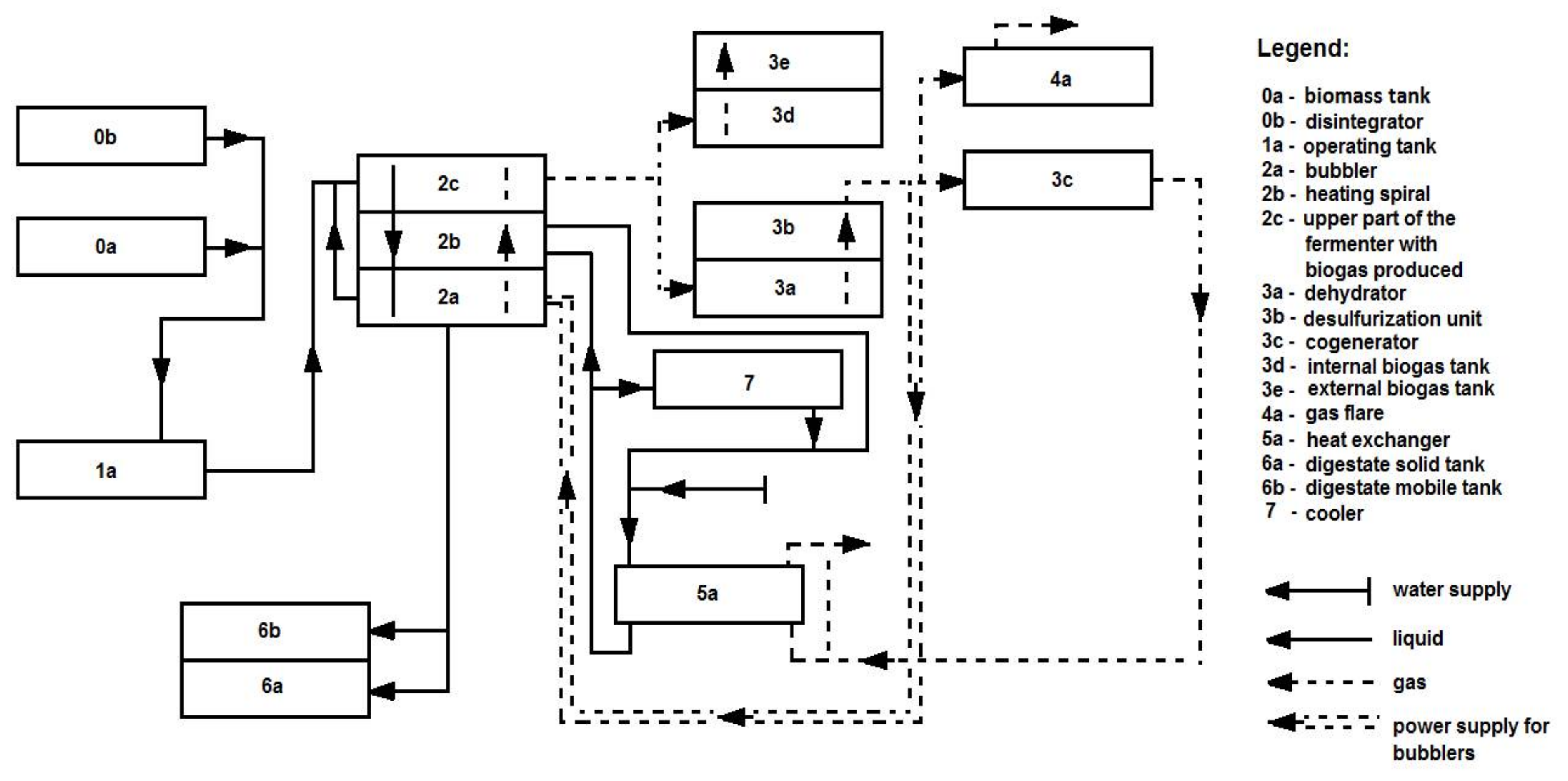

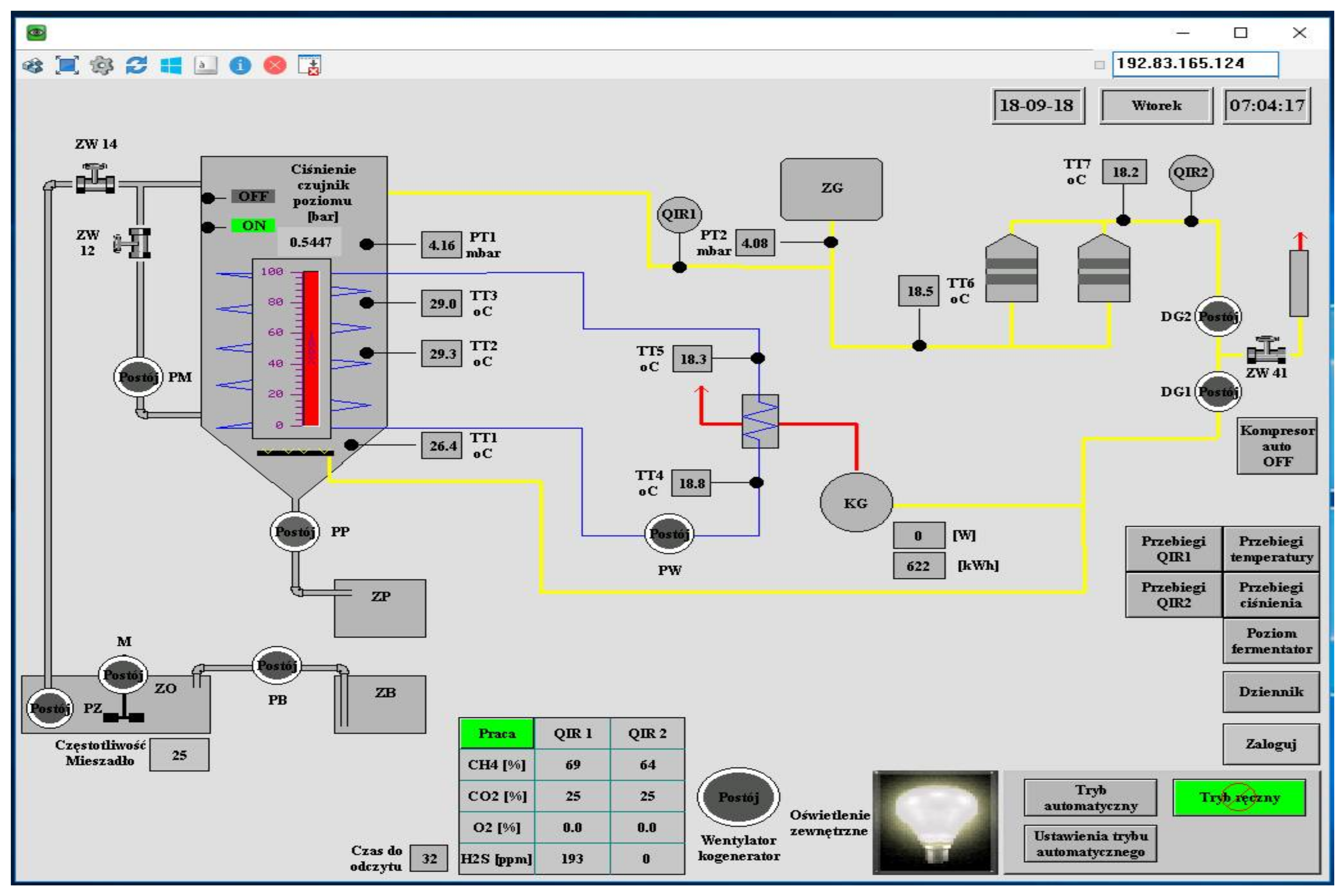


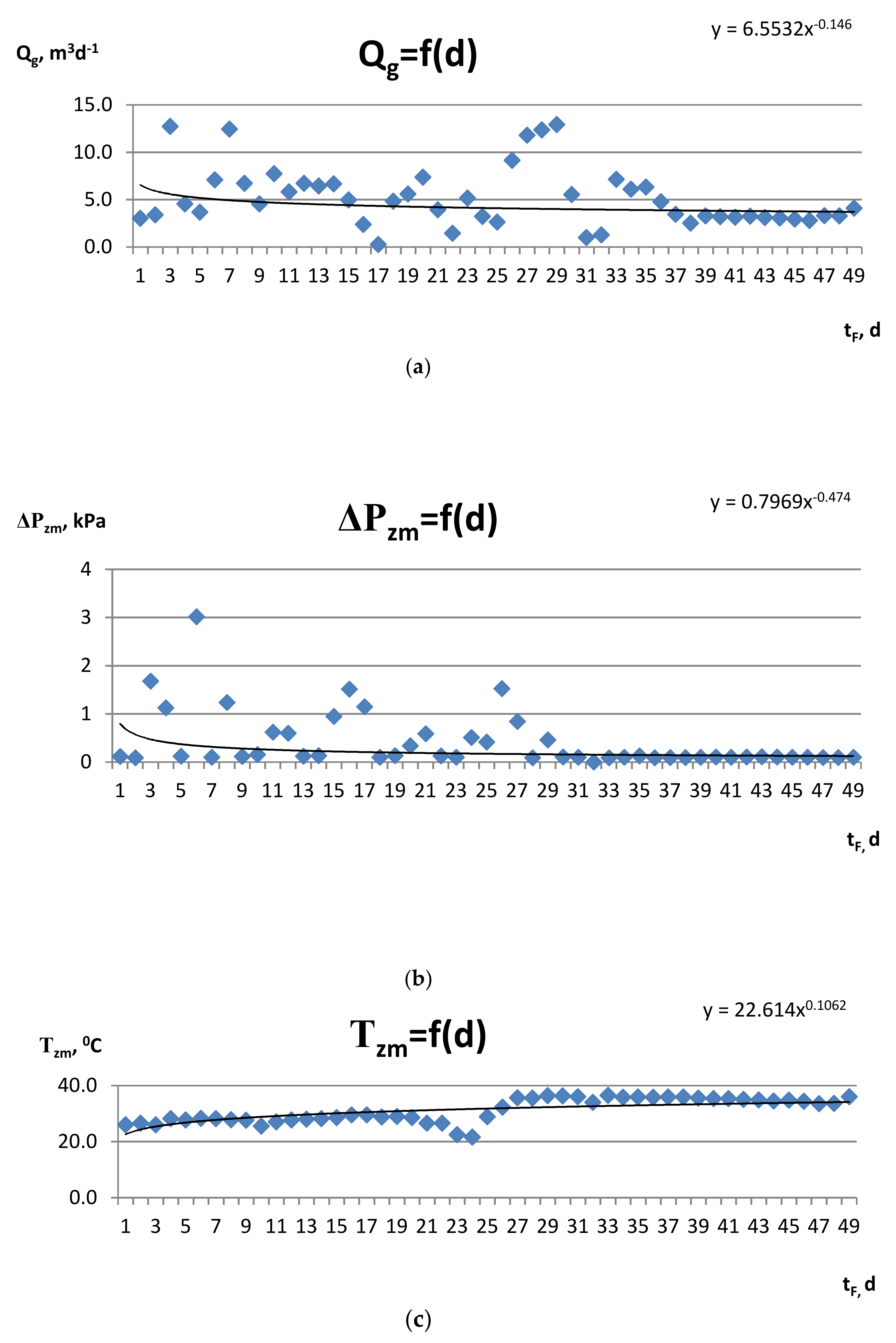
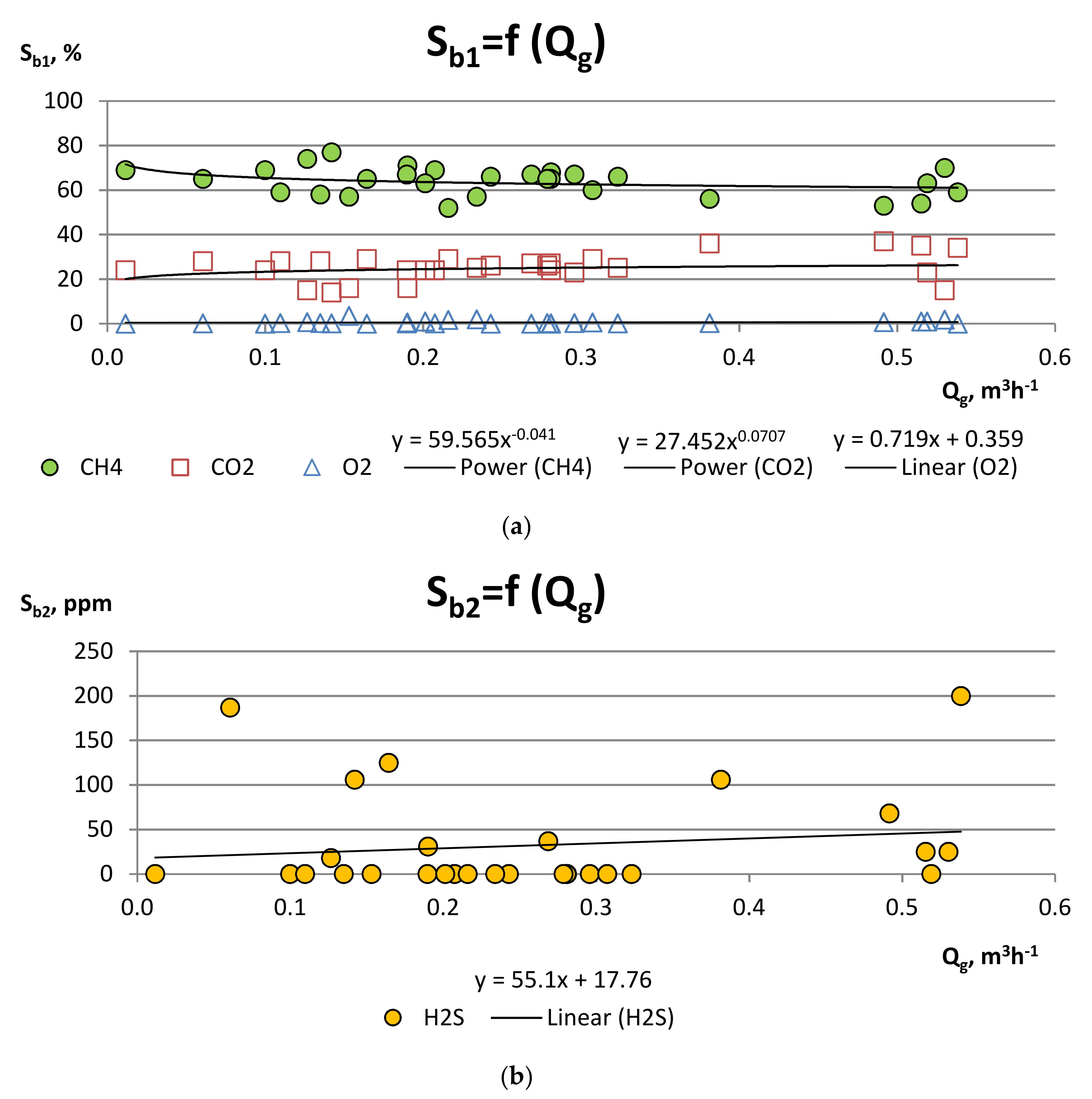
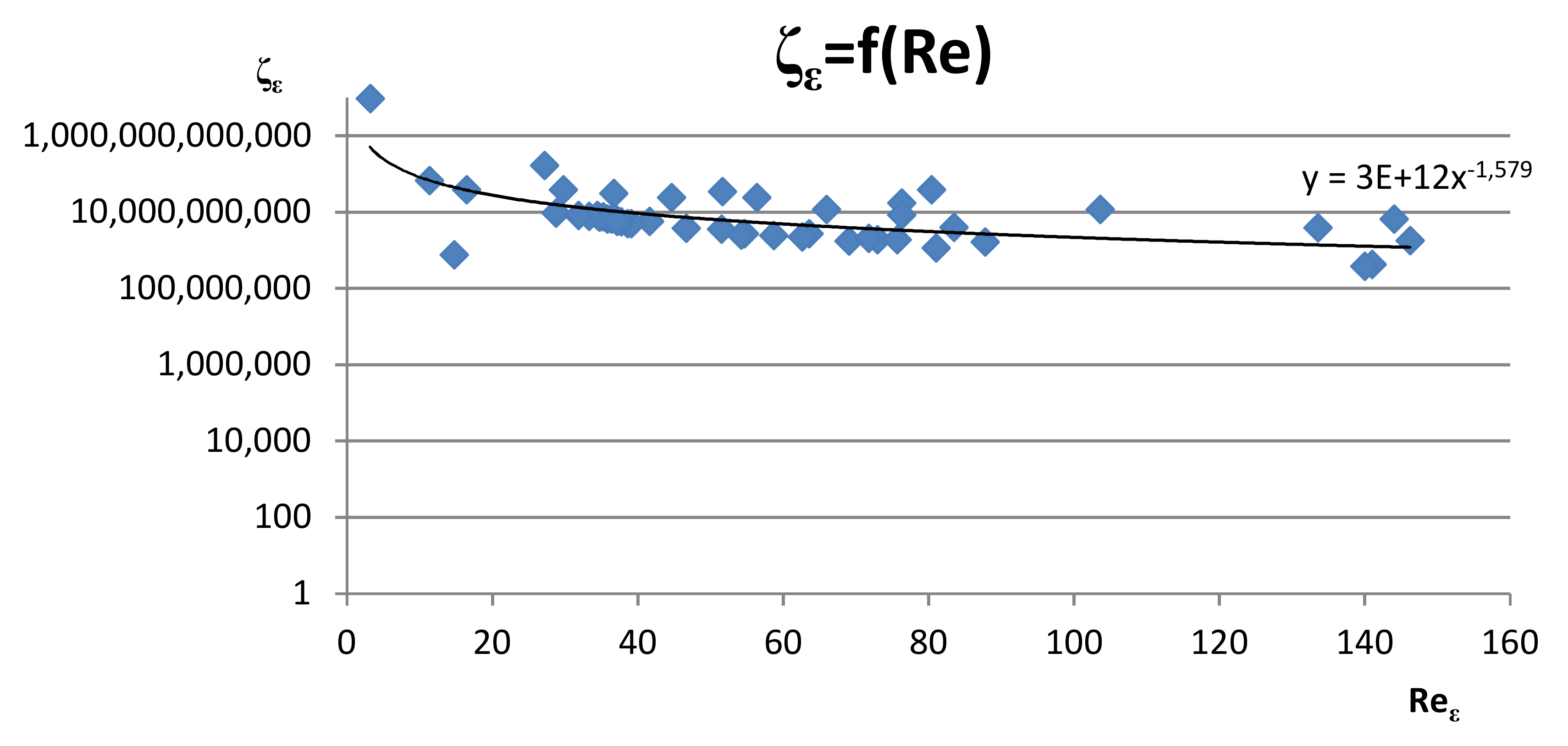


| Component | Unit | Value |
|---|---|---|
| Metabolic energy | MJ | 11.50 |
| Crude protein | % | 39.34 |
| lysine | % | 4.60 |
| Methionine | % | 1.08 |
| Threonine | % | 2.38 |
| Tryptophan | % | 0.55 |
| Crude fiber | % | 3.95 |
| Crude ash | % | 81.41 |
| Calcium (Ca) | % | 4.45 |
| Phosphorus (P) | % | 0.98 |
| Sodium (Na) | % | 0.90 |
| Raw oils and fats | % | 1.50 |
| Vitamin E | mg | 700.00 |
| Enzymes | (+/−) | + |
| Phytobiotic | (+/−) | + |
