Prospective of Upfront Nitrogen (N2) Removal in LNG Plants: Technical Communication
Abstract
:1. Introduction
2. Advances on State-of-the-Art
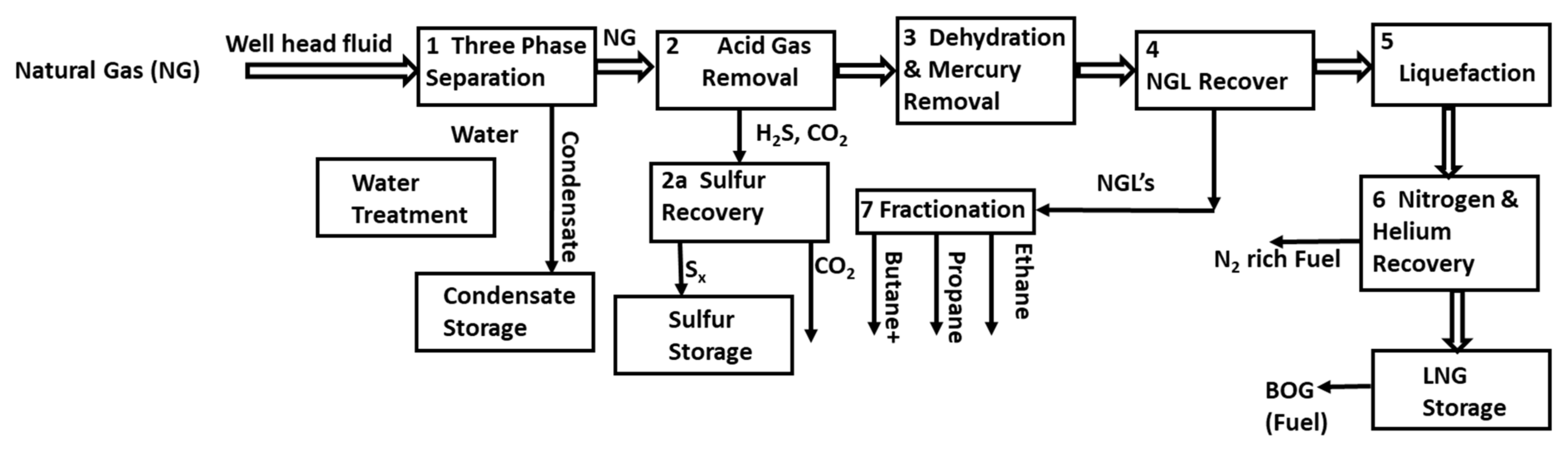
3. Upfront N2 Removal Methodology
4. Literature Analysis of the Upfront N2 Removal Technologies
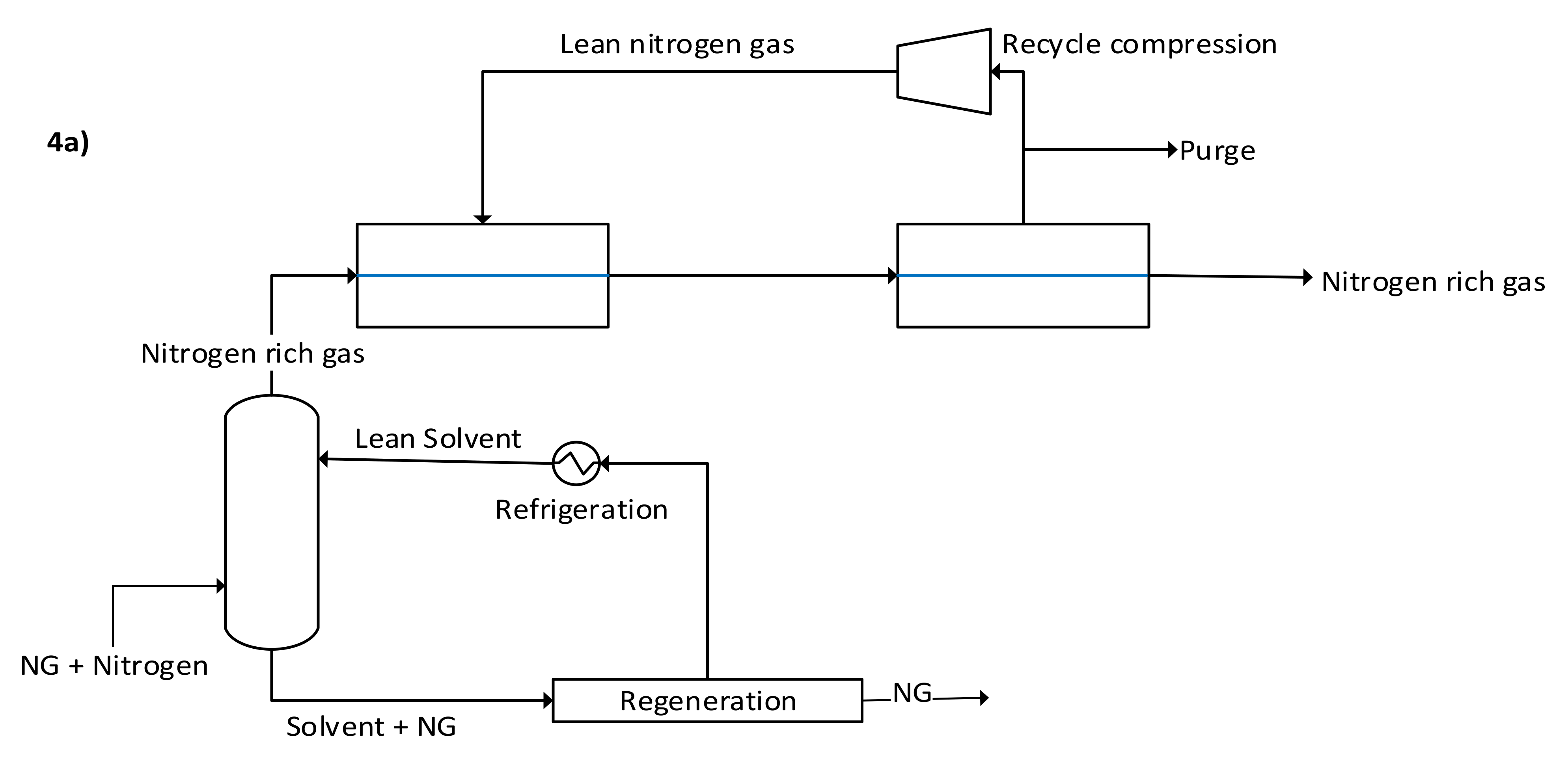
4.1. Physical Separation Technologies
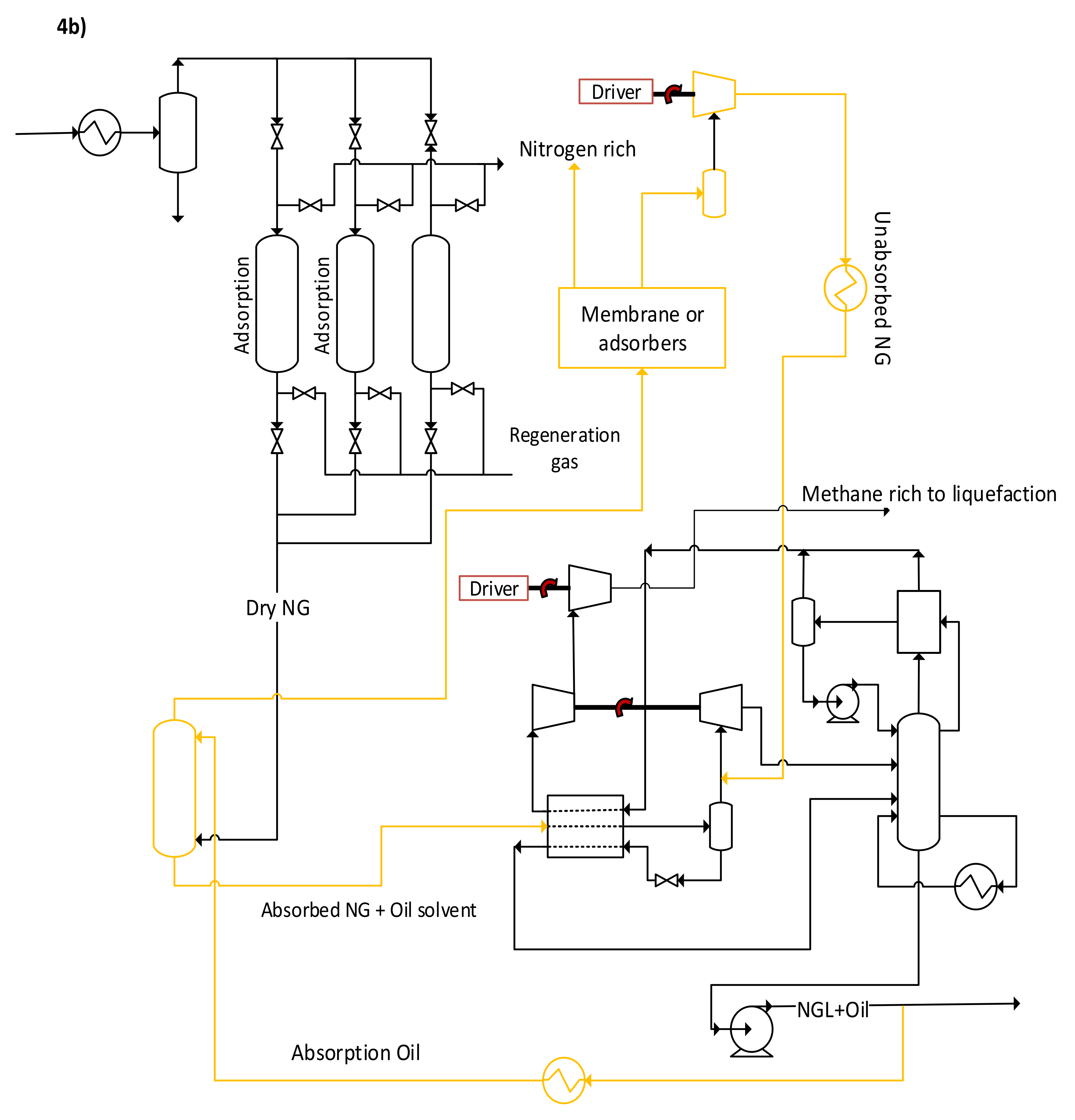
4.1.1. Adsorption
- Porous-aromatic frameworks (PAFs): Open-structure aromatic frameworks that are superior over MOFs and zeolites in terms of ultrahigh surface area and high stability [16].
- Carbon molecular sieves (CMS): Solid adsorbents manufactured from organic sources such as coconut shell and coal, differing from AC in having a narrower range of pore size [19,20]. It has been stated that they possess a kinetic selectivity for N2 over methane [21]. The TRL of the CMS is ranged from 6–9.
4.1.2. Membranes
- Mixed-matrix membranes (MMMs): Membranes fabricated from organic polymers and inorganic materials to increase the permselectivity of the membrane where inorganic materials could be zeolites, MOFs, or CMS [44].
