Promoted Disappearance of CO2 Hydrate Self-Preservation Effect by Surfactant SDS
Abstract
:1. Introduction
2. Materials and Methods
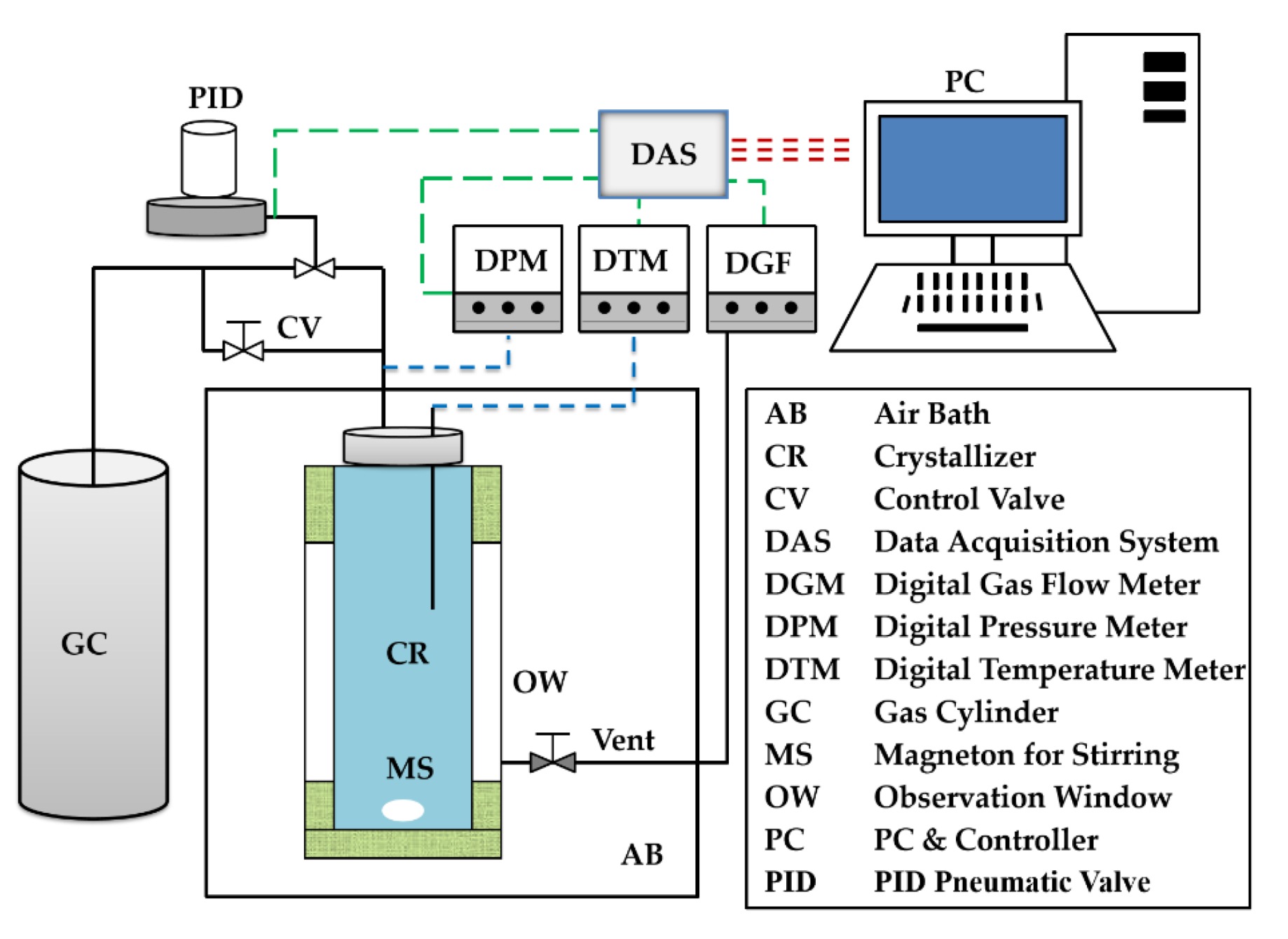
2.1. Experimental Apparatus and Materials
2.2. Experimental Procedure
2.3. Calculation Method
3. Results
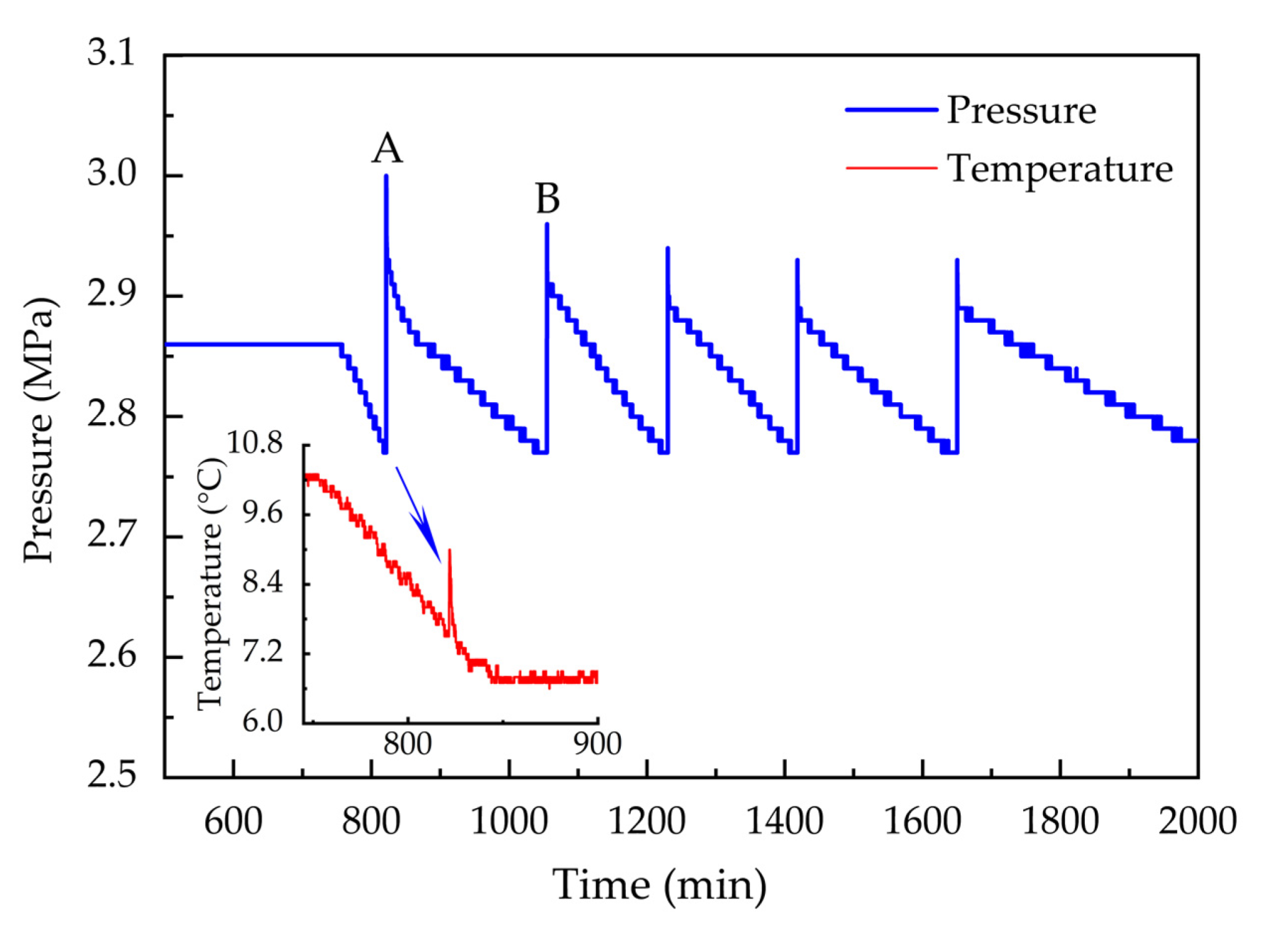
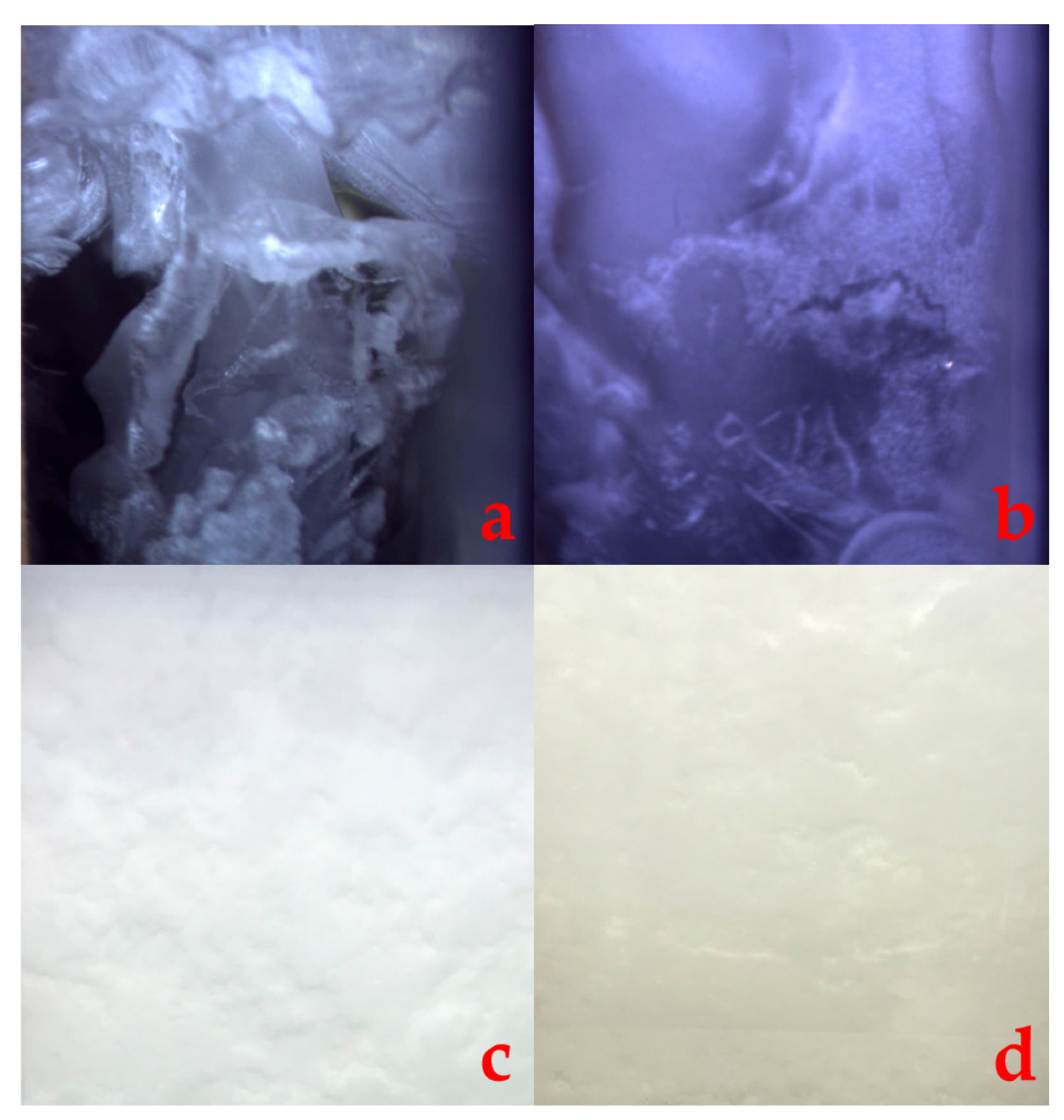
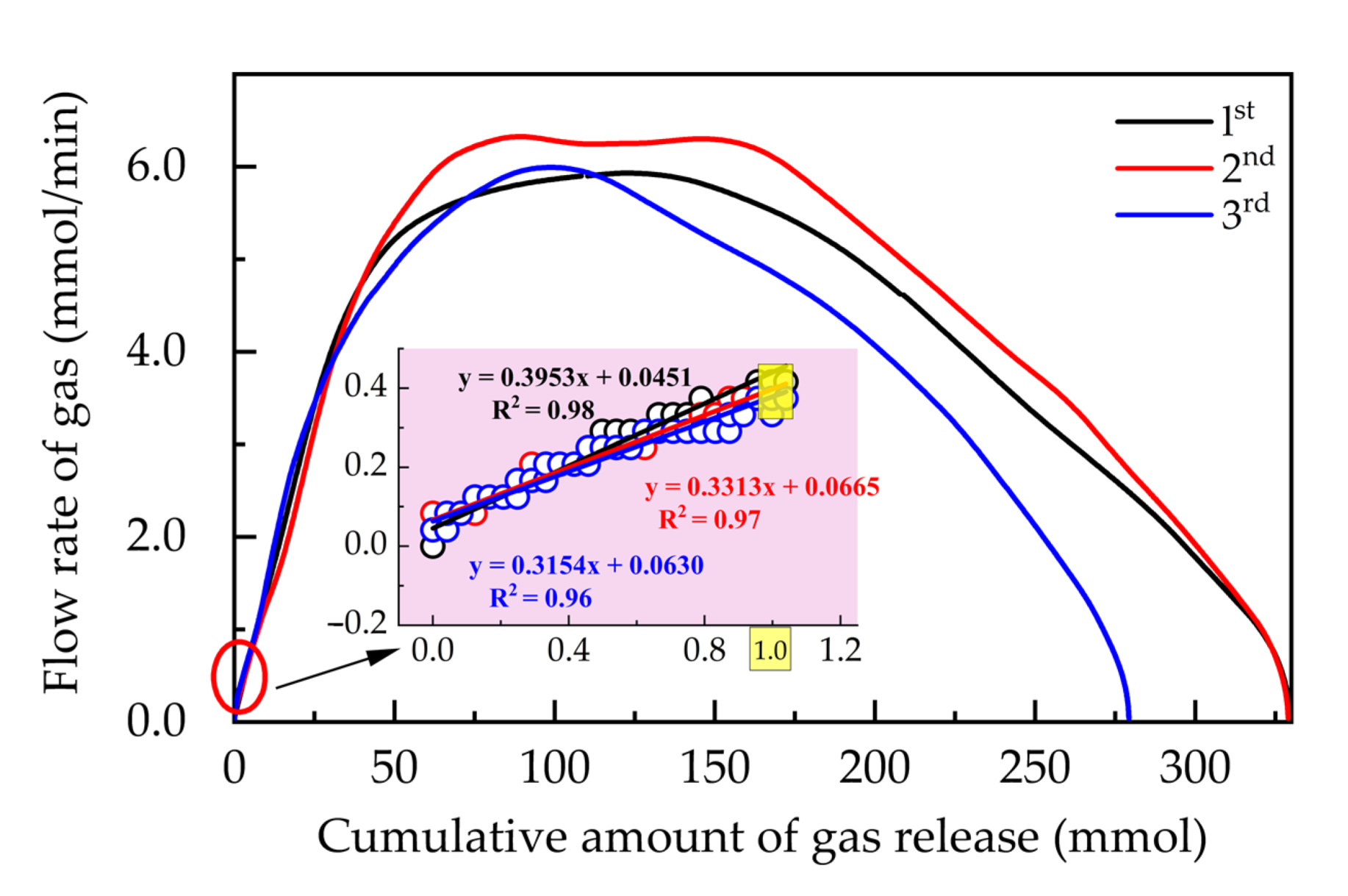
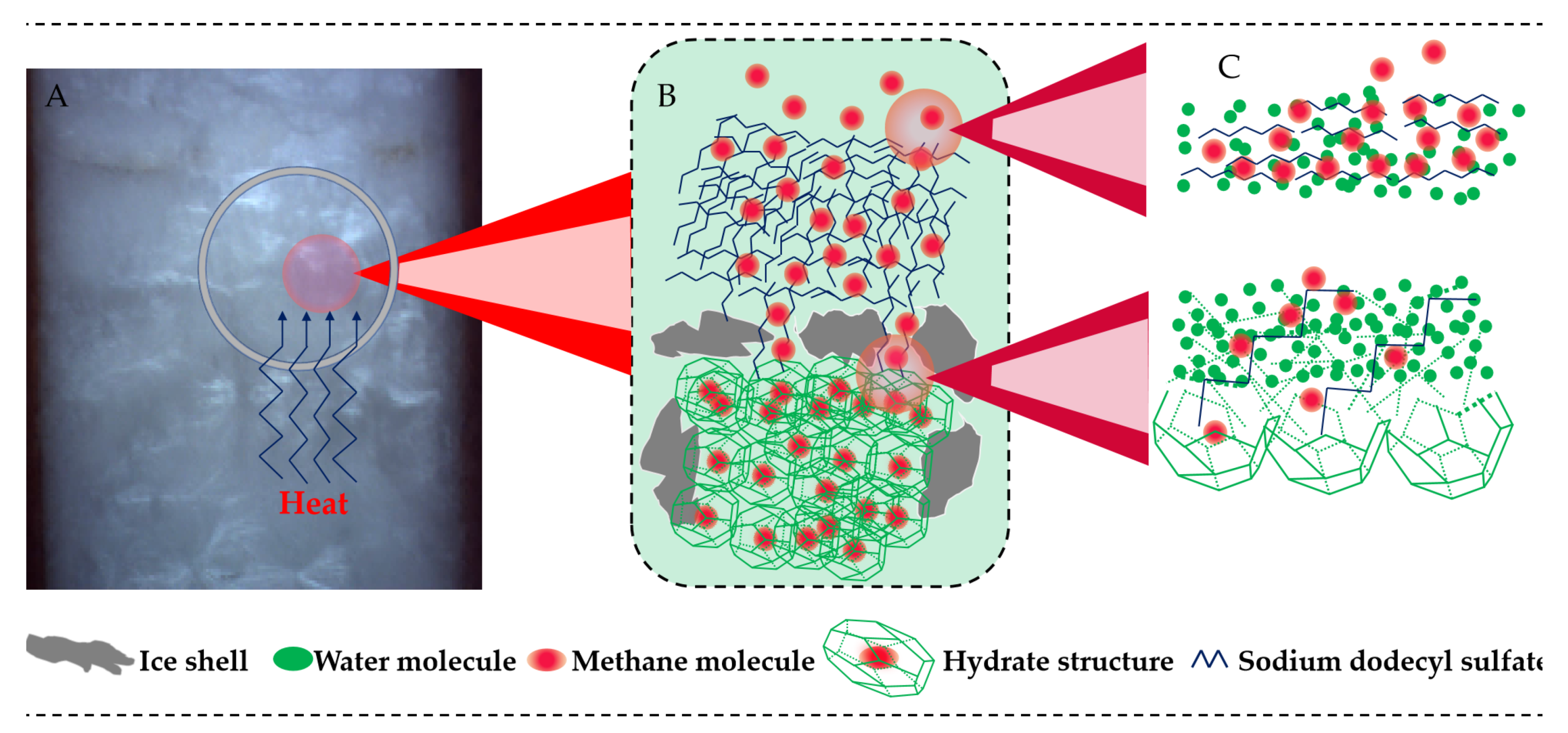