| Optiferm F1 25–50 kg | Optiferm F2 50–90 kg | Optiferm F3 90–115 kg | ||||||
|---|---|---|---|---|---|---|---|---|
| Barley | 35.5 | 30 | 25 | 30 | 25 | 35 | 35 | 35 |
| Wheat | 15 | 15 | 10 | 10 | 10 | 10 | 0 | 5 |
| Triticale | 25 | 27.5 | 35 | 30 | 30 | 25 | 30.5 | 35.5 |
| Rye | 0 | 0 | 0 | 0 | 10 | 5 | 10 | 5 |
| Wheat bran | 6 | 0 | 6 | 8 | 0 | 10 | 12 | 7 |
| Maize | 0 | 10 | 5.5 | 6.5 | 10 | 0 | 0 | 0 |
| Soybean oil | 1 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 |
| 600+ | 17.5 | 17.5 | 17.5 | 15 | 15 | 15 | 12.5 | 12.5 |
| Porker | Substrate Volume | Cycle |
|---|---|---|
| quantity | time | |
| 3500 pieces | 1400 m3 | 1 year |
| 1 piece | 0.4 m3 | 90 days |
| 1 piece | 0.4 L | 1 day |
| Measurement No. | Equivalent Velocity | Pressure Measured | Composition of Biogas | |||
|---|---|---|---|---|---|---|
| Fermentation Time Counted Daily | CH4 | CO2 | O2 | H2S | ||
| t, d | wε, m·s−1 | ∆Pzm, Pa | Sb1, % | Sb2, ppm | ||
| 1 | 0.00016 | 118 | 74 | 15 | 0.6 | 18 |
| 2 | 0.00017 | 91 | 77 | 14 | 0.1 | 106 |
| 3 | 0.00065 | 1681 | 70 | 15 | 1.8 | 25 |
| 4 | 0.00023 | 1127 | 71 | 16 | 0.5 | 31 |
| 5 | 0.00019 | 123 | 57 | 16 | 3.5 | 0 |
| 6 | 0.00036 | 3017 | 67 | 23 | 0.2 | 0 |
| 7 | 0.00064 | 104 | 63 | 23 | 0.9 | 0 |
| 8 | 0.00034 | 1240 | 68 | 24 | 0.1 | 0 |
| 9 | 0.00023 | 117 | 67 | 24 | 0.1 | 0 |
| 10 | 0.00040 | 155 | 66 | 25 | 0.1 | 0 |
