Links between Process Performance and Microbial Community of Pennisetum Hybrid Co-Digested with Municipal Solid Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Inocula
2.2. Experimental Setup and Procedure
2.3. Analytical Methods
2.4. Analytical Methods for Microbial Communities
3. Results and Discussion
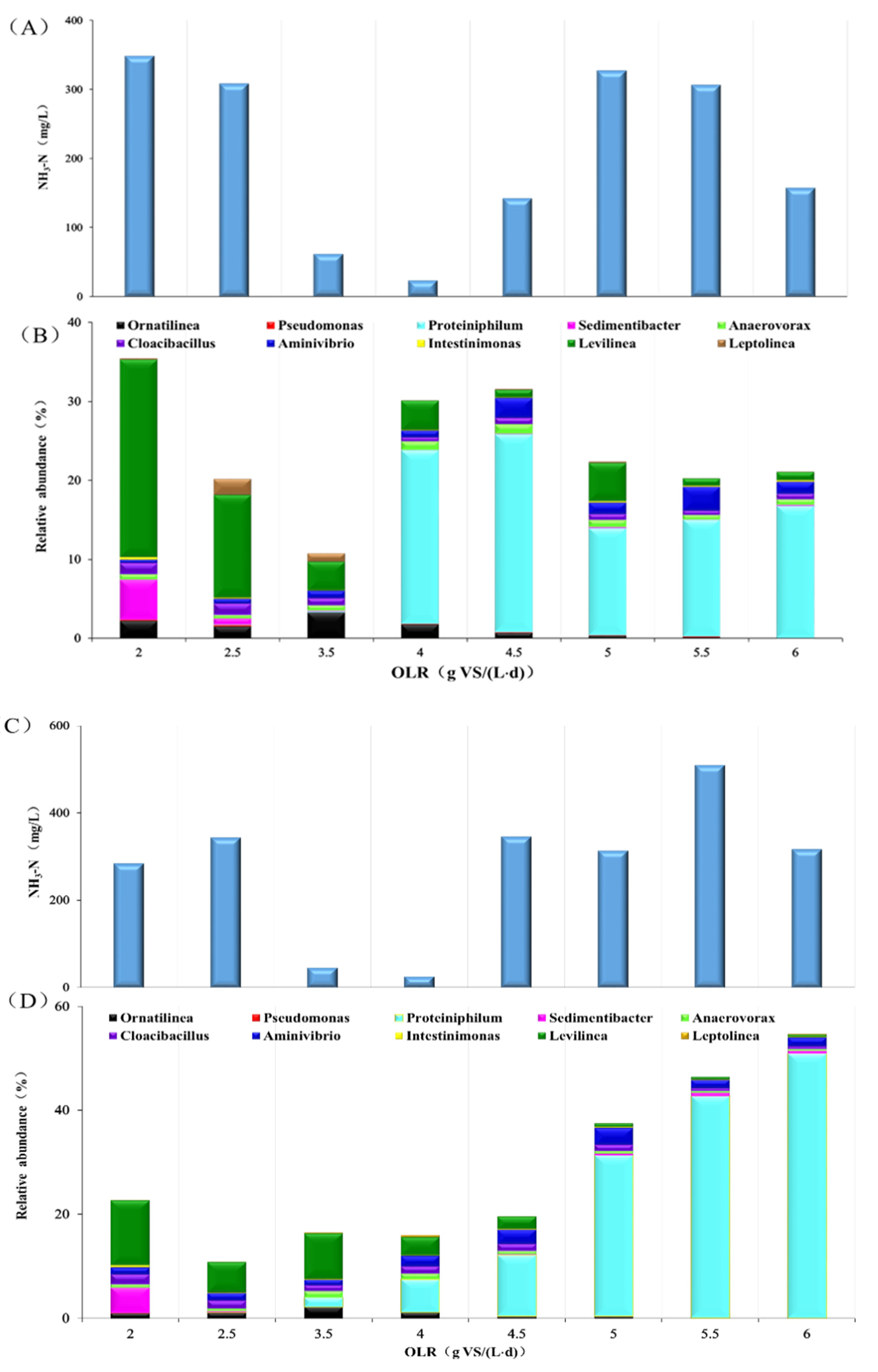
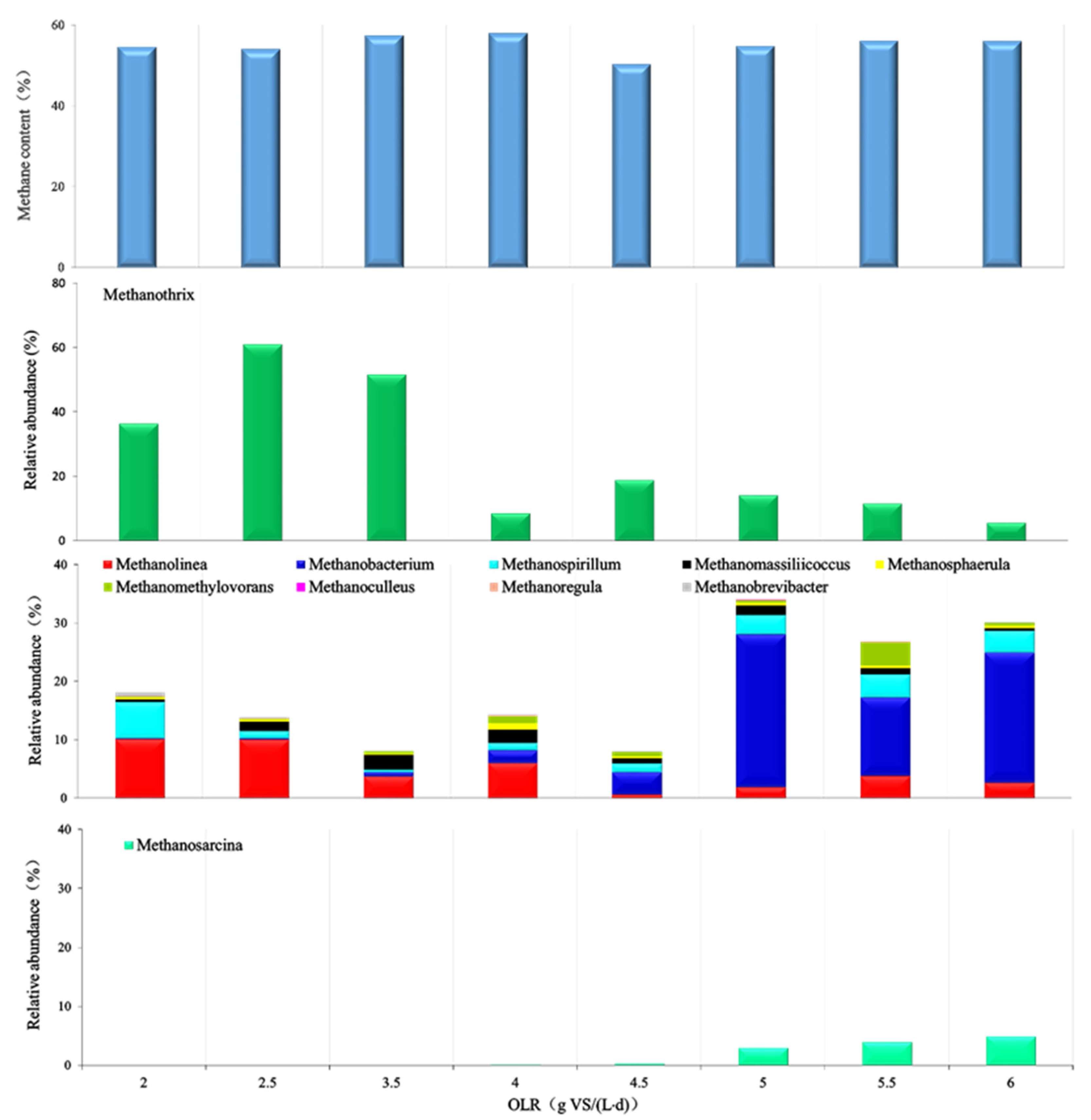
3.1. Performance, Process Parameters, and the Microbial Community of GM11
3.1.1. GM11 Performance
3.1.2. Changes in Microbial Community during Digestion
3.2. Performance, Process, and Microbial Community of GM31
3.2.1. GM31 Performance
3.2.2. Changes of Microbial Community of GM31 during the Process
3.3. Comparison of the Performance and Shift of Microorganisms at Different Mixture Ratios and OLRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surendra, K.C.; Ogoshi, R.; Zaleski, H.M.; Hashimoto, A.G.; Khanal, S.K. High yielding tropical energy crops for bioenergy production: Effects of plant components, harvest years and locations on biomass composition. Bioresour. Technol. 2018, 251, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe−N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbial fuel cell. Int. J. Agric. Biol. Eng. 2020, 13, 207–214. [Google Scholar]
- Masse, D.; Gilbert, Y.; Savoie, P.; Belanger, G.; Parent, G.; Babineau, D. Methane yield from switchgrass and reed canarygrass grown in Eastern Canada. Bioresour. Technol. 2011, 102, 10286–10292. [Google Scholar] [CrossRef]
- Masse, D.; Gilbert, Y.; Savoie, P.; Belanger, G.; Parent, G.; Babineau, D. Methane yield from switchgrass harvested at different stages of development in Eastern Canada. Bioresour. Technol. 2010, 101, 9536–9541. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; Li, C.; Liu, J.; Yi, Z.L.; Han, Y.J. Changes in composition, cellulose degradability and biochemical methane potential of Miscanthus species during the growing season. Bioresour. Technol. 2017, 235, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Thamsiriroj, T.; Nizami, A.S.; Murphy, J.D. Why does mono-digestion of grass silage fail in long term operation? Appl. Energy 2012, 95, 64–76. [Google Scholar] [CrossRef]