3.1. CO2 Hydrate Formation and Disappearance of Its Self-Preservation Effect
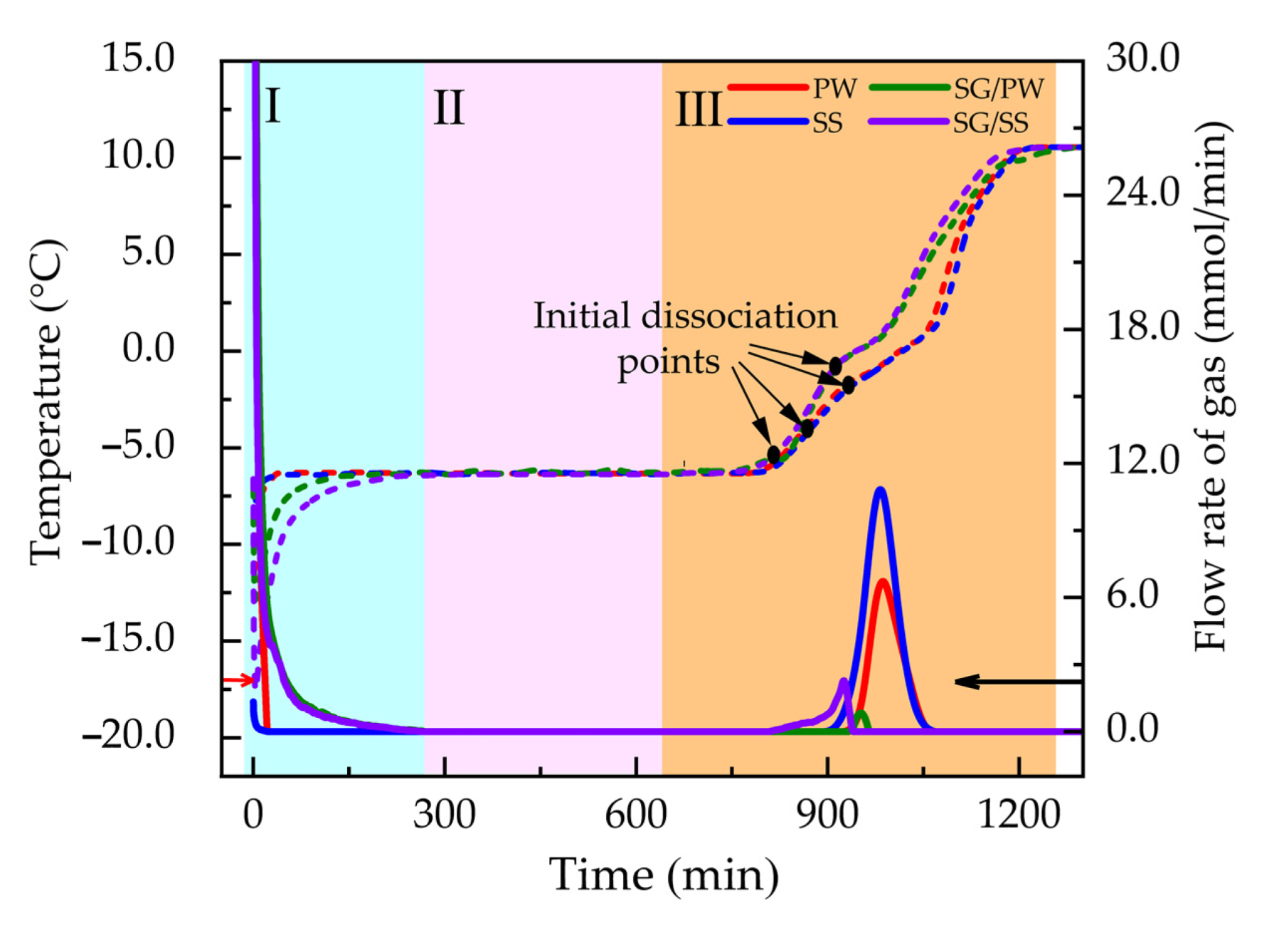
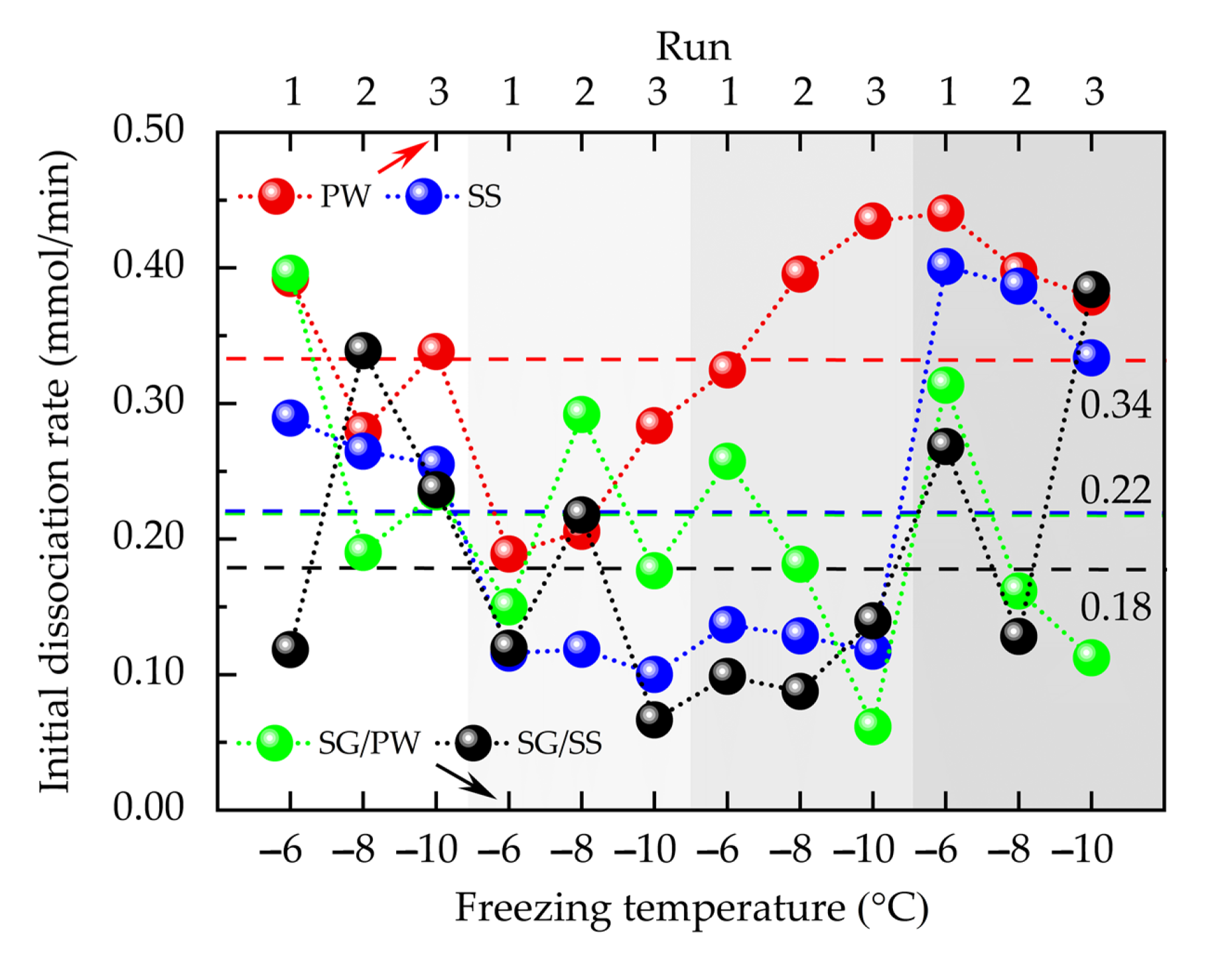
3.2. Influence of Medium and Experimental Conditions on IDT and IDR
3.3. Relations between IDR and Ratio of Total Dissociation Time to Ice Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: New York, NY, USA, 2003; pp. 685–692. [Google Scholar]
- Reed, S.K.; Westacott, R.E. The interface between water and a hydrophobic gas. Phys