4.1.3. Hybrid Upfront N2 Removal Technologies
4.1.4. Distillation
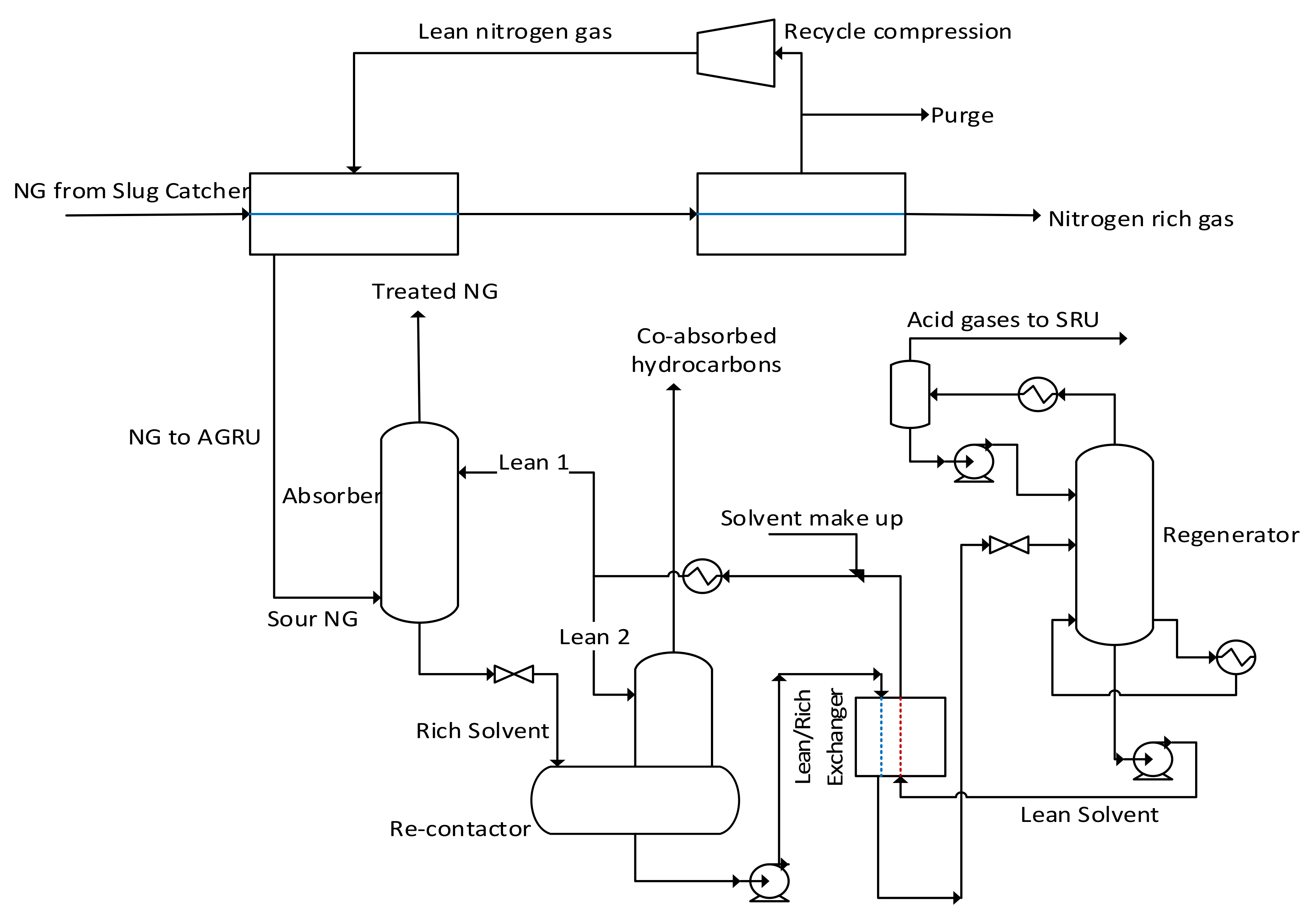
4.2. Chemical Separation Technologies
4.3. Gas Hydrate Technology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| CBM | Coal-bed methane |
| CMS | Carbon molecular sieves |
| HCs | Hydrocarbons |
| LNG | Liquefied natural gas |
| NG | Natural gas |
| PSA | Pressure swing adsorption |
| C3/MR | Propane/mixed refrigerant |
| BOG | Boil-off gas |
| AGR | Acid gas removal |
| MOFs | Metal-organic frameworks |
| CBM | Coal-bed methane |
| SAPO | Silicoaluminophosphate |
| NRU | Nitrogen removal unit |
References
- Osorio-Tejada, J.L.; Llera-Sastresa, E.; Scarpellini, S. Liquefied natural gas: Could it be a reliable option for road freight transport in the EU? Renew. Sustain. Energy Rev. 2017, 71, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Kurle, Y.M.; Wang, S.; Xu, Q. Dynamic simulation of LNG loading, BOG generation, and BOG recovery at LNG exporting terminals. Comput. Chem. Eng. 2017, 97, 47–58. [Google Scholar] [CrossRef]
- Kuo, J.; Wang, K.; Chen, C. Pros and cons of different Nitrogen Removal Unit (NRU) technology. J. Nat. Gas Sci. Eng. 2012, 7, 52–59. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. On the optimal design of membrane-based gas separation processes. J. Membr. Sci. 2017, 526, 118–130. [Google Scholar] [CrossRef]
- Ohs, B.; Lohaus, J.; Wessling, M. Optimization of membrane based nitrogen removal from natural gas. J. Membr. Sci. 2016, 498, 291–301. [Google Scholar] [CrossRef]
- Saleman, T.L.; Li, G.K.; Rufford, T.E.; Stanwix, P.L.; Chan, K.I.; Huang, S.H.; May, E.F. Capture of low grade methane from nitrogen gas using dual-reflux pressure swing adsorption. Chem. Eng. J. 2015, 281, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Anna, H.R.S.; Barreto, A.G., Jr.; Tavares, F.W.; do Nascimento, J.F. Methane/nitrogen separation through pressure swing adsorption process from nitrogen-rich streams. Chem. Eng. Process. Process Intensif. 2016, 103, 70–79. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bouabidi, Z.; Katebah, M.A.; Hussein, M.M.; Shazed, A.R.; Al-musleh, E.I. Towards improved and multi-scale liquefied natural gas supply chains: Thermodynamic analysis. Comput. Chem. Eng. 2021, 107359. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Corbin, D.R.; Shiflett, M.B. A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas. Ind. Eng. Chem. Res. 2020, 59, 13355–13369. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, D.; He, D.; Xing, J.; Zeng, S.; Xu, S.; Xu, Y.; Liu, Z. Decorated Traditional Zeolites with Subunits of Metal–Organic Frameworks for CH4/N2 Separation. Angew. Chem. Int. Ed. 2019, 58, 10241–10244. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, S.-Y.; Yoon, T.-U.; Kim, M.-B.; Park, W.; Han, H.H.; Kong, C.-I.; Park, C.-Y.; Kim, J.-H.; Bae, Y.-S. Improved methane/nitrogen separation properties of zirconium-based metal–organic framework by incorporating highly polarizable bromine atoms. Chem. Eng. J. 2020, 399, 125717. [Google Scholar] [CrossRef]
- Silva, J.A.C.; Ferreira, A.; Mendes, P.A.P.; Cunha, A.F.; Gleichmann, K.; Rodrigues, A.E. Adsorption Equilibrium and Dynamics of Fixed Bed Adsorption of CH4/N2 in Binderless Beads of 5A Zeolite. Ind. Eng. Chem. Res. 2015, 54, 6390–6399. [Google Scholar] [CrossRef]
- Ben, T.; Qiu, S. Porous aromatic frameworks: Synthesis, structure and functions. CrystEngComm 2013, 15, 17–26. [Google Scholar] [CrossRef]
- Su, W.; Yao, L.; Ran, M.; Sun, Y.; Liu, J.; Wang, X. Adsorption Properties of N2, CH4, and CO2 on Sulfur-Doped Microporous Carbons. J. Chem. Eng. Data 2018, 63, 2914–2920. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Zhang, P.; Xu, M.; Tan, T.; Wang, J.; Deng, Q.; Zhang, L.; Wan, Y.; Deng, S. Facile preparation of N and O-rich porous carbon from palm sheath for highly selective separation of CO2/CH4/N2 gas-mixture. Chem. Eng. J. 2020, 399, 125812. [Google Scholar] [CrossRef]
