Evaluation of Temperature Influence on Electrochemical Processes Occurring in a Lithium-Ion Supercapacitor with the Use of Dynamic Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
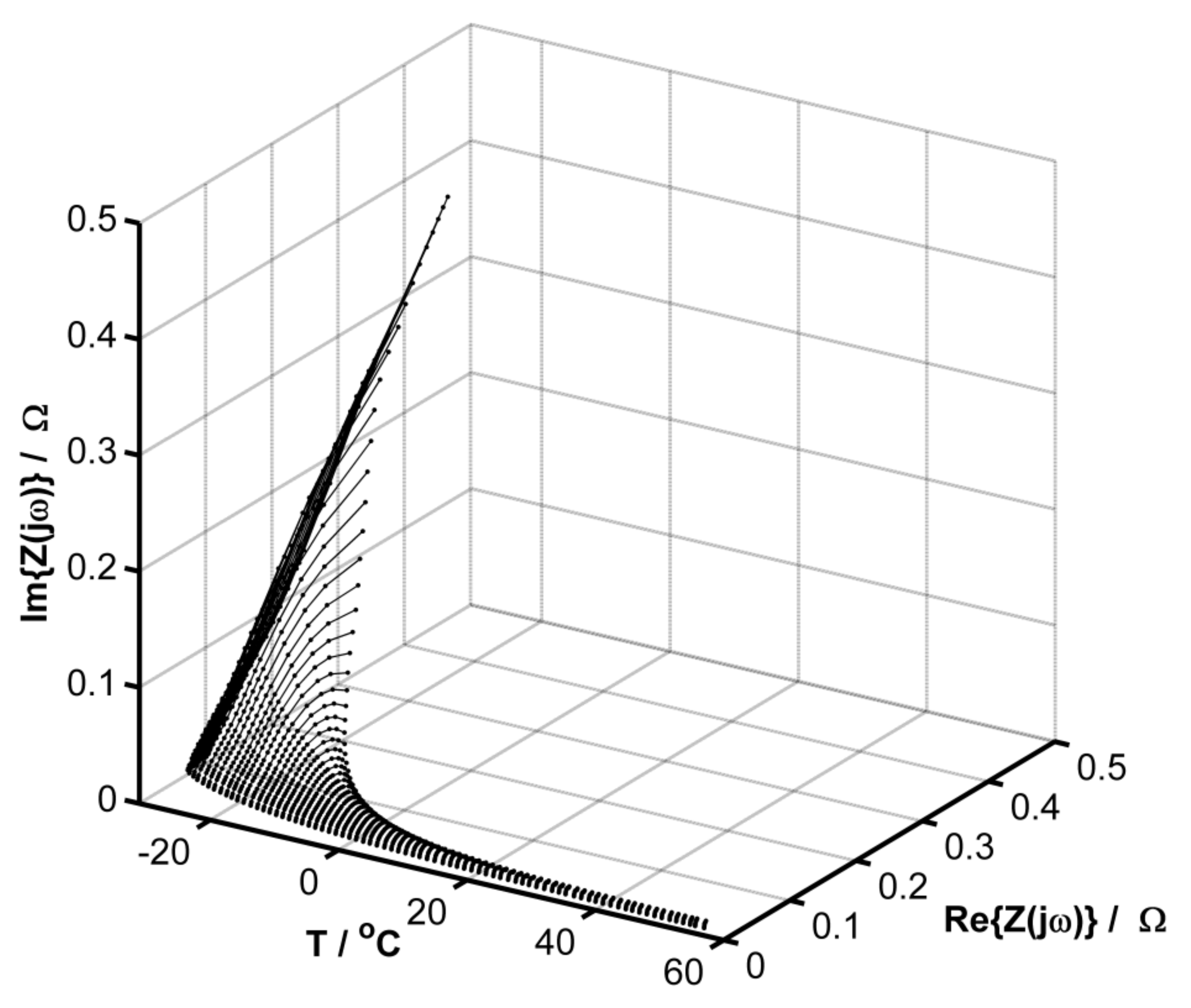
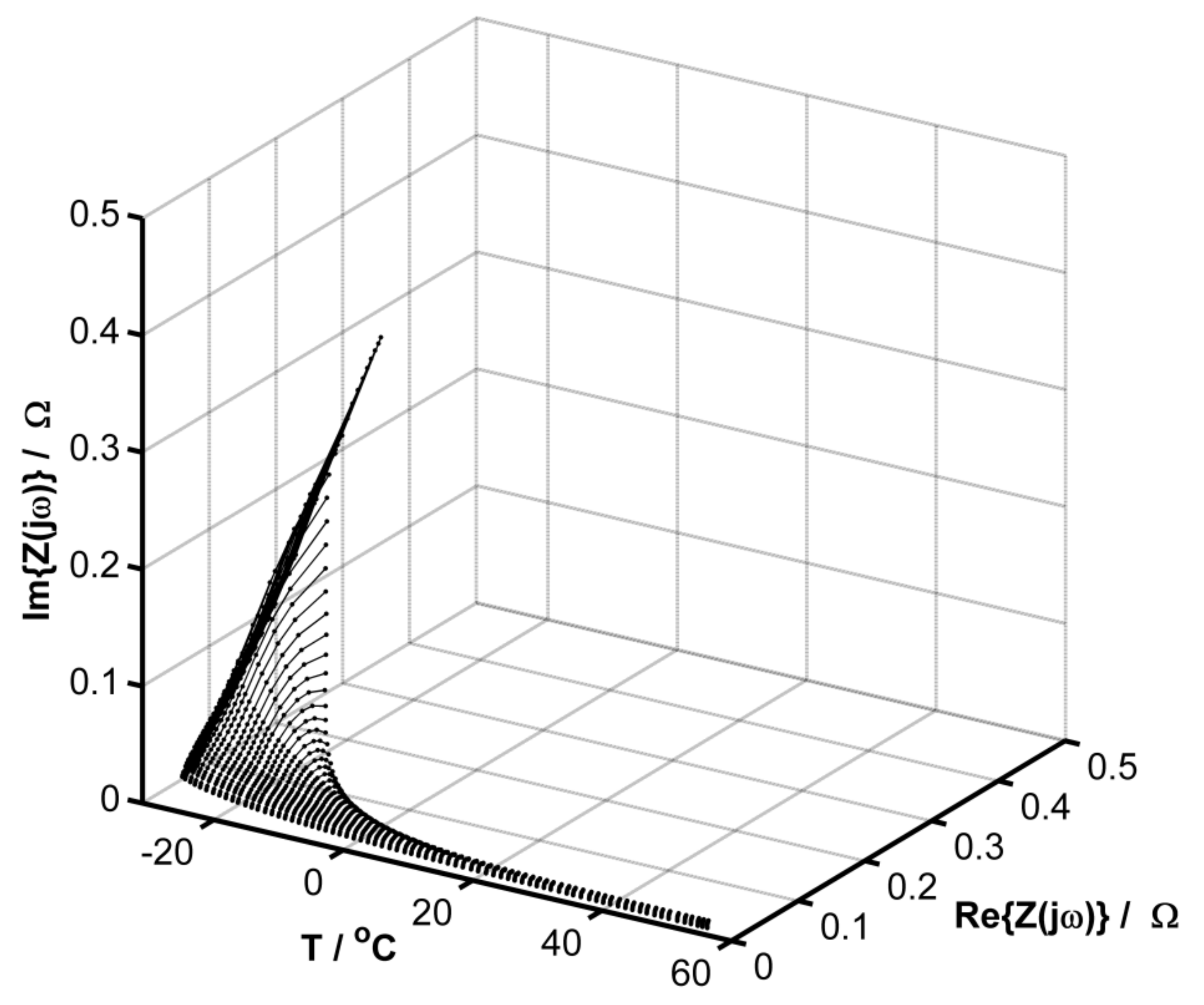
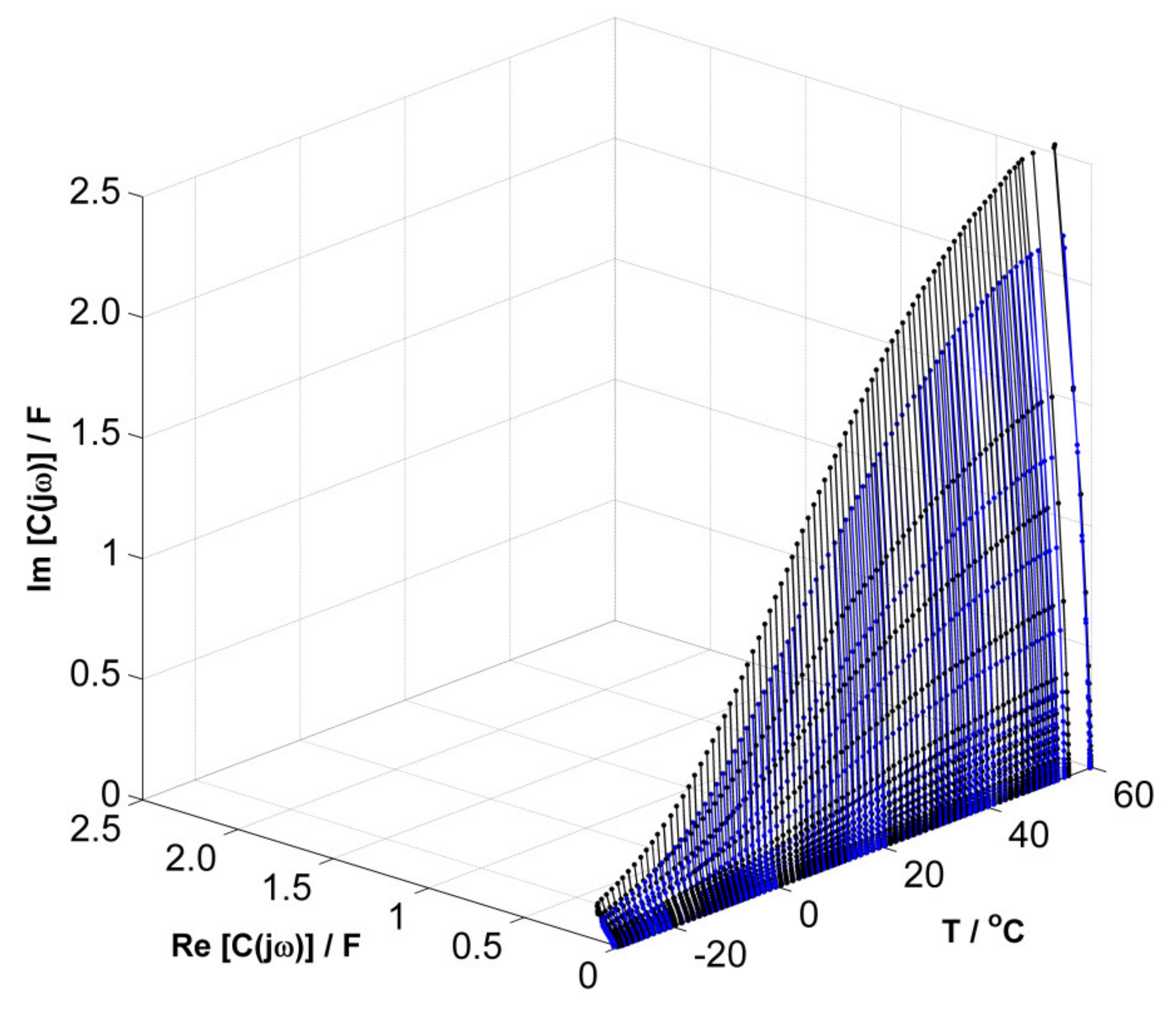