- Wall, D.M.; Allen, E.; Straccialini, B.; O’Kiely, P.; Murphy, J.D. The effect of trace element addition to mono-digestion of grass silage at high organic loading rates. Bioresour. Technol. 2014, 172, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.N.I.; Ab Wahid, Z. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Hidaka, T.; Wang, F.; Sakurai, K.; Tsumori, J.; Minamiyama, M. Anaerobic codigestion of grass and sewage sludge: Laboratory experiments and feasibility analysis. Water Environ. Res. 2016, 88, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Pehme, S.; Veromann, E.; Hamelin, L. Environmental performance of manure co-digestion with natural and cultivated grass—A consequential life cycle assessment. J. Clean. Prod. 2017, 162, 1135–1143. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.; Zhang, Y.G. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Gu, B.X.; Jiang, S.Q.; Wang, H.K.; Wang, Z.B.; Jia, R.F.; Yang, J.; He, S.; Cheng, R. Characterization, quantification and management of China’s municipal solid waste in spatiotemporal distributions: A review. Waste Manag. 2017, 61, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Li, A. Semi-continuous anaerobic digestion of extruded OFMSW: Process performance and energetics evaluation. Bioresour. Technol. 2018, 247, 103–115. [Google Scholar] [CrossRef]
- Negi, S.; Dhar, H.; Hussain, A.; Kumar, S. Biomethanation potential for co-digestion of municipal solid waste and rice straw: A batch study. Bioresour. Technol. 2018, 254, 139–144. [Google Scholar] [CrossRef]
- Abudi, Z.N.; Hu, Z.Q.; Sun, N.; Xiao, B.; Rajaa, N.; Liu, C.X.; Guo, D.B. Batch anaerobic co-digestion of OFMSW (organic fraction of municipal solid waste), TWAS (thickened waste activated sludge) and RS (rice straw): Influence of TWAS and RS pretreatment and mixing ratio. Energy 2016, 107, 131–140. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, L.; Li, A.M. Anaerobic co-digestion of food waste with MSW incineration plant fresh leachate: Process performance and synergistic effects. Chem. Eng. J. 2015, 259, 795–805. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.F.; Hou, X.C.; Wu, J.Y.; Fan, X.F.; Jiang, F.; Tao, P.; Wang, F.; Peng, P.; Yang, F.X.; et al. Exploring surface characterization and electrostatic property of Hybrid Pennisetum during alkaline sulfite pretreatment for enhanced enzymatic hydrolysability. Bioresour. Technol. 2017, 244, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.H.; Kang, X.H.; Sun, Y.M.; Yuan, Z.H.; Xing, T.; Lin, R.C. Improving methane production from Pennisetum hybrid by monitoring plant height and ensiling pretreatment. Renew. Energy 2019, 141, 57–63. [Google Scholar] [CrossRef]
