Grown and Characterization of ZnO Aligned Nanorod Arrays for Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
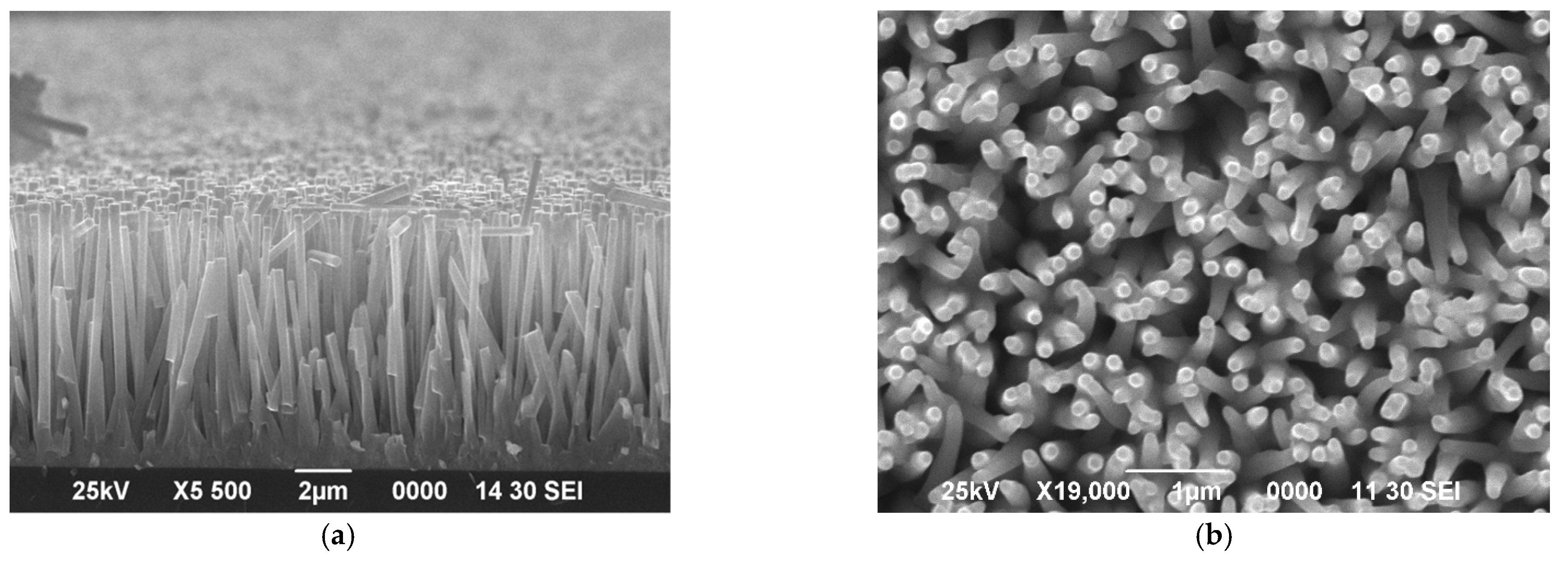
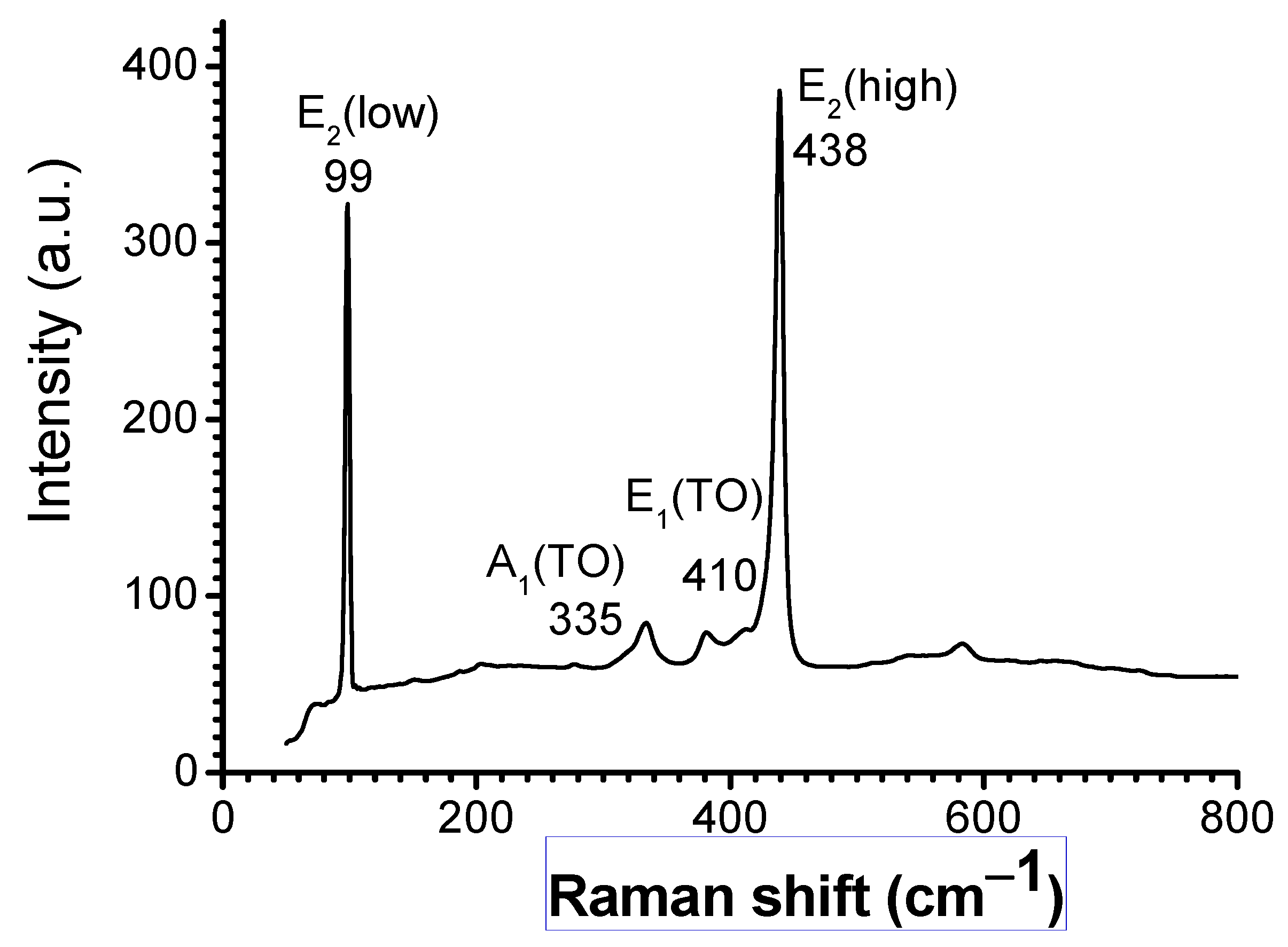
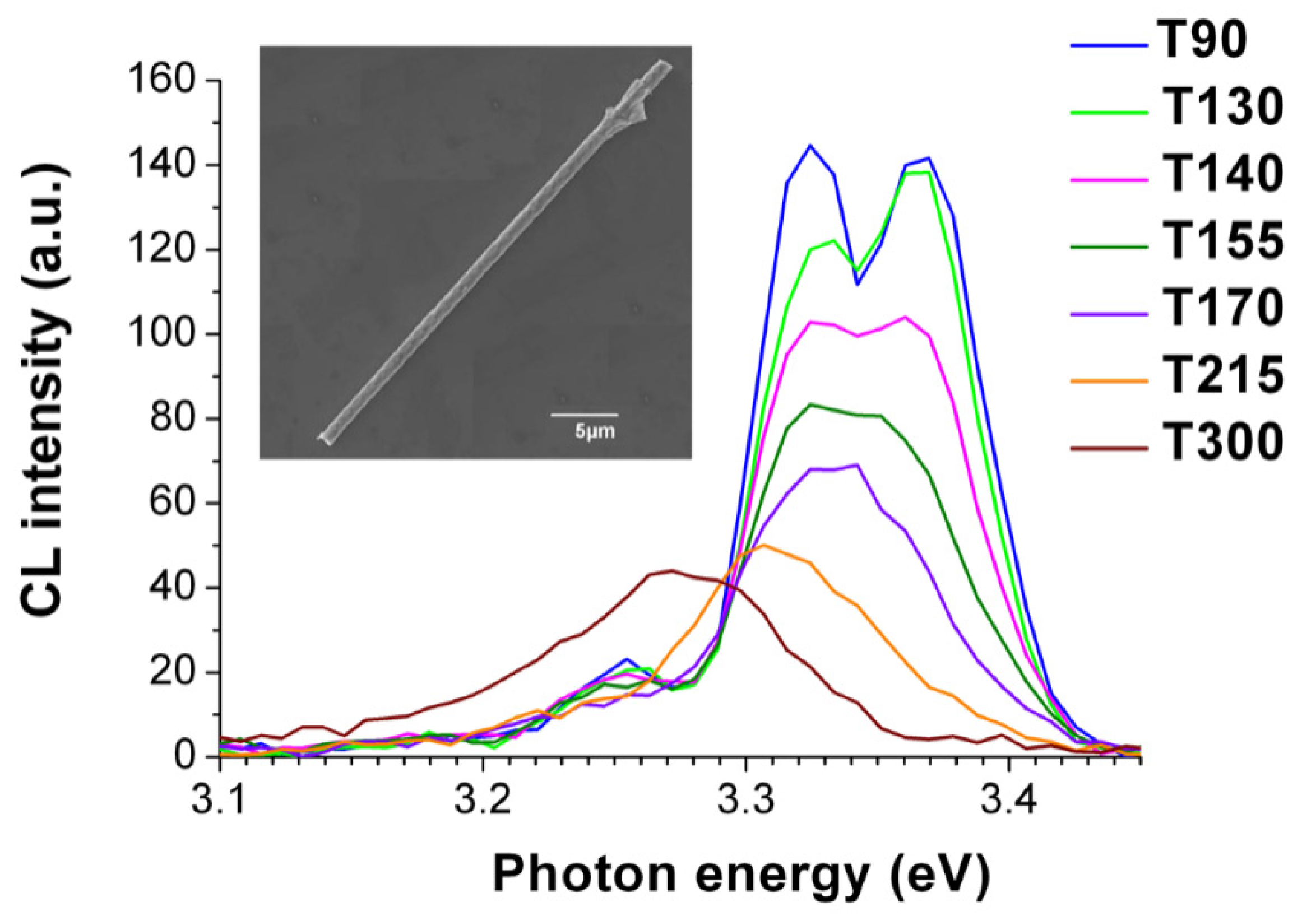
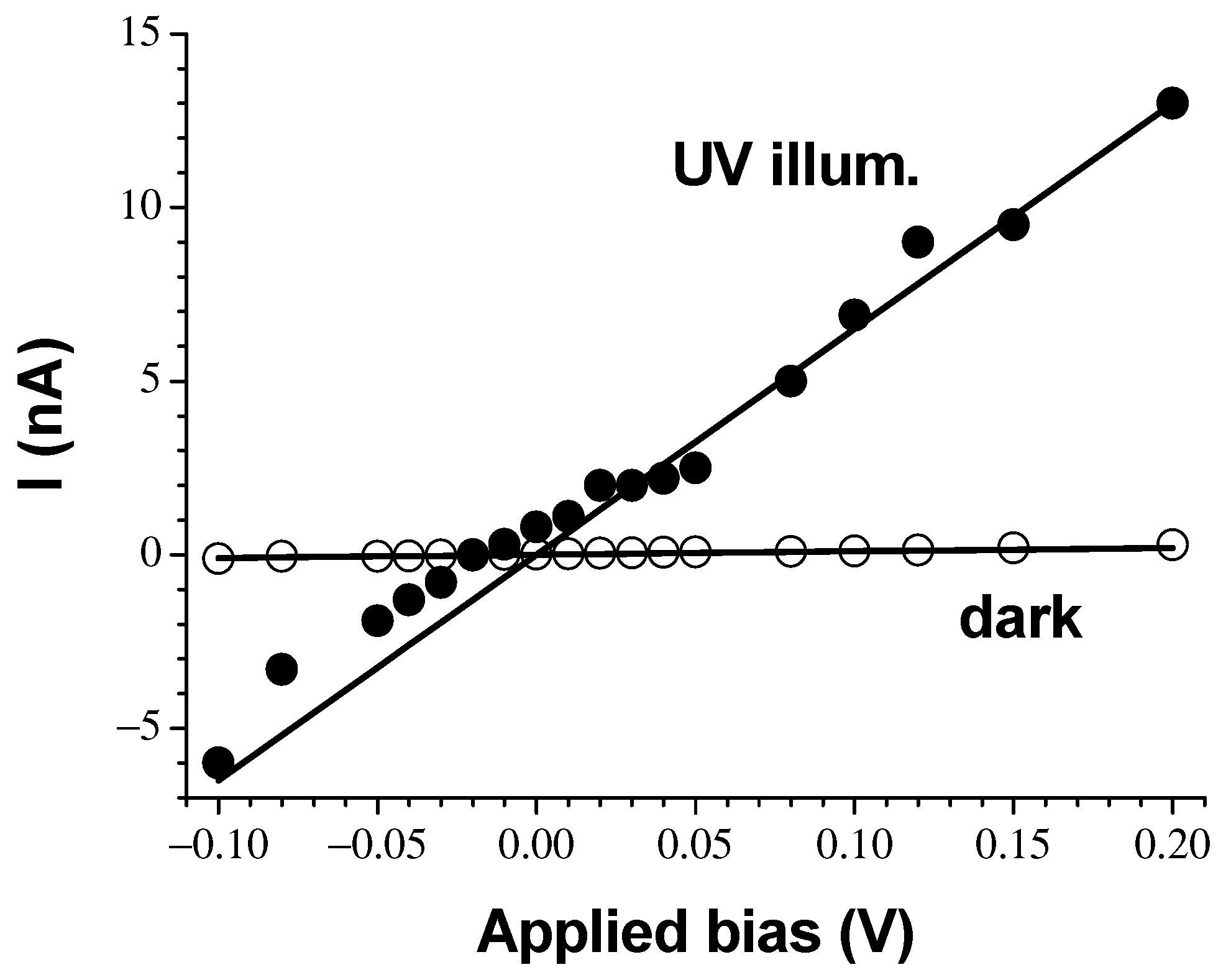
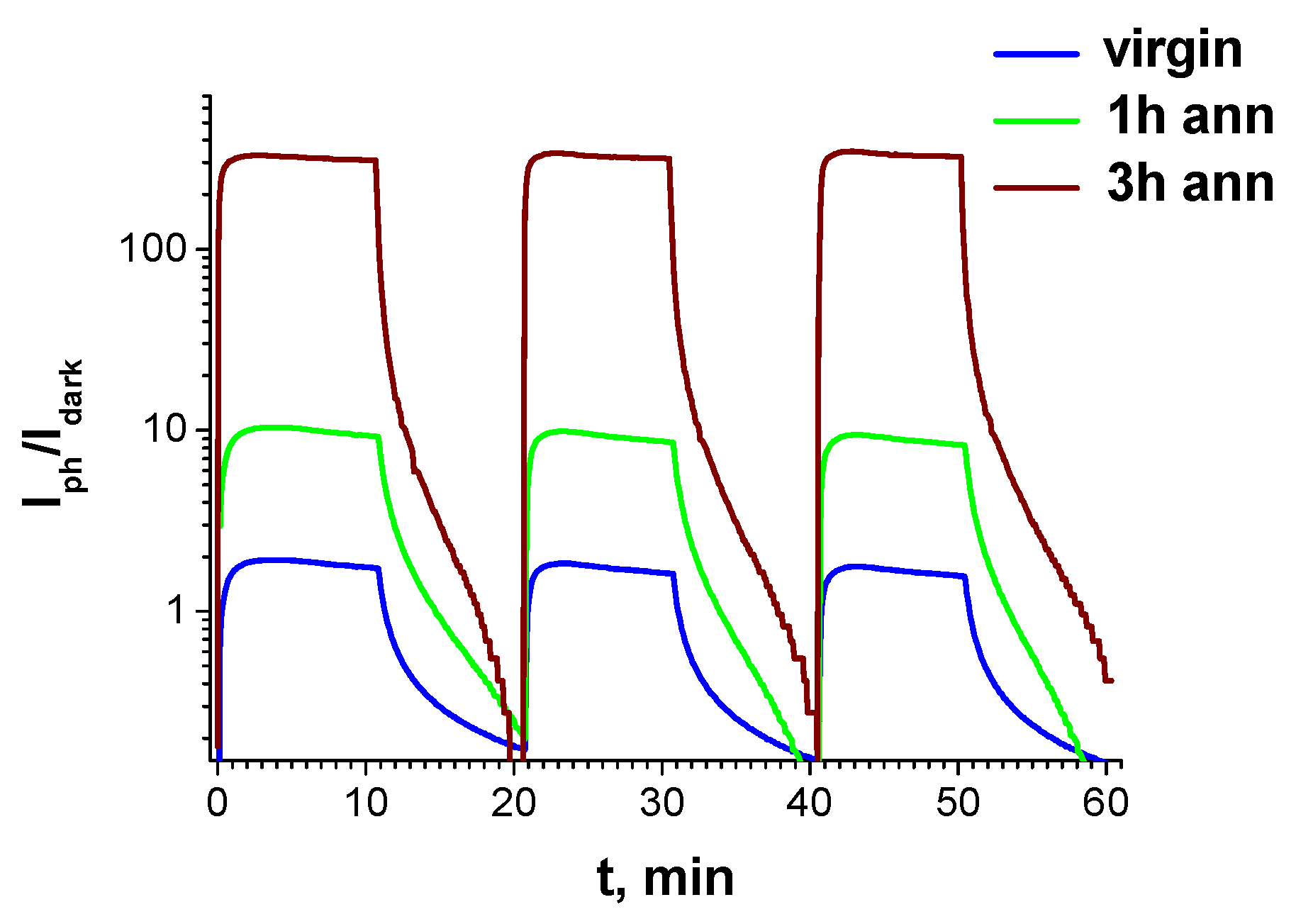
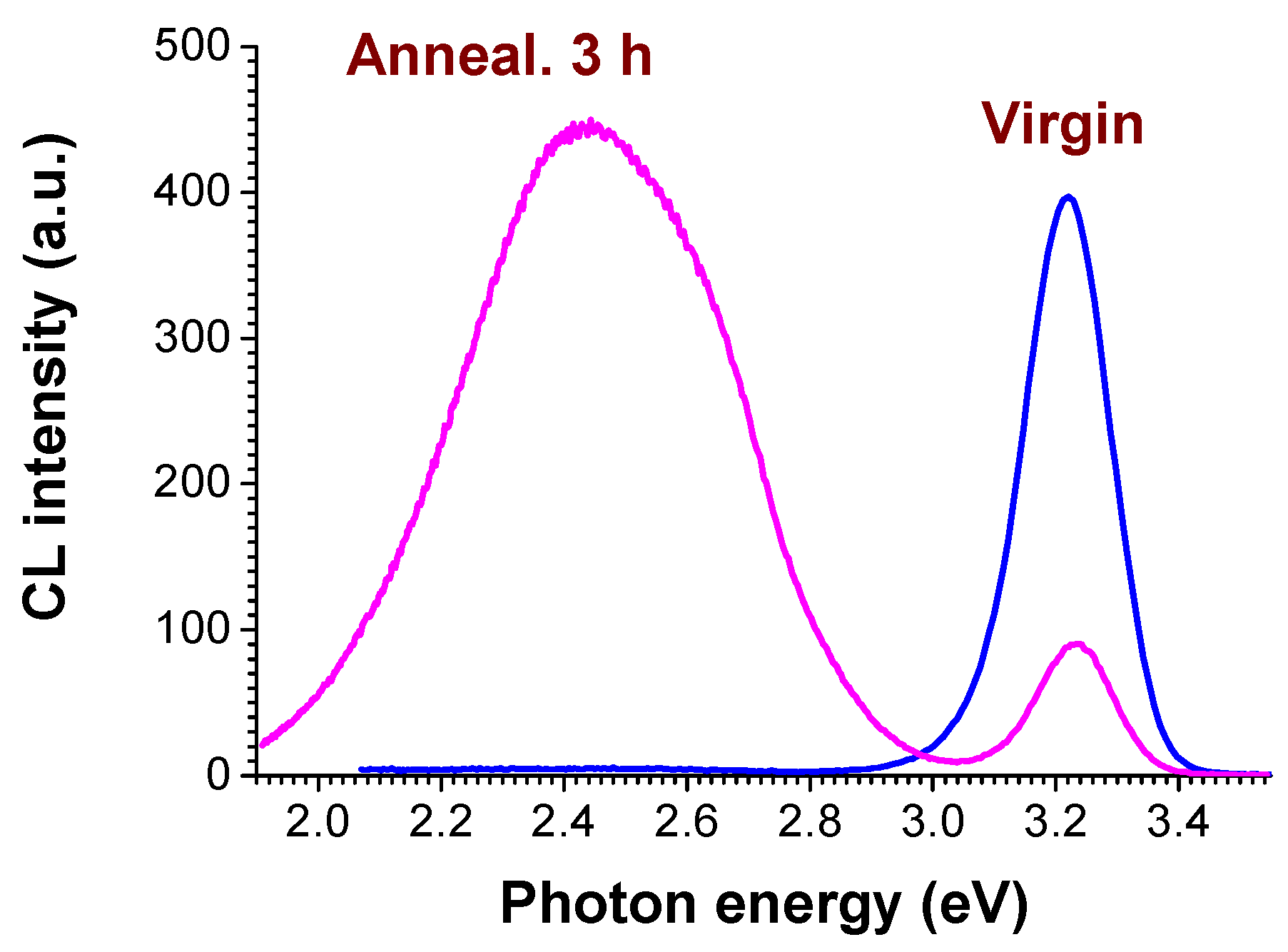
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proceed. 2020, in press. [Google Scholar] [CrossRef]
- Cui, J.; Gibson, U. Low-temperature fabrication of single-crystal ZnO nanopillar photonic bandgap structures. Nanotechnology 2007, 18, 155302. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, H.; Fu, Z.; Yang, B.; Zuo, J.; Fu, S. Enhanced photoluminescence from three-dimensional ZnO photonic crystals. Solid State Commun. 2006, 139, 218–221. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Progr. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Willander, M.; Zhao, Q.X.; Hu, Q.