| 11 | 0.00030 | 621 | 66 | 26 | 0 | 0 |
| 12 | 0.00034 | 601 | 65 | 27 | 0 | 0 |
| 13 | 0.00033 | 124 | 67 | 27 | 0 | 37 |
| 14 | 0.00034 | 133 | 65 | 26 | 0.3 | 0 |
| 15 | 0.00025 | 949 | 69 | 24 | 0.1 | 0 |
| 16 | 0.00012 | 1518 | 69 | 24 | 0 | 0 |
| 17 | 0.00001 | 1149 | 69 | 24 | 0 | 0 |
| 18 | 0.00025 | 100 | 63 | 24 | 0.9 | 0 |
| 19 | 0.00029 | 136 | 57 | 25 | 1.9 | 0 |
| 20 | 0.00038 | 340 | 60 | 29 | 0.5 | 0 |
| 21 | 0.00020 | 592 | 65 | 29 | 0 | 125 |
| 22 | 0.00007 | 128 | 65 | 28 | 0.1 | 187 |
| 23 | 0.00026 | 103 | 52 | 29 | 1.6 | 0 |
| 24 | 0.00017 | 515 | 58 | 28 | 0.3 | 0 |
| 25 | 0.00013 | 420 | 59 | 28 | 0.3 | 0 |
| 26 | 0.00047 | 1526 | 56 | 36 | 0.3 | 106 |
| 27 | 0.00060 | 847 | 53 | 37 | 0.7 | 68 |