- Li, L.H.; Li, Y.; Sun, Y.M.; Yuan, Z.H.; Kang, X.H.; Zhang, Y.; Yang, G.X. Effect of bioaugmentation on the microbial community and mono-digestion performance of Pennisetum hybrid. Waste Manag. 2018, 78, 741–749. [Google Scholar]
- Zhou, S.; Geng, B.; Li, M.; Li, Z.; Liu, X.; Guo, H. Comprehensive analysis of environmental factors mediated microbial community succession in nitrogen conversion and utilization of ex situ fermentation system. Sci. Total Environ. 2021, 769, 145219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Kang, X.; Yuan, Y.; Du, M. Short chain fatty acids accumulation and microbial community succession during ultrasonic-pretreated sludge anaerobic fermentation process: Effect of alkaline adjustment. Int. Biodeterior. 2014, 94, 128–133. [Google Scholar] [CrossRef]
- Shi, R.; Han, Z.; Li, H.; Wang, S.; Guo, N.; Zhang, Y. Carbon emission and energy potential of a novel spatiotemporally anaerobic/semi-aerobic bioreactor for domestic waste treatment. Waste Manag. 2020, 114, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Llamas, M.; Magdalena, J.A.; Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Insights on the microbial communities developed during the anaerobic fermentation of raw and pretreated microalgae biomass. Chemosphere 2021, 263, 127942. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhao, Y.; Meng, X.; Zhang, Y.; Lu, C.; Hu, Y.; Cui, Z.; Wang, X. Achieve clean and efficient biomethane production by matching between digestate recirculation and straw-to-manure feeding ratios. J. Clean. Prod. 2020, 263, 121414. [Google Scholar] [CrossRef]
- Li, L.H.; Sun, Y.M.; Yuan, Z.H.; Kong, X.Y.; Wang, Y. Influence of harvest period and frequency on methane yield of Pennisetum hybrids. J. Energy Eng. 2016, 142, 04015033. [Google Scholar] [CrossRef]