-H.; Klason, P.; Kuzmin, V.; Al-Hilli, S.M.; Nur, O.; Lozovik, Y.E. Fundamentals and properties of zinc oxide nanostructures: Optical and sensing applications. Superlattices Microstruct. 2008, 43, 352–361. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, P. Comparison of the structural and optical properties of ZnO thin films deposited by three different methods for optoelectronic applications. Superlattices Microstruct. 2013, 64, 283–293. [Google Scholar] [CrossRef]
- Sohn, J.I.; Choi, S.S.; Morris, S.M.; Bendall, J.S.; Coles, H.J.; Hong, W.-K.; Jo, G.; Lee, T.; Welland, M.E. Novel Nonvolatile Memory with Multibit Storage Based on a ZnO Nanowire Transistor. Nano Lett. 2010, 10, 4316–4320. [Google Scholar] [CrossRef]
- Singh, S.K.; Hazra, P. Performance of RF sputtered p-Si/n-ZnO nanoparticle thin film heterojunction diodes in high temperature environment. Appl. Surf. Sci. 2017, 400, 206–211. [Google Scholar] [CrossRef]
- Pietruszka, R.; Luka, G.; Kopalko, K.; Zielony, E.; Bieganski, P.; Placzek-Popko, E.; Godlewski, M. Photovoltaic and photoelectrical response of n-ZnO/p-Si heterostructures with ZnO films grown by an Atomic Layer Deposition method. Mater. Sci. Semicond. Proces. 