| 28 | 0.00063 | 92 | 54 | 35 | 0.9 | 25 |
| 29 | 0.00066 | 463 | 59 | 34 | 0 | 200 |
| 30 | 0.00028 | 106 | 38 | 11 | 6.8 | 0 |
| 31 | 0.00005 | 104 | 38 | 11 | 6.8 | 0 |
| 32 | 0.00042 | 2 | 51 | 21 | 2.1 | 0 |
| 33 | 0.00037 | 93 | 34 | 25 | 5.2 | 0 |
| 34 | 0.00031 | 103 | 44 | 38 | 1.4 | 0 |
| 35 | 0.00032 | 128 | 51 | 36 | 0.2 | 0 |
| 36 | 0.00024 | 91 | 67 | 25 | 0 | 568 |
| 37 | 0.00018 | 94 | 67 | 26 | 0 | 1068 |
| 38 | 0.00013 | 94 | 66 | 25 | 0.4 | 600 |
| 39 | 0.00017 | 100 | 67 | 24 | 0.2 | 550 |
| 40 | 0.00016 | 106 | 68 | 23 | 0.1 | 556 |
| 41 | 0.00016 | 103 | 67 | 23 | 0.2 | 500 |
| 42 | 0.00017 | 107 | 68 | 23 | 0.2 | 418 |