- Li, L.H.; Li, Y.; Sun, Y.M.; Yuan, Z.H.; Lv, P.M.; Kang, X.H.; Zhang, Y.; Yang, G.X. Influence of the feedstock ratio and organic loading rate on the Co-digestion performance of Pennisetum hybrid and cow manure. Energy Fuel 2018, 32, 5171–5180. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Sun, Y.; Kang, X.; Jiang, J.; Yuan, Z.; Liu, D. A comparative study of performance and microbial community at different mixture ratio and organic loading rate. Chemosphere 2020, 247, 125871. [Google Scholar]
- Streitwieser, D.A. Comparison of the anaerobic digestion at the mesophilic and thermophilic temperature regime of organic wastes from the agribusiness. Bioresour. Technol. 2017, 241, 985–992. [Google Scholar] [CrossRef]
- Yang, P.; Peng, Y.; Tan, H.; Liu, H.; Wu, D.; Wang, X.; Li, L.; Peng, X. Foaming mechanisms and control strategies during the anaerobic digestion of organic waste: A critical review. Sci. Total Environ. 2021, 779, 146531. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mei, Z.; He, W.; Yuan, Y.; Yan, Z.; Li, J.; Liu, X. Biogas production from thermophilic codigestion of air-dried rice straw and animal manure. Int. J. Energy Res. 2016, 40, 1245–1254. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.K.; Li, X.K. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, X.Y.; Wang, X.M.; Di Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 248, 20–28. [Google Scholar] [CrossRef]
- Martin-Gonzalez, L.; Font, X.; Vicent, T. Alkalinity ratios to identify process imbalances in anaerobic digesters treating source-sorted organic fraction of municipal wastes. Biochem. Eng. J. 2013, 76, 1–5. [Google Scholar] [CrossRef]
- Ferrer, I.; Vazquez, F.; Font, X. Long term operation of a thermophilic anaerobic reactor: Process stability and efficiency at decreasing sludge retention time. Bioresour. Technol. 2010, 101, 2972–2980. [Google Scholar] [CrossRef]
- Ma, X.X.; Yu, M.; Song, N.; Xu, B.H.; Gao, M.; Wu, C.F.; Wang, Q.H. Effect of ethanol pre-fermentation on organic load rate and stability of semi-continuous anaerobic digestion of food waste. Bioresour. Technol. 2020, 299, 122587. [Google Scholar] [CrossRef]
- Mashava, A. Operations Manual: Anaerobic Sludge Digestion Pilot Plant Ethekwini Municipality Department of Water and Sanitation—Southern Wastewater Treatment Plant; EPA: Washington, DC, USA, 2017.