- Ghazi-MirSaeed, M.; Matavos-Aramyan, S. Gaseous Mixtures Separation via Chemically-Activated Nano Silica-Modified Carbon Molecular Sieves. Silicon 2020. [Google Scholar] [CrossRef]
- Lemcoff, N.O. Nitrogen separation from air by pressure swing adsorption. In Studies in Surface Science and Catalysis; Dąbrowski, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 120, pp. 347–370. [Google Scholar]
- Effendy, S.; Xu, C.; Farooq, S. Optimization of a Pressure Swing Adsorption Process for Nitrogen Rejection from Natural Gas. Ind. Eng. Chem. Res. 2017, 56, 5417–5431. [Google Scholar] [CrossRef]
- Mitariten, M.; Dolan, W. Nitrogen Removal from Natural Gas with the Molecular Gate Technology. In Proceedings of the Laurance Reid Gas Conditioning Conference, Norman, OK, USA, 2001. [Google Scholar]
- Neishabori Salehi, R.; Rahimpour, F.; Sharifnia, S. Adsorption of carbon dioxide, nitrogen and methane on modified titanosilicate type molecular sieves. J. Nat. Gas Sci. Eng. 2017, 46, 730–737. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Yang, Y.; Kong, X.-M.; Li, P.; Yu, J.-G.; Ribeiro, A.M.; Rodrigues, A.E. Adsorption of Pure and Binary CO2, CH4, and N2 Gas Components on Activated Carbon Beads. J. Chem. Eng. Data 2015, 60, 2684–2693. [Google Scholar] [CrossRef]
- Chen, G.; An, Y.; Shen, Y.; Wang, Y.; Tang, Z.; Lu, B.; Zhang, D. Effect of pore size on CH4/N2 separation using activated carbon. Chin. J. Chem. Eng. 2020, 28, 1062–1068. [Google Scholar] [CrossRef]
- Peng, X.; Jin, Q. Ideal adsorbed solution theory, two-dimensional equation of state, and molecular simulation for separation of H2/N2/O2/CH4/CO in graphite nanofiber and C60 intercalated graphite. Sep. Purif. Technol. 2020, 237, 116369. [Google Scholar] [CrossRef]
- Jia, X.; Yuan, N.; Wang, L.; Yang, J.; Li, J. (CH3)2NH-Assisted Synthesis of High-Purity Ni-HKUST-1 for the Adsorption of CO2, CH4, and N2. Eur. J. Inorg. Chem. 2018, 2018, 1047–1052. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Yang, Q.; Liu, D. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation. Chem. Eng. J. 2021, 408, 127294. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Wang, J.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework. AIChE J. 2018, 64, 3681–3689. [Google Scholar] [CrossRef]
- Pillai, R.S.; Yoon, J.W.; Lee, S.-J.; Hwang, Y.K.; Bae, Y.-S.; Chang, J.-S.; Maurin, G. N2 Capture Performances of the Hybrid Porous MIL-101(Cr): From Prediction toward Experimental Testing. J. Phys. Chem. C 2017, 121, 22130–22138. [Google Scholar] [CrossRef]
- Hu, J.; Wu, F.; Gu, C.; Liu, J. Computational Design of Porous Framework Materials with Transition-Metal Alkoxide Ligands for Highly Selective Separation of N2 over CH4. Ind. Eng. Chem. Res. 2021, 60, 378–386. [Google Scholar] [CrossRef]
- Weh, R.; Xiao, G.; Islam, M.A.; May, E.F. Nitrogen Rejection by Dual Reflux Pressure Swing Adsorption Using Engelhard Titanosilicate Type 4. Ind. Eng. Chem. Res. 2020, 59, 22573–22581. [Google Scholar] [CrossRef]
- Henrique, A.; Karimi, M.; Silva, J.A.C.; Rodrigues, A.E. Analyses of Adsorption Behavior of CO2, CH4, and N2 on Different Types of BETA Zeolites. Chem. Eng. Technol. 2019, 42, 327–342. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.A.; Tezel, F.H. Cation exchange modification of clinoptilolite—Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater. 2018, 262, 235–250. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Mujčin, M.; Abou-Zeid, C.; Tezel, F.H. Cation exchange modification of clinoptilolite–thermodynamic effects on adsorption separations of carbon dioxide, methane, and nitrogen. Microporous Mesoporous Mater. 2019, 274, 327–341. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhai, C.; Sun, J.; Panezai, H.; Bai, S. Nanosol precursor as structural promoter for clinoptilolite via hydrothermal synthesis and resulting effects on selective adsorption of CH4 and N2. Microporous Mesoporous Mater. 2020, 294, 109913. [Google Scholar] [CrossRef]