3. Results and Discussion
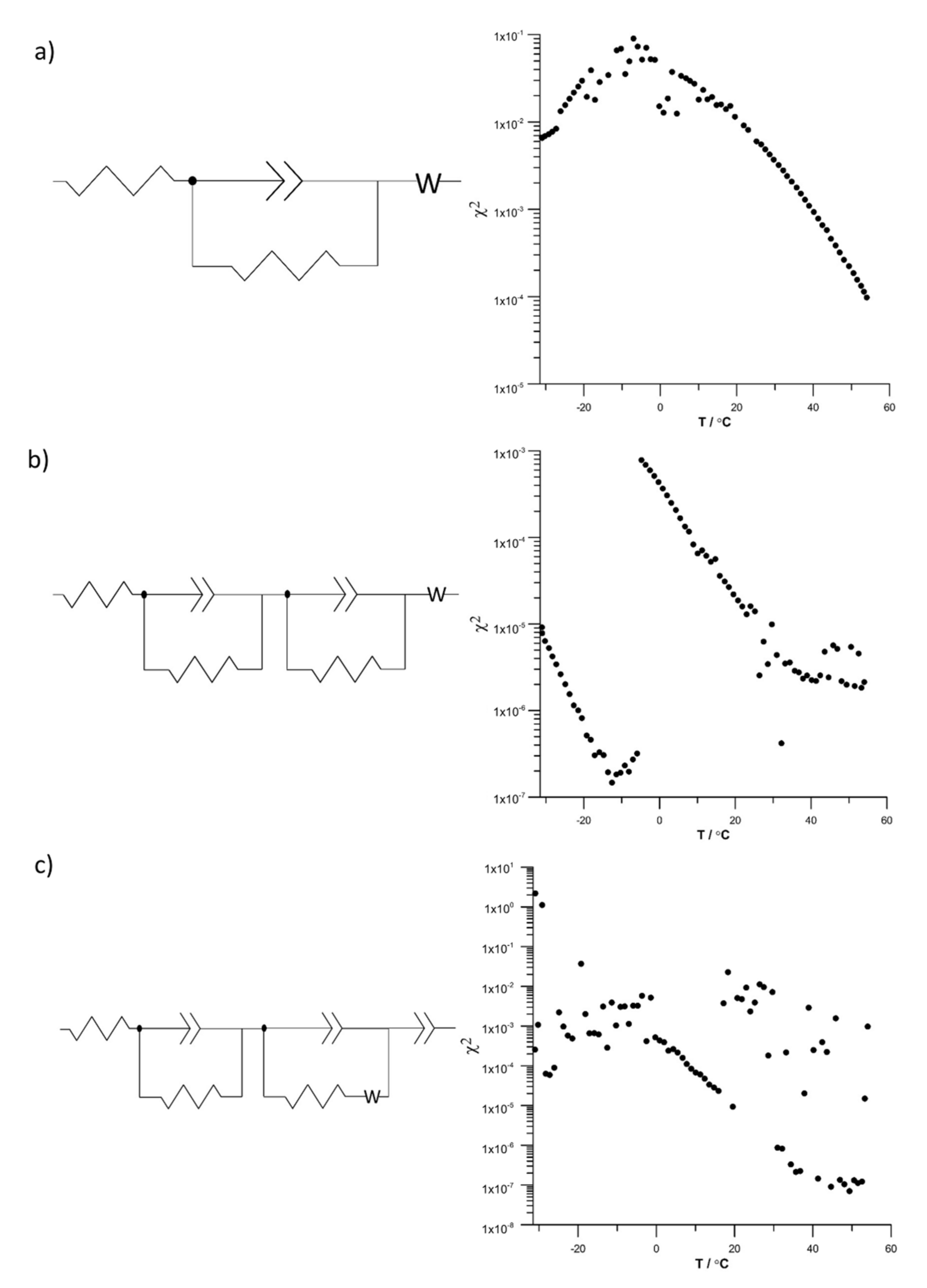
Evaluation of the Equivalent Circuit Fitting Quality Using the DEIS Method
4. Conclusions
- Temperature has a much greater effect on impedance spectrum than the state of charge level of a supercapacitor.