2014, 25, 190–196. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Su, T.-H.; Lin, J.-C.; Su, Y.-C. Photocurrent stability and responsivity in the n-type Si/ZnO-doped conducting polymer photovoltaic device. Synth. Metals 2012, 162, 406–409. [Google Scholar] [CrossRef]
- Shukla, R.K.; Srivastava, A.; Srivastava, A.; Dubey, K.C. Growth of transparent conducting nanocrystalline Al doped ZnO thin films by pulsed laser deposition. J. Cryst. Growth 2006, 294, 427–431. [Google Scholar] [CrossRef]
- Mohammad, M.T.; Hashim, A.A.; Al-Maamory, M.H. Highly conductive and transparent ZnO thin films prepared by spray pyrolysis technique. Mater. Chem. Phys. 2006, 99, 382–387. [Google Scholar] [CrossRef]
- Lee, C.-T.; Chiu, Y.-S. Piezoelectric ZnO-nanorod-structured pressure sensors using GaN-based field-effect-transistor. Appl. Phys. Lett. 2015, 106, 073502. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Lee, J.S.; Jang, J. Highly sensitive, wearable and wireless pressure sensor using free-standing ZnO nanoneedle/PVDF hybrid thin film for heart rate monitoring. Nano Energy 2016, 22, 95–104. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Le, A.T.; Ahmadipour, M.; Pung, S.-Y. A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J. Alloys Compd. 2020, 844, 156172. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Wei, Z.-R.; Li, Z.-Q.; Dong, G.-Y. Room-temperature ferromagnetism in Fe-doped, Fe- and Cu-codoped ZnO diluted magnetic semiconductor. Mater. Lett. 2007, 61, 3605–3607. [Google Scholar] [CrossRef]
- Qi, B.; Olafsson, S.; Gislason, H.P. Vacancy defect-induced d0 ferromagnetism in undoped ZnO nanostructures: Controversial origin and challenges. Progr. Mater. Sci. 2017, 90, 45–74. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Qurashi, A.; Khanday, F.A. ZnO nanostructures based biosensors for cancer and infectious disease applications: Perspectives, prospects and promises. Trends Anal. Chem. 2017, 86, 1–13. [Google Scholar] [CrossRef]