- Fischer, M. Porous aluminophosphates as adsorbents for the separation of CO2/CH4 and CH4/N2 mixtures—A Monte Carlo simulation study. Sustain. Energy Fuels 2018, 2, 1749–1763. [Google Scholar] [CrossRef]
- Sun, T.; Hu, J.; Ren, X.; Wang, S. Experimental Evaluation of the Adsorption, Diffusion, and Separation of CH4/N2 and CH4/CO2 Mixtures on Al-BDC MOF. Sep. Sci. Technol. 2015, 50, 874–885. [Google Scholar] [CrossRef]
- Yang, Z.; Ning, H.; Liu, J.; Meng, Z.; Li, Y.; Ju, X.; Chen, Z. Surface modification on semi-coke-based activated carbon for enhanced separation of CH4/N2. Chem. Eng. Res. Des. 2020, 161, 312–321. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Xing, R.; Zhang, D.-H.; Shen, Y.-H.; Fu, Q.; Ding, Z.-Y.; Tian, C.-X. Vacuum pressure swing adsorption system for N2/CH4 separation under uncertainty. Chem. Eng. Res. Des. 2019, 142, 245–256. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.A.E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Katebah, M.A.; Hussein, M.M.; Shazed, A.; Bouabidi, Z.; Al-musleh, E.I. Rigorous simulation, energy and environmental analysis of an actual baseload LNG supply chain. Comput. Chem. Eng. 2020, 141, 106993. [Google Scholar] [CrossRef]
- Race, J.M.; Wetenhall, B.; Seevam, P.N.; Downie, M.J. Towards a CO 2 pipeline specification: Defining tolerance limits for impurities. J. Pipeline Eng. 2012, 11. [Google Scholar]
- Wang, S.; Guo, Q.; Liang, S.; Li, P.; Li, X.; Luo, J. [Ni3(HCOO)6]/Poly(styrene-b-butadiene-b-styrene) Mixed-Matrix Membranes for CH4/N2 Gas Separation. Chem. Eng. Technol. 2018, 41, 353–366. [Google Scholar] [CrossRef]
- Tanis, I.; Brown, D.; Neyertz, S.; Heck, R.; Mercier, R.; Vaidya, M.; Ballaguet, J.-P. A comparison of pure and mixed-gas permeation of nitrogen and methane in 6FDA-based polyimides as studied by molecular dynamics simulations. Comput. Mater. Sci. 2018, 141, 243–253. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.-C. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Miricioiu, M.G.; Niculescu, V.-C.; Filote, C.; Raboaca, M.S.; Nechifor, G. Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation. Membranes 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J. Membr. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Zhao, S.; Cao, X.; Ma, Z.; Wang, Z.; Qiao, Z.; Wang, J.; Wang, S. Mixed-matrix membranes for CO2/N2 separation comprising a poly (vinylamine) matrix and metal–organic frameworks. Ind. Eng. Chem. Res. 2015, 54, 5139–5148. [Google Scholar] [CrossRef]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Pinnau, I.; He, Z.; Amo, K.D.; DaCosta, A.R.; Wijmans, J.G.; Baker, R.W. Membrane separation of nitrogen from natural gas: A case study from membrane synthesis to commercial deployment. J. Membr. Sci. 2010, 346, 270–279. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Alam, S.F.; Kim, M.-Z.; Kim, Y.J.; Rehman, A.U.; Devipriyanka, A.; Sharma, P.; Yeo, J.-G.; Lee, J.-S.; Kim, H.; Cho, C.-H. A new seeding method, dry rolling applied to synthesize SAPO-34 zeolite membrane for nitrogen/methane separation. J. Membr. Sci. 2020, 602, 117825. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Liu, B.; Wang, B.; Zhou, R. An Effective Approach to Synthesize High-Performance SSZ-13 Membranes Using the Steam-Assisted Conversion Method for N2/CH4 Separation. Energy Fuels 2020, 34, 16502–16511. [Google Scholar] [CrossRef]