Chem Chem Phys. 2008, 10, 4614–4622. [Google Scholar] [CrossRef] [PubMed]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers. Manag. 2003, 20, 3151–3175. [Google Scholar] [CrossRef]
- Rajnauth, J.J.; Barrufet, M.A. Monetizing gas: Focusing on developments in gas hydrate as a mode of transportation. Energy Sci. Technol. 2012, 4, 61–68. [Google Scholar]
- Safari, S.; Varaminian, F. Study the kinetics and thermodynamics conditions for CO2 hydrate formation in orange juice concentration. Innov. Food Sci. Emerg. Technol. 2019, 57, 102155. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Moon, S.; Koh, D.Y.; Hong, S.; Park, Y. One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending. Energy Storage Mater. 2020, 1, 655–661. [Google Scholar] [CrossRef]
- Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A.; Lee, J.W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 7, 2315–23192. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.J.; Zhang, P.; Linga, P. Semiclathrate hydrate process for pre-combustion capture of CO2 at near ambient temperatures. Appl Energ. 2017, 194, 267–278. [Google Scholar] [CrossRef]
- Qi, Y.; Ota, M.; Zhang, H. Molecular dynamics simulation of replacement of CH4 in hydrate with CO2. Energy Convers. Manag. 2011, 7, 2682–2687. [Google Scholar] [CrossRef]
- Xu, C.G.; Cai, J.; Yu, Y.S.; Yan, K.F.; Li, X.S. Effect of pressure on methane recovery from natural gas hydrates by methane-carbon dioxide replacement. Appl. Energy 2018, 217, 527–536. [Google Scholar] [CrossRef]
- IEA. Energy Technology Perspectives 2015: Mobilising Innovation to Accelerate Climate Action; International Energy Agency: Paris, France, 2015. Available online: https://www.researchgate.net/publication/311746701_Energy_Technology_Perspectives_2015_-_Mobilising_Innovation_to_Accelarate_Climate_Action/citations (accessed on 25 June 2015).