- Wei, L.L.; Qin, K.; Ding, J.; Xue, M.; Yang, C.Y.; Jiang, J.Q.; Zhao, Q.L. Optimization of the co-digestion of sewage sludge, maize straw and cow manure: Microbial responses and effect of fractional organic characteristics. Sci. Rep. UK 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Sun, F.R.; Yu, J.D.; Cai, Y.F.; Luo, X.S.; Cui, Z.J.; Hu, Y.G.; Wang, X.F. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]
- Jehlee, A.; Khongkliang, P.; Suksong, W.; Rodjaroen, S.; Waewsak, J.; Reungsang, A.; Thong, S.O. Biohythane production from Chlorella sp biomass by two-stage thermophilic solid-state anaerobic digestion. Int. J. Hydrogen. Energy 2017, 42, 27792–27800. [Google Scholar] [CrossRef]
- Cardinali-Rezende, J.; Rojas-Ojeda, P.; Nascimento, A.M.A.; Sanz, J.L. Proteolytic bacterial dominance in a full-scale municipal solid waste anaerobic reactor assessed by 454 pyrosequencing technology. Chemosphere 2016, 146, 519–525. [Google Scholar] [CrossRef]
- Gao, Z.M.; Liu, X.; Ruan, L.W. Genome sequence of Anaerophaga sp. strain HS1, a novel, moderately thermophilic, strictly anaerobic bacterium isolated from hot spring sediment. J. Bacteriol. 2011, 193, 5572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.Q.; Shen, Q.Y.; Chen, G.J.; Du, Z.J. Mariniphaga sediminis sp. nov., isolated from coastal sediment. Int. J. Syst. Evol. Micr. 2015, 65, 2908–2912. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, Q.H.; Chen, Y.X.; Xu, M.S.; Shen, M.; Gao, R.; Gan, S.Q. Role of age-related shifts in rumen bacteria and methanogens in methane production in cattle. Front. Microbiol. 2017, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Maspolim, Y.; Zhou, Y.; Guo, C.H.; Xiao, K.K.; Ng, W.J. The effect of pH on solubilization of organic matter and microbial community structures in sludge fermentation. Bioresour. Technol. 2015, 190, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.J.; Liu, W.Z.; Varrone, C.; Wang, Y.Z.; Wang, A.J.; Yue, X.P. Evaluation of surfactants on waste activated sludge fermentation by pyrosequencing analysis. Bioresour. Technol. 2015, 192, 835–840. [Google Scholar] [CrossRef]
- Ma, K.L.; Li, X.K.; Wang, K.; Meng, L.W.; Liu, G.G.; Zhang, J. Establishment of thermophilic anaerobic terephthalic acid degradation system through one-step temperature increase startup strategy—Revealed by Illumina Miseq Sequencing. Chemosphere 2017, 184, 951–959. [Google Scholar] [CrossRef]