- Montes Luna, A.D.J.; Castruita de León, G.; García Rodríguez, S.P.; Fuentes López, N.C.; Pérez Camacho, O.; Perera Mercado, Y.A. Na+/Ca2+ aqueous ion exchange in natural clinoptilolite zeolite for polymer-zeolite composite membranes production and their CH4/CO2/N2 separation performance. J. Nat. Gas Sci. Eng. 2018, 54, 47–53. [Google Scholar] [CrossRef]
- Montes Luna, A.D.J.; Fuentes López, N.C.; Castruita de León, G.; Pérez Camacho, O.; Yeverino Miranda, C.Y.; Perera Mercado, Y.A. PBI/Clinoptilolite mixed-matrix membranes for binary (N2/CH4) and ternary (CO2/N2/CH4) mixed gas separation. J. Appl. Polym. Sci. 2021, 138, 50155. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wu, T.; Song, S.; Wang, B.; Zhong, S.; Zhou, R. High-performance SSZ-13 membranes prepared using ball-milled nanosized seeds for carbon dioxide and nitrogen separations from methane. Chin. J. Chem. Eng. 2020, 28, 1285–1292. [Google Scholar] [CrossRef]
- Nagesh Rao, H.; Karimi, I.A. A superstructure-based model for multistream heat exchanger design within flow sheet optimization. AIChE J. 2017, 63, 3764–3777. [Google Scholar] [CrossRef]
- Hamedi, H.; Karimi, I.A.; Gundersen, T. Optimal cryogenic processes for nitrogen rejection from natural gas. Comput. Chem. Eng. 2018, 112, 101–111. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, Y.; Lee, W.B. Integrated Process Design and Optimization of Nitrogen Recovery in Natural Gas Processing. Ind. Eng. Chem. Res. 2019, 58, 1658–1674. [Google Scholar] [CrossRef]
- Ghorbani, B.; Hamedi, M.-H.; Amidpour, M. Development and optimization of an integrated process configuration for natural gas liquefaction (LNG) and natural gas liquids (NGL) recovery with a nitrogen rejection unit (NRU). J. Nat. Gas Sci. Eng. 2016, 34, 590–603. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Sharifzadeh, M.M.M.; Ansarinasab, H. Investigation of a novel integrated process configuration for natural gas liquefaction and nitrogen removal by advanced exergoeconomic analysis. Appl. Therm. Eng. 2018, 128, 1249–1262. [Google Scholar] [CrossRef]
- Friesen, D.T.; Babcock, W.C.; Edlund, D.J.; Miller, W.K.; Lyon, D.K. Liquid Absorbent Solutions for Separating Nitrogen from Natural Gas; Bend Research, Inc.: Bend, OR, USA, 1997. [Google Scholar]
- Li, Z.; Xiao, G.; Graham, B.; Li, G.; May, E.F. Nitrogen Sorption in a Transition Metal Complex Solution for N2 Rejection from Methane. Ind. Eng. Chem. Res. 2019, 58, 13284–13293. [Google Scholar] [CrossRef]
- Li, Z. Separation of Nitrogen from Natural Gas: Conventional and Emerging Technologies; The University of Western Australia: Perth, Australia, 2018. [Google Scholar]
- Gilbertson, J.D.; Szymczak, N.K.; Crossland, J.L.; Miller, W.K.; Lyon, D.K.; Foxman, B.M.; Davis, J.; Tyler, D.R. Coordination Chemistry of H2 and N2 in Aqueous Solution. Reactivity and Mechanistic Studies Using trans-FeII(P2)2X2-Type Complexes (P2 = a Chelating, Water-Solubilizing Phosphine). Inorg. Chem. 2007, 46, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Bomberger, D.C.; Bomben, J.L.; Amirbahman, A.; Asaro, M. Nitrogen Removal from Natural Gas: Phase II; Federal Energy Technology Center: Morgantown, WV, USA, 1999. [Google Scholar]
- Gu, Q.; Shang, J.; Hanif, A.; Li, G.; Shirazian, S. Theoretical Study of Moisture-Pretreated Lithium as Potential Material for Natural Gas Upgrading. Ind. Eng. Chem. Res. 2018, 57, 15512–15521. [Google Scholar] [CrossRef]
- Ballard, A.; Sloan, E., Jr. The next generation of hydrate prediction: An overview. J. Supramol. Chem. 2002, 2, 385–392. [Google Scholar] [CrossRef]
- Jhaveri, J.; Robinson, D.B. Hydrates in the methane-nitrogen system. Can. J. Chem. Eng. 1965, 43, 75–78. [Google Scholar] [CrossRef]
- Dong, Q.; Su, W.; Liu, X.; Liu, J.; Sun, Y. Separation of the N2/CH4 mixture through hydrate formation in ordered mesoporous carbon. Adsorpt. Sci. Technol. 2014, 32, 821–832. [Google Scholar] [CrossRef]