- The behavior of a lithium-ion supercapacitor changes with temperature. At high temperatures, the operation is similar to that of conventional double-layer capacitors, where non-Faraday processes determine the operation of the device. At lower temperatures, however, the processes corresponding to the chemistry of lithium-ion batteries appear to be much more significant, which translates into a substantial increase in impedance value.
- High dependence of lithium-ion supercapacitors on temperature significantly hinders the correct selection of an equivalent circuit that describes their operation in the entire temperature range.
- With proper monitoring and energy management, the devices have the capability to fully exploit their potential. Dynamic Electrochemical Impedance Spectroscopy, which provides key information about the operation of the devices in a very fast time, seems to be the ideal tool for this purpose. The proposed methodology can be a very useful monitoring tool in automotive applications, where lithium–ion capacitors are subjected to work in a very wide temperature range.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mi, Z.; Guan, D.; Liu, Z.; Liu, J.; Viguié, V.; Fromer, N.; Wang, Y. Cities: The core of climate change mitigation. J. Clean. Prod. 2019, 207, 582–589. [Google Scholar] [CrossRef]
- Darowicki, K.; Janicka, E.; Mielniczek, M.; Zielinski, A.; Gawel, L.; Mitzel, J.; Hunger, J. Implementation of DEIS for reliable fault monitoring and detection in PEMFC single cells and stacks. Electrochim. Acta 2018, 292, 383–389. [Google Scholar] [CrossRef]
- Chen, W.; Liang, J.; Yang, Z.; Li, G. A review of lithium-ion battery for electric vehicle applications and beyond. Energy Procedia 2019, 158, 4363–4368. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Lü, X.; Qu, Y.; Wang, Y.; Qin, C.; Liu, G. A comprehensive review on hybrid power system for PEMFC-HEV: Issues and strategies. Energy Convers. Manag. 2018, 171, 1273–1291. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Ottaviano, A. Optimization of a PEMFC/battery pack power system for a bus application. Appl. Energy 2012, 97, 777–784. [Google Scholar] [CrossRef]
- Hong, H.; Jiang, Q. model predictive control-based coordinated control algorithm with a hybrid energy storage system to smooth wind power fluctuations. Energies 2019, 12, 4591. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Bonnet, C.; Mukherjee, M.; Raël, S.; Lapicque, F. Direct hybridization of pemfc and supercapacitors: Effect of excess hydrogen on a single cell fuel cell durability and its feasibility on fuel cell stack. Electrochim. Acta 2019, 310, 213–220. [Google Scholar] [CrossRef]
- Lopez Lopez, G.; Schacht Rodriguez, R.; Alvarado, V.M.; Gomez-Aguilar, J.F.; Mota, J.E.; Sandoval, C. Hybrid PEMFC-supercapacitor system: Modeling and energy management in energetic macroscopic representation. Appl. Energy 2017, 205, 1478–1494. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Han, Y.; Li, M.; Chen, W. A state machine strategy based on droop control for an energy management system of pemfc-battery-supercapacitor hybrid tramway. Int. J. Hydrog. Energy 2016, 41, 16148–16159. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, Y.; Li, X.; Liu, Z.; Chen, W.; Liu, Y.; Zhou, D. Development of master-slave energy management strategy based on fuzzy logic hysteresis state machine and differential power processing compensation for a pemfc-lib-sc hybrid tramway. Appl. Energy 2017, 206, 346–363. [Google Scholar] [CrossRef]
- Mutarraf, M.; Terriche, Y.; Niazi; Khan, F.; Vasquez, J.; Guerrero, J. Control of hybrid diesel/PV/battery/ultra-capacitor systems for future shipboard microgrids. Energies 2019, 12, 3460. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.; Sakka, M.A.; Van Mierlo, J.; Van den Bossche, P.; Gualous, H. Electric and thermal characterization of advanced hybrid li-ion capacitor rechargeable energy storage system. In Proceedings of the 4th International Conference on Power Engineering, Energy and Electrical Drives, IEEE, Istanbul, Turkey, 13–17 May 2013; pp. 1574–1580. [Google Scholar]
- Oca, L.; Guillet, N.; Tessard, R.; Iraola, U. Lithium-ion capacitor safety assessment under electrical abuse tests based on ultrasound characterization and cell opening. J. Energy Storage 2019, 23, 29–36. [Google Scholar] [CrossRef]
- Karimi, D.; Khaleghi, S.; Behi, H.; Beheshti, H.; Hosen, M.S.; Akbarzadeh, M.; Van Mierlo, J.; Berecibar, M. Lithium-ion capacitor lifetime extension through an optimal thermal management system for smart grid applications. Energies 2021, 14, 2907. [Google Scholar] [CrossRef]
- Firouz, Y.; Omar, N.; Timmermans, J.-M.; Van den Bossche, P.; Van Mierlo, J. Lithium-ion capacitor–characterization and development of new electrical model. Energy 2015, 83, 597–613. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Li, C.; Wang, K.; Sun, X.; Huo, Q.; Wei, T.; Ma, Y. Equivalent circuit models and parameter identification methods for lithium-ion capacitors. J. Energy Storage 2019, 24, 100762. [Google Scholar] [CrossRef]
- Barcellona, S.; Piegari, L. A lithium-ion capacitor model working on a wide temperature range. J. Power Sources 2017, 342, 241–251. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Jiang, L.; Tian, Y.; Xue, R.; Hao, L.; Liu, W.; Yi, B. Electrochemical performance of lithium ion capacitors using aqueous Electrolyte at High Temperature. J. Renew. Sustain. Energy 2013, 5, 021404. [Google Scholar] [CrossRef] [Green Version]
- El Ghossein, N.; Sari, A.; Venet, P. Lifetime prediction of lithium-ion capacitors based on accelerated aging tests. Batteries 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Nakata, S. Investigation of charging efficiency of a lithium-ion capacitor during galvanostatic charging method. Materials 2019, 12, 3191. [Google Scholar] [CrossRef] [Green Version]
- Nakata, S. Characteristic of an adiabatic charging reversible circuit with a lithium ion capacitor as an energy storage device. Results Phys. 2018, 10, 964–966. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Shi, Z.; Xu, Z. Electrochemical behavior of lithium ion capacitor under low temperature. J. Electroanal. Chem. 2018, 817, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Darowicki, K. Theoretical description of the measuring method of instantaneous impedance spectra. J. Electroanal. Chem. 2000, 486, 101–105. [Google Scholar] [CrossRef]
- Darowicki, K.; Orlikowski, J.; Lentka, G. Instantaneous impedance spectra of a non-stationary model electrical system. J. Electroanal. Chem. 2000, 486, 106–110. [Google Scholar] [CrossRef]
- Darowicki, K.; Janicka, E.; Slepski, P. Study of direct methanol fuel cell process dynamics using dynamic electrochemical impedance spectroscopy. Int. J. Electrochem. Sci. 2012, 7, 12090–12097. [Google Scholar]
- Slepski, P.; Janicka, E.; Darowicki, K.; Pierozynski, B. Impedance monitoring of fuel cell stacks. J. Solid State Electrochem. 2015, 19, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Darowicki, K.; Gawel, L. Impedance measurement and selection of electrochemical equivalent circuit of a working PEM fuel cell cathode. Electrocatalysis 2017, 8, 235–244. [Google Scholar] [CrossRef]