- Xia, X.X.; Zhang, J.C.; Song, T.Z.; Lu, Y.H. Stimulation of Smithella-dominating propionate oxidation in a sediment enrichment by magnetite and carbon nanotubes. Environ. Microbiol. Rep. 2019, 11, 236–248. [Google Scholar] [CrossRef]
- Podosokorskaya, O.A.; Bonch-Osmolovskaya, E.A.; Novikov, A.A.; Kolganova, T.V.; Kublanov, I.V. Ornatilinea apprima gen. nov., sp. nov., a cellulolytic representative of the class Anaerolineae. Int. J. Syst. Evol. Micr. 2013, 63, 86–92. [Google Scholar] [CrossRef]
- He, Q.M.; Li, L.; Peng, X.Y. Early warning indicators and microbial mechanisms for process failure due to organic overloading in food waste digesters. J. Environ. Eng. 2017, 143, 04017077. [Google Scholar] [CrossRef]
- Feng, S.S.; Hong, X.J.; Wang, T.; Huang, X.; Tong, Y.J.; Yang, H.L. Reutilization of high COD leachate via recirculation strategy for methane production in anaerobic digestion of municipal solid waste: Performance and dynamic of methanogen community. Bioresour. Technol. 2019, 288, 121509. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.C.; Guo, X.L.; Zuo, J.N.; Wang, Y.J.; Zhang, M.Y. A comparative study of thermophilic and mesophilic anaerobic co-digestion of food waste and wheat straw: Process stability and microbial community structure shifts. Waste Manag. 2018, 75, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar] [CrossRef] [PubMed]




| Material | TS/% | VS/% | pH | C/% | N/% | C/N |
|---|---|---|---|---|---|---|
| Pennisetum hybrid | 17.64 ± 0.68 | 16.55 ± 0.66 | 3.48 | 41.76 ± 0.25 | 0.975 ± 0.035 | 42.86 ± 1.81 |
| 15.87 ± 0.62 | 12.81 ± 0.45 | - | 39.59 ± 0.04 | 0.915 ± 0.021 | 43.28 ± 0.96 | |
| 20.94 ± 0.84 | 18.17 ± 0.81 | 5.04 | 43.41 ± 0.54 | 0.695 ± 0.02 | 62.50 ± 2.68 | |
| 19.05 ± 1.15 | 16.48 ± 1.18 | 4.88 | 40.51 ± 0.33 | 0.655 ± 0.021 | 61.88 ± 2.51 | |
| 16.68 ± 0.49 | 13.61 ± 0.96 | 4.35 | 38.98 ± 0.57 | 0.735 ± 0.021 | 53.06 ± 2.31 | |
| MSW | 29.42 ± 0.19 | 20.21 ± 0.06 | - | 35.97 ± 0.51 | 1.25 ± 0.28 | 29.57 ± 6.14 |
| 28.11 ± 1.03 | 20.55 ± 0.85 | - | 32.84 ± 1.17 | 1.46 ± 0.01 | 22.49 ± 1.02 | |
| 32.62 ± 0.59 | 24.02 ± 0.22 | - | 35.27 ± 0.51 | 1.75 ± 0.04 | 20.16 ± 0.78 | |