- Chatti, I.; Delahaye, A.; Fournaison, L.; Petitet, J.-P. Benefits and drawbacks of clathrate hydrates: A review of their areas of interest. Energy Convers. Manag. 2005, 46, 1333–1343. [Google Scholar] [CrossRef]
- Happel, J.; Hnatow, M.A.; Meyer, H. The Study of Separation of Nitrogen from Methane by Hydrate Formation Using a Novel Apparatus a. Ann. New York Acad. Sci. 1994, 715, 412–424. [Google Scholar] [CrossRef]
- Waycuilis, J.J.; York, S.D. Production of a Gas Hydrate Slurry Using a Fluidized Bed Heat Exchanger. U.S. Patent No. 6,350,928, 26 February 2002. [Google Scholar]
- Cai, J.; Xu, C.-G.; Chen, Z.-Y.; Li, X.-S. Recovery of methane from coal-bed methane gas mixture via hydrate-based methane separation method by adding anionic surfactants. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1019–1026. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B.; Li, Y.; Yuan, G.; Xu, Z.; Guo, X.; Li, X.; Lan, W.; Yang, L. Enhanced separation of coal bed methane via bioclathrates formation. Fuel 2019, 243, 10–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, J.; Zhang, B.; Linga, P. Coal mine gas separation of methane via clathrate hydrate process aided by tetrahydrofuran and amino acids. Appl. Energy 2021, 287, 116576. [Google Scholar] [CrossRef]
- Sun, Q.; Azamat, A.; Chen, B.; Guo, X.; Yang, L. The effects of alkyl polyglucosides on the formation of CH4 hydrate and separation of CH4/N2 via hydrates formation. Sep. Sci. Technol. 2020, 55, 81–87. [Google Scholar] [CrossRef]

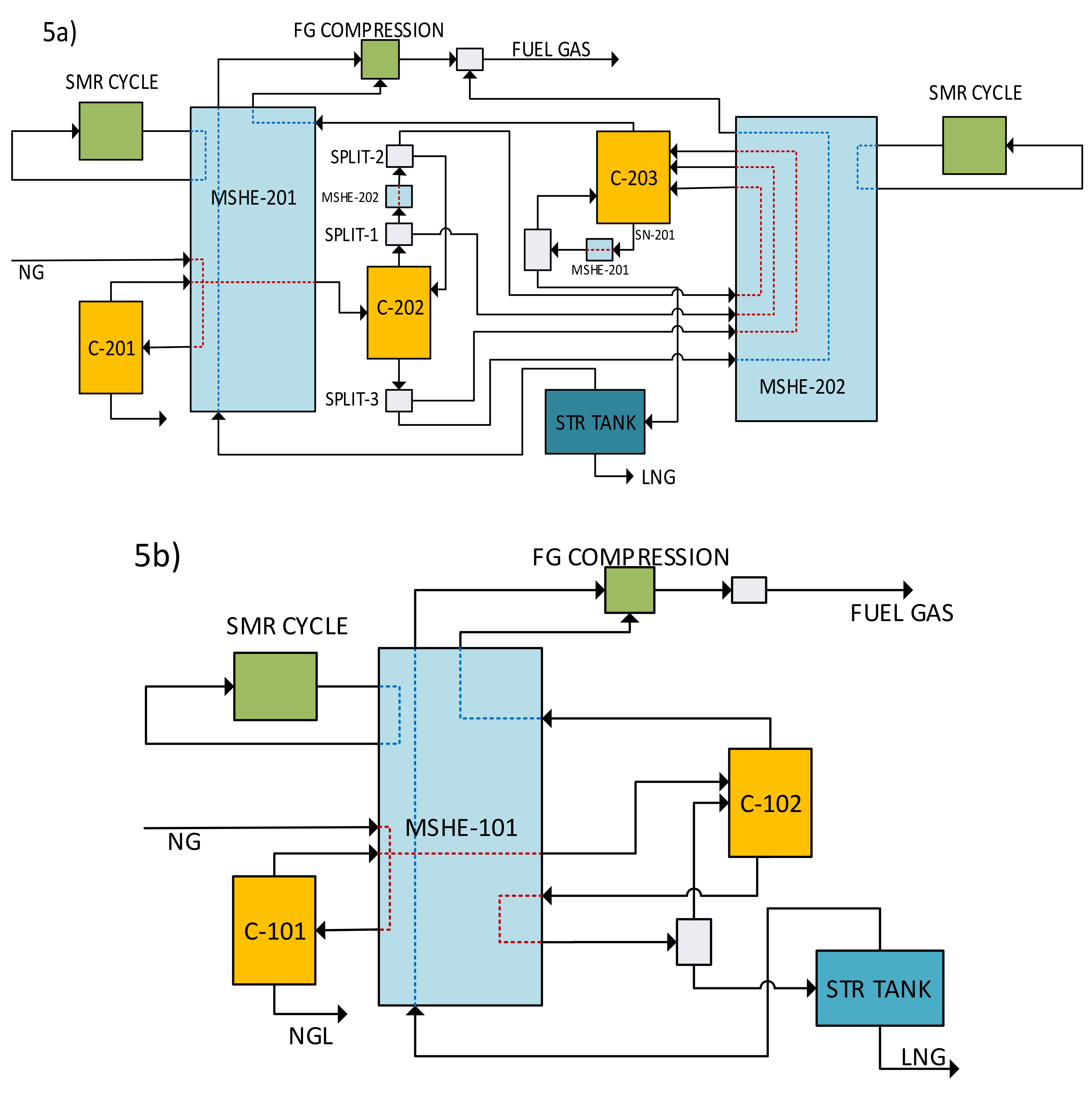
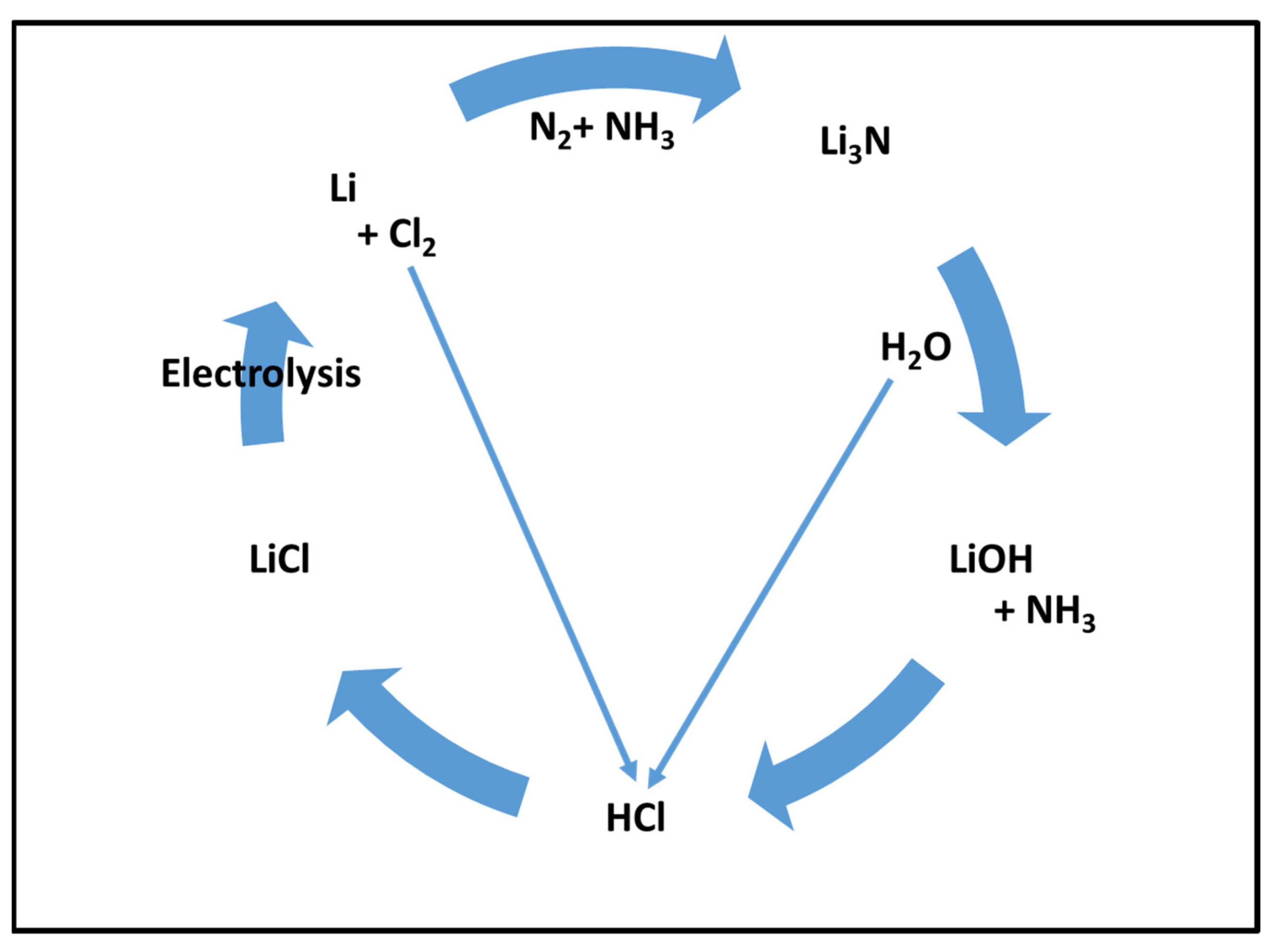
| Adsorbent Type | Adsorbent Name | Feed (by mol) | Operating Conditions | Favored Component | Best Selectivity | Type of Work | Reference |
|---|---|---|---|---|---|---|---|
| AC | — | 50% CH4 50% N2 | 1 bar 30 °C | CH4 | 5.5 | Experimental | [24] |
| SMAC | — | 1 bar 25 °C | CH4 | 3.644 | Experimental | [17] | |
| — | — | 3 bar 25 °C | N2 | 7.64 | GCMC and MD simulation | [25] | |
| CMS | — | — | 40 bar 25 °C | N2 | 2.1 | Experimental | [19] |
| Takeda CMS | 90% CH4 10% N2 | 3–16 bar | N2 | * 97.9% CH4 for 10% N2 in the feed | simulation | [21] † | |
| Graphite nanofibers | — | 50% CH4 50% N2 | 0–15 bar 25 °C | N2 | 5 to 8 | GCMC simulation | [26] |
| MOF | Ni-HKUST-1 | 50% CH4 50% N2 | 1 bar 0 °C | CH4 | 5.36 | Experimental | [27] |
| SBMOF-1 | 50% CH4 50% N2 | 1 bar 25 °C | CH4 | 11.5 | Experimental | [28] | |
| — | 90% CH4 10% N2 | 1 bar 0 °C and 25 °C | CH4 | 28 @ 0 °C and 16 @ 25 °C | Experimental | [29] | |
| Zr-based MOF | 50% CH4 50% N2 | 1–15 bar 25 °C | CH4 | 5.06 @ 1 bar and 5.63 @ 15 bar | Experimental and Molecular simulation | [14] | |
| MIL-101(Cr) | 90% CH4/10% N2 80% CH4/20% N2 | 0.1–10 bar | N2 | 5–10 @ 20% N2 and 10 °C 1.32 @ 10% N2 and 250 °C | Experimental and GCMC simulation | [30] | |
| PAF | PAF-302 PAF-303 PAF-304 | 10–90% N2 and CH4 | 1 bar 25 °C | N2 | 18.3 to 118.2 from 90% N2 to 10% N2 | GCMC simulation | [31] |
| Titanosilicate | ETS-10 | 70% CH4 15% CO2 10% N2 5% He | 10 bar 4 °C | CO2 then N2 over CH4 | 8.35 for CO2 and 1.24 for N2 | Experimental | [23] |
| ETS-4 | 85% CH4 15% N2 | 1 bar and 20 bar 20 °C | N2 | * 95.7% CH4 99% CH4 recovery | Numerical simulation | [32] | |
| — | 85% CH4 15% N2 | 7 bar | CO2 then N2 | * 90–93% CH4 recovery 4% N2 in sales gas | — | [22] | |
| Zeolite | BETA | — | 40 °C, 100 °C, 150 °C | CH4 | 1.27–2.05 | Experimental | [33] |
| CLINO | — | 0–10 bar 30 °C | CH4-Cs+ exchanged clinoptilolite | 20.6 in vacuum and 5.7 @ 10 bar | Experimental | [34] | |
| CLINO | — | 0–10 bar 0 °C and 100 °C | CH4-Cs+ exchanged clinoptilolite | 1 @ 0 °C and 10 bar 10 @ 100 °C and 0.001 bar | Experimental | [35] | |
| CLINO | — | 1 bar 0 °C and 25 °C | CH4 | 1.2–2.6 | Experimental | [36] | |