| 43 | 0.00016 | 116 | 67 | 22 | 0.4 | 243 |
| 44 | 0.00016 | 110 | 65 | 22 | 0.7 | 162 |
| 45 | 0.00015 | 104 | 64 | 23 | 0.9 | 106 |
| 46 | 0.00014 | 101 | 64 | 23 | 0.8 | 131 |
| 47 | 0.00017 | 98 | 64 | 24 | 0.6 | 125 |
| 48 | 0.00017 | 97 | 64 | 24 | 0.5 | 106 |
| 49 | 0.00021 | 101 | 64 | 24 | 0.5 | 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, K.; Kapłan, M.; Syrotyuk, S.; Konieczny, R.; Anders, D.; Dybek, B.; Karwacka, A.; Wałowski, G. Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed. Energies 2021, 14, 3538. https://doi.org/10.3390/en14123538
Klimek K, Kapłan M, Syrotyuk S, Konieczny R, Anders D, Dybek B, Karwacka A, Wałowski G. Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed. Energies. 2021; 14(12):3538. https://doi.org/10.3390/en14123538
Chicago/Turabian StyleKlimek, Kamila, Magdalena Kapłan, Serhiy Syrotyuk, Ryszard Konieczny, Dorota Anders, Barbara Dybek, Agnieszka Karwacka, and Grzegorz Wałowski. 2021. "Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed" Energies 14, no. 12: 3538. https://doi.org/10.3390/en14123538
APA StyleKlimek, K., Kapłan, M., Syrotyuk, S., Konieczny, R., Anders, D., Dybek, B., Karwacka, A., & Wałowski, G. (2021). Production of Agricultural Biogas with the Use of a Hydrodynamic Mixing System of a Polydisperse Substrate in a Reactor with an Adhesive Bed. Energies, 14(12), 3538. https://doi.org/10.3390/en14123538