- Yi, G.-C.; Wang, C.; Park, W.I. ZnO nanorods: Synthesis, characterization and applications. Semicond. Sci. Technol. 2005, 20, S22–S34. [Google Scholar] [CrossRef]
- Ho, S.-T.; Wang, C.-Y.; Liu, H.-L.; Lin, H.-N. Catalyst-free selective-area growth of vertically aligned zinc oxide nanowires. Chem. Phys. Lett. 2008, 463, 141–144. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redkin, A.N.; Ryzhova, M.V.; Yakimov, E.E.; Gruzintsev, A.N. Aligned Arrays of Zinc Oxide Nanorods on Silicon Substrates. Semiconductors 2013, 47, 252–258. [Google Scholar] [CrossRef]
- Red’kin, A.N.; Gruzintsev, A.N.; Yakimov, E.E.; Kononenko, O.V.; Roshchupkin, D.V. Vapor-Phase Synthesis of Aligned Zinc Oxide Nanorod Arrays on Various Substrates. Inorg. Mater. 2011, 47, 740–745. [Google Scholar] [CrossRef]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Roldan Cuenya, B.; et al. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Serrano, J.; Manjón, F.G.; Romero, A.H.; Widulle, F.; Lauck, R.; Cardona, M. Dispersive Phonon Linewidths: The E2 Phonons of