- Darowicki, K.; Janicka, E.; Mielniczek, M.; Zielinski, A.; Gawel, L.; Mitzel, J.; Hunger, J. The influence of dynamic load changes on temporary impedance in hydrogen fuel cells, selection and validation of the electrical equivalent circuit. Appl. Energy 2019, 251, 113396. [Google Scholar] [CrossRef]
- Slepski, P.; Darowicki, K.; Janicka, E.; Sierczynska, A. Application of electrochemical impedance spectroscopy to monitoring discharging process of nickel/metal hydride battery. J. Power Sources 2013, 241, 121–126. [Google Scholar] [CrossRef]
- Wysocka, J.; Krakowiak, S.; Ryl, J.; Darowicki, K. Investigation of the electrochemical behaviour of AA1050 aluminium alloy in aqueous alkaline solutions using dynamic electrochemical impedance spectroscopy. J. Electroanal. Chem. 2016, 778, 126–136. [Google Scholar] [CrossRef]
- Smith, P.H.; Tran, T.N.; Jiang, T.L.; Chung, J. Lithium-ion capacitors: Electrochemical performance and thermal behavior. J. Power Sources 2013, 243, 982–992. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P. Development of a capacitance versus voltage model for lithium-ion capacitors. Batteries 2020, 6, 54. [Google Scholar] [CrossRef]
- Navid, Q.; Hassan, A. An accurate and precise grey box model of a low-power lithium-ion battery and capacitor/supercapacitor for accurate estimation of state-of-charge. Batteries 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Z.; Sun, X.; An, Y.; Zhang, X.; Wang, K.; Dong, C.; Huo, Q.; Wei, T.; Ma, Y. Experimental study of thermal charge–discharge behaviors of pouch lithium-ion capacitors. J. Energy Storage 2019, 25, 100902. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, B.; Chen, M.; Wang, X.; Xing, T.; Liu, M.; Wang, X. Porous activated carbon derived from chinese-chive for high energy hybrid lithium-ion capacitor. J. Power Sources 2018, 398, 128–136. [Google Scholar] [CrossRef]
- Guo, X.; Gong, R.; Qin, N.; Jin, L.; Zheng, J.; Wu, Q.; Zheng, J.P. The influence of electrode matching on capacity decaying of hybrid lithium ion capacitor. J. Electroanal. Chem. 2019, 845, 84–91. [Google Scholar] [CrossRef]
- Darowicki, K.; Gawel, L.; Mielniczek, M.; Janicka, E.; Zielinski, A.; Mitzel, J.; Hunger, J. An integral-differential method for impedance determination of the hydrogen oxidation process in the presence of carbon monoxide in the proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2020, 45, 27551–27562. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielniczek, M.; Janicka, E.; Gawel, L.; Darowicki, K. Evaluation of Temperature Influence on Electrochemical Processes Occurring in a Lithium-Ion Supercapacitor with the Use of Dynamic Electrochemical Impedance Spectroscopy. Energies 2021, 14, 3807. https://doi.org/10.3390/en14133807
Mielniczek M, Janicka E, Gawel L, Darowicki K. Evaluation of Temperature Influence on Electrochemical Processes Occurring in a Lithium-Ion Supercapacitor with the Use of Dynamic Electrochemical Impedance Spectroscopy. Energies. 2021; 14(13):3807. https://doi.org/10.3390/en14133807
Chicago/Turabian StyleMielniczek, Michal, Ewa Janicka, Lukasz Gawel, and Kazimierz Darowicki. 2021. "Evaluation of Temperature Influence on Electrochemical Processes Occurring in a Lithium-Ion Supercapacitor with the Use of Dynamic Electrochemical Impedance Spectroscopy" Energies 14, no. 13: 3807. https://doi.org/10.3390/en14133807