| 26.82 ± 0.03 | 19.51 ± 1.70 | - | 37.13 ± 1.03 | 0.92 ± 0.12 | 41.01 ± 6.52 |
| OLR /VS·L−1·d−1 | GM11 | GM31 | ||||
|---|---|---|---|---|---|---|
| Volumetric Biogas Yield /m3·m−3·d−1 | Specific Biogas Yield /mL·g VS−1 | Specific Methane Yield /mL·g VS−1 | Volumetric Biogas Yield /m3·m−3·d−1 | Specific Biogas Yield /mL·g VS−1 | Specific Methane Yield /mL·g VS−1 | |
| 2.0 | 1.03 ± 0.12 | 512.60 ± 57.95 | 279.53 ± 33.81 | 1.08 ± 0.10 | 538.79 ± 49.43 | 290.67 ± 30.33 |
| 2.5 | 1.25 ± 0.14 | 515.73 ± 58.95 | 278.01 ± 33.02 | 1.44 ± 0.16 | 525.70 ± 70.81 | 272.18 ± 51.79 |
| 3.5 | 1.67 ± 0.15 | 471.78 ± 42.68 | 271.17 ± 28.29 | 1.69 ± 0.13 | 502.29 ± 44.98 | 262.75 ± 27.98 |
| 4.0 | 1.70 ± 0.15 | 424.62 ± 37.84 | 246.15 ± 23.12 | 1.76 ± 0.30 | 439.46 ± 75.19 | 233.48 ± 50.45 |
| 4.5 | 1.78 ± 0.12 | 395.21 ± 27.61 | 199.81 ± 33.00 | 1.84 ± 0.18 | 404.10 ± 38.93 | 236.23 ± 21.78 |
| 5.0 | 1.91 ± 0.33 | 382.83 ± 66.43 | 210.35 ± 41.77 | 2.25 ± 0.31 | 450.52 ± 61.48 | 259.35 ± 37.18 |
| 5.5 | 2.36 ± 0.22 | 428.90 ± 40.37 | 241.62 ± 33.87 | 2.48 ± 0.17 | 421.04 ± 30.04 | 238.66 ± 34.34 |
| 6.0 | 2.67 ± 0.24 | 445.43 ± 40.23 | 249.31 ± 22.75 | 2.55 ± 0.26 | 424.67 ± 44.01 | 250.04 ± 30.21 |
| 7.0 | 2.83 ± 0.15 | 403.94 ± 21.90 | 224.27 ± 12.16 | 2.71 ± 0.13 | 387.78 ± 18.74 | 241.89 ± 11.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; He, S.; Li, L.; Sun, Y.; Ren, H. Links between Process Performance and Microbial Community of Pennisetum Hybrid Co-Digested with Municipal Solid Waste. Energies 2021, 14, 3651. https://doi.org/10.3390/en14123651
Zhao Q, He S, Li L, Sun Y, Ren H. Links between Process Performance and Microbial Community of Pennisetum Hybrid Co-Digested with Municipal Solid Waste. Energies. 2021; 14(12):3651. https://doi.org/10.3390/en14123651
Chicago/Turabian StyleZhao, Quanlin, Shuibin He, Lianhua Li, Yongming Sun, and Haiwei Ren. 2021. "Links between Process Performance and Microbial Community of Pennisetum Hybrid Co-Digested with Municipal Solid Waste" Energies 14, no. 12: 3651. https://doi.org/10.3390/en14123651
APA StyleZhao, Q., He, S., Li, L., Sun, Y., & Ren, H. (2021). Links between Process Performance and Microbial Community of Pennisetum Hybrid Co-Digested with Municipal Solid Waste. Energies, 14(12), 3651. https://doi.org/10.3390/en14123651