ZnO. Phys. Rev. Lett. 2003, 90, 055510. [Google Scholar] [CrossRef] [Green Version]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruzintsev, A.N.; Red’kin, A.N.; Yakimov, E.E.; Yakimov, E.B. Cathodoluminescence study of individual ZnO nanorods. Phys. Status Solidi C 2011, 8, 1403–1406. [Google Scholar] [CrossRef]
- Reddy, N.K.; Ahsanulhaq, Q.; Kim, J.H.; Devika, M.; Hahn, Y.B. Selection of non-alloyed Ohmic contacts for ZnO nanostructure based devices. Nanotechnology 2007, 18, 445710. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Bao, X.-Y.; Kim, H.; Lo, Y.; Wang, D. Nanowire Photodetectors. J. Nanosci. Nanotechnol. 2010, 10, 1430–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire Ultraviolet Photodetectors and Optical Switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Shasti, M.; Dariani, R.S. Study of growth time and post annealing effect on the performance of ZnO nanorods ultraviolet photodetector. J. Appl. Phys. 2017, 121, 064503. [Google Scholar] [CrossRef]
- Kim, D.; Leem, J.-Y. Improving of the Rise and Decay Rates of an Ultraviolet Photodetector Using Stepwise Annealed ZnO Nanorods. Phys. Status Solidi A 2019, 216, 1800929. [Google Scholar] [CrossRef]
- Noh, Y.; Jeong, H.; Lee, D. Enhanced ultraviolet photodetector using zinc oxide nanowires with intense pulsed light post-treatment. J. Alloys Compd. 2021, 871, 159537. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, H.; Jiang, W.; Xu, L.; Hou, H. Photoresponse and decay mechanism of an individual ZnO nanowire UV sensor. Sensor Actuat. A. 2012, 174, 43–46. [Google Scholar] [CrossRef]
- Lao, C.S.; Park, M.-C.; Kuang, Q.; Deng, Y.; Sood, A.K.; Polla, D.L.; Wang, Z.L. Giant Enhancement in UV Response of ZnO Nanobelts by Polymer Surface-Functionalization. J. Am. Chem. Soc. 2007, 129, 12096–12097. [Google Scholar] [CrossRef] [Green Version]
- Hsu, N.E.; Hung, W.K.; Chen, Y.F. Origin of defect emission identified by polarized luminescence from aligned ZnO nanorods. J. Appl. Phys. 2004, 96, 4671–4673. [Google Scholar] [CrossRef]
- Hullavarad, S.; Hullavarad, N.; Look, D.; Claflin, B. Persistent Photoconductivity Studies in Nanostructured ZnO UV Sensors. Nanoscale Res. Lett. 2009, 4, 1421–1427. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, A.; Aslam, M. Defect induced high photocurrent in solution grown vertically aligned ZnO nanowire array films. J. Appl. Phys. 2012, 112, 054316. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redkin, A.N.; Yakimov, E.E.; Evstafieva, M.V.; Yakimov, E.B. Grown and Characterization of ZnO Aligned Nanorod Arrays for Sensor Applications. Energies 2021, 14, 3750. https://doi.org/10.3390/en14133750
Redkin AN, Yakimov EE, Evstafieva MV, Yakimov EB. Grown and Characterization of ZnO Aligned Nanorod Arrays for Sensor Applications. Energies. 2021; 14(13):3750. https://doi.org/10.3390/en14133750
Chicago/Turabian StyleRedkin, Arkady N., Eugene E. Yakimov, Maria V. Evstafieva, and Eugene B. Yakimov. 2021. "Grown and Characterization of ZnO Aligned Nanorod Arrays for Sensor Applications" Energies 14, no. 13: 3750. https://doi.org/10.3390/en14133750