| zeolite framework | AIPO | 85% CH4 15% N2 | 10 bar 25 °C | CH4 | 3.8 | GCMC simulation | [37] |
| Zeolites with MOF subunits | ZIF | 50% CH4 50% N2 | 1 bar 25 °C | CH4 | 8.44 | molecular simulation and experimental | [13] |
| Membrane Type | Membrane Name | Feed (by mol) | Feed Pressure and Temperature | Favored Component | Permselectivity | Type of Work | Reference |
|---|---|---|---|---|---|---|---|
| Fluorinated polyimides | 6FDA-durene | 66% CH4 34% N2 | up to 60 bar 65 °C | N2 | 1 | Molecular dynamics simulations | [45] |
| MMM | [Ni3(HCOO)6]/SBS-5 MMM | 50% CH4 50% N2 | 1 bar 25 °C | CH4 | 2.9 | Experimental | [44] |
| Amide and CLINO | 85% CH4 10% CO2 5% N2 | 10 bar 35 °C | CO2 then N2 | 31.77 for CO2 and 1.87 for N2 | Experimental | [56] | |
| polybenzimidazole (PBI)-based with natural zeolite | binary (10% N2 90% CH4) and ternary (5% CO2 10% N2 85% CH4) | 3.5 bar, 10 bar, 20 bar 35 °C | CO2 then N2 | 22.38 for N2 in the binary mixture @ 3.5 bar and 12.82 for N2 in the ternary mixture @ 20 bar | Experimental | [57] | |
| perfluorinated polymers | Hyflon® AD 60 | 20% N2 20% CO2 60% CH4 | 14 bar 22 °C | CO2 then N2 | 2.3 for N2 | Experimental | [52] |
| polymer membrane | PDMS | 3% C3H8 87% CH4 10% N2 | 14 bar 23 °C | CH4 | 3–3.5 | Experimental | [52] |
| — | 10% N2 90% CH4 10 MMscfd | 32 bar 30 °C | CH4 | 3 for CH4 * ≤4% N2 | Commercial simulation package (ChemCad 5.5) | [52] † | |
| — | 0.2 MMscfd gas having 7% N2 | — | HCs | * <3.8% N2 80% HC recovery | Field (Southern Kentucky) | [52] | |
| — | 12 MMscfd 16% N2 900 Btu/scf | 67.5 bar | CH4 and heavier HCs | * 9% N2 990 Btu/scf 95% HC recovery | Field (Rio Bista, California) | [52] | |
| Zeolite membrane | SAPO-34 | — | 3 bar 40 °C | N2 | 4.38 | Experimental | [54] |
| SSZ-13 | 90% CH4 10% N2 | ∆P = 2 bar 25 °C | N2 | 11.8 and 5 @ ∆P = 25 bar | Experimental | [55] | |
| SSZ-13 | 50% CH4 50% N2 | 7 bar 25 °C | N2 | 13 | Experimental | [58] |
| Separation Method | Feed | Operating Conditions | Solvent/Media | Favored Content | Selectivity/Product Purity/HHV | Type of Work | Ref |
|---|---|---|---|---|---|---|---|
| Absorption | — | 3–30 bar 30 °C | TMC Solution (K- [RuII(EDTA)]) | N2 | 1.7–2.4 | Experimental | [65] |
| — | Low T and P | FeII phosphine complexes | N2 | — | Experimental | [67] | |
| 15% N2 85% CH4 | 69 bar 20 °C | Ligands and TMCs solution | N2 | 5.75 | Experimental | [64] | |
| 1 MM SCFD 20% N2 80% CH4 | 69 bar 20 °C | Organometallic complex solution (Mo) | N2 | * 4% N2 96% CH4 | Experimental | [68] † | |
| Adsorption | 20 SCCM 10% N2 90% CH4 | 8.8 bar 60 °C | Lithium | N2 | * 2% N2 | Experimental | [66] † |
| (i) 10% N2 90% CH4 (ii) pretreated NG (4% N2, 95.9% CH4, CO2 and H2O) | (i) 25 °C, 80 bar (ii) 25 °C, 1 bar | Moisture-pretreated lithium | N2 | * <0.5% N2 | Numerical simulation | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomani, F.; Othman, A.; Pal, A.; Al-Musleh, E.I.; Karimi, I.A. Prospective of Upfront Nitrogen (N2) Removal in LNG Plants: Technical Communication. Energies 2021, 14, 3616. https://doi.org/10.3390/en14123616
Almomani F, Othman A, Pal A, Al-Musleh EI, Karimi IA. Prospective of Upfront Nitrogen (N2) Removal in LNG Plants: Technical Communication. Energies. 2021; 14(12):3616. https://doi.org/10.3390/en14123616
Chicago/Turabian StyleAlmomani, Fares, Asmaa Othman, Ajinkya Pal, Easa I. Al-Musleh, and Iftekhar A. Karimi. 2021. "Prospective of Upfront Nitrogen (N2) Removal in LNG Plants: Technical Communication" Energies 14, no. 12: 3616. https://doi.org/10.3390/en14123616
APA StyleAlmomani, F., Othman, A., Pal, A., Al-Musleh, E. I., & Karimi, I. A. (2021). Prospective of Upfront Nitrogen (N2) Removal in LNG Plants: Technical Communication. Energies, 14(12), 3616. https://doi.org/10.3390/en14123616