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.J.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 1-3, 91–96. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A review of clathrate hydrate based desalination to strengthen energy-water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Kondo, W.; Ogawa, H.; Ohmura, R.; Mori, Y.H. Clathrate hydrate formation from a hydrocarbon gas mixture: Evolution of gas-phase composition in a hydrate-forming reactor. Energy Fuel. 2010, 12, 6375–6383. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, G.Y.; Guo, X.Q.; Liu, A.X. Experiments on the continuous separation of gas mixtures via dissolution and hydrate formation in the presence of THF. Fluid Phase Equilib. 2014, 1, 250–256. [Google Scholar] [CrossRef]
- Horii, S.; Ohmura, R. Continuous separation of CO2 from a H2+CO2 gas mixture using clathrate hydrate. Appl. Energy 2018, 225, 78–84. [Google Scholar] [CrossRef]
- Sun, Q.B.; Kang, Y.T. Experimental correlation for the formation rate of CO2 hydrate with THF (tetrahydrofuran) for cooling application. Energy 2015, 91, 712–719. [Google Scholar] [CrossRef]
- Monammadi, A.; Jodat, A. Investigation of the kinetics of TBAB + carbon dioxide semiclathrate hydrate in presence of tween 80 as a cold storage material. J. Mol. Liq. 2019, 293, 111433. [Google Scholar] [CrossRef]
- Castellani, B.; Rossi, F.; Filipponi, M.; Nicolini, A. Hydrate-based removal of carbon dioxide and hydrogen sulphide from biogas mixtures: Experimental investigation and energy evaluations. Biomass Bioenergy 2014, 70, 330–338. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Nicolini, A.; Rossi, F. Energy and environmental analysis of membrane-based CH4-CO2 replacement processes in natural gas hydrates. Energies 2019, 12, 850. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, G.; Goh, M.N.; Arumuganainar, S.; Zhang, Y.; Linga, P. Ultra-rapid uptake and the highly stable storage of methane as combustible ice. Ultra-rapid uptake and the highly stable storage of methane as combustible ice. Energy Environ. Sci. 2020, 13, 4946–4961. [Google Scholar] [CrossRef]
- Davidson, D.W.; Garg, S.K.; Gough, S.R.; Handa, Y.P.; Ratcliffe, C.I.; Ripmeester, J.A.; Tse, J.S.; Lawson, W.F. Laboratory analysis of naturally occurring gas hydrate from sediment of the Gulf Mexico. Geochim. Cosmochim. 1986, 50, 619–623. [Google Scholar] [CrossRef]
- HandaInd, Y.P. A calorimetric study of naturally occurring gas hydrate. Ind. Eng. Chem. Res. 1988, 5, 872–874. [Google Scholar] [CrossRef]
- Yakushev, V.S.; Istomin, V.A. Gas hydrate self-preservation effect. In Physics and Chemistry of Ice; Hokkaido University Press: Sapporo, Japan, 1992; pp. 136–140. [Google Scholar]
- Takeya, S.; Shimada, W.; Kamata, Y.; Ebinuma, T.; Uchida, T. in situ x-ray diffraction measurements of the self-preservation effect of CH4 hydrate. J. Phys. Chem. A. 2001, 10, 9756–9759. [Google Scholar] [CrossRef]
- Takeya, S.; Ebinuma, T.; Uchida, T. Self-preservation effect and dissociation rates of CH4 hydrate. J. Cryst. Growth 2002, 237, 379–382. [Google Scholar]
- Takeya, S.; Uchida, T.; Nagao, J.; Ohmura, R.; Shimada, W.; Kamata, Y.; Ebinuma, T.; Narita, H. Particle size effect of hydrate for self-preservation. Chem. Eng. Sci. 2005, 60, 1383–1387. [Google Scholar] [CrossRef]
- Takeya, S.; Ripmeester, J.A. Dissociation behavior of clathrate hydrates to ice and dependence on guest molecules. Angew. Chem. Int. 2008, 47, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Takeya, S.; Ripmeester, J.A. Dissociation of clathrate hydrates below the ice point. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Anomalous preservation of pure methane hydrate at 1 atm. J. Phys. Chem. B. 2001, 9, 1756–1762. [Google Scholar] [CrossRef]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Temperature, pressure, and compositional effects on anomalous or “self” preservation of gas hydrate. Can. J. Phys. 2003, 1, 271–283. [Google Scholar] [CrossRef]
- Shirota, H.; Aya, I.; Namie, S. Measurement of methane hydrate dissociation for application to natural gas storage and transportation. In Proceedings of the Fourth International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002. [Google Scholar]
- Falenty, A.; Kuhs, W.F. Self-Preservation of CO2 gas hydrates-surface microstructure and ice perfection. J. Phys. Chem. B 2009, 113, 15975–15988. [Google Scholar] [CrossRef]
- Wu, Q.B.; Wang, Y.M.; Zhan, J. Effect of rapidly depressurizing and rising temperature on methane hydrate dissociation. J. Energy Chem. 2012, 21, 91–97. [Google Scholar] [CrossRef]
- Gregor, R.; Robert, E.; Markus, E.; Falenty, A.; Hamann, R.; Kähler, N.; Kuhs, W.F.; Osterkamp, H.; Windmeier, C. Methane Hydrate Pellet Transport Using the Self-Preservation Effect: A Techno-Economic Analysis. Energies 2012, 7, 2499–2523. [Google Scholar]
- Chuvilin, E.; Istomin, V.; Buhanov, B.; Guryeva, O.; Takeya, S.; Hachikubo, A. Experimental study of self-preservation mechanisms and relict gas hydrates formation in porous media. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 2–7 May 2010. [Google Scholar]
- Takeya, K.; Takahashi, R.; Kawase, K. Terahertz time domain spectroscopy on methane hydrate. In Proceedings of the International Conference on Infrared Millimeter and Terahertz Waves, Copenhagen, Denmark, 25–30 September 2016. [Google Scholar]
- Zhang, G.; Rogers, R.E. Ultra-stability of gas hydrates at 1 atm and 268.2 K. Chem. Eng. Sci. 2008, 63, 2066–2074. [Google Scholar] [CrossRef]
- Nagashima, H.D.; Takeya, S.; Uchida, T.; Ohmura, R. Preservation of carbon dioxide clathrate hydrate in the presence of trehalose under freezer conditions. Sci. Rep. 2016, 6, 19354. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Influence of contact medium and surfactants on carbon dioxide clathrate hydrate kinetics. Fuel 2013, 105, 664–671. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, S.; Kumar, R.; Rangsunvigit, P.; Linga, P. Enhanced clathrate hydrate formation kinetics at near ambient temperatures and moderate pressures: Application to natural gas storage. Fuel 2016, 182, 907–919. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Enhanced carbon dioxide hydrate formation kinetics in a fixed bed reactor filled with metallic packing. Chem. Eng. Sci. 2015, 122, 78–85. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Muruganathan, R.M.; Rossen, W.R.; Krastev, R. Effect of gas type on foam film permeability and its implications for foam flow in porous media. Adv. Colloid Interface Sci. 2011, 168, 71–78. [Google Scholar] [CrossRef]
- Babu, P.; Yee, D.; Linga, P.; Palmer, A.; Khoo, B.C.; Tan, T.S.; Rangsunvigit, P. Morphology of methane hydrate formation in porous media. Energy Fuels 2013, 27, 3364–3372. [Google Scholar] [CrossRef]
- Mekala, P.; Babu, P.; Sangwai, J.S.; Linga, P. Formation and dissociation kinetics of methane hydrates in seawater and silica sand. Energy Fuels 2014, 28, 2708–2716. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Roy, S.; Kumar, R. Methane hydrate formation in a test sediment of sand and clay at various levels of water saturation. Can. J. Chem. 2015, 93, 1742–1773. [Google Scholar] [CrossRef]
- Starling, K.E.; Kwok, Y. Thermo data for LPG. I. Equation of state and computer prediction. Hydrocarbon. Process. 1971, 50, 101–104. [Google Scholar]
- Zhang, C.S.; Fan, S.S.; Liang, D.Q.; Guo, K.H. Effect of additives on formation of natural gas hydrate. Fuel 2004, 83, 2115–2121. [Google Scholar] [CrossRef]
- Albertí, M.; Pirani, F.; Laganà, A. Carbon dioxide clathrate hydrates: Selective role of intermolecular interactions and action of the SDS catalyst. J. Phys. Chem. A 2013, 117, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Zhou, H.; Wang, P.F.; Song, Y.C. Effects of additives on continuous hydrate-based flue gas separation. Appl. Energy. 2018, 221, 374–385. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M.; Manakov, A.Y.; Adamova, T.P. Synergistic effect of combination of surfactant and oxide powder on enhancement of gas hydrates nucleation. J. Energy Chem. 2017, 26, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Kvamme, B.; Coffin, R.B.; Zhao, J.Z.; Wei, N.; Zhou, S.W.; Li, Q.P.; Saeidi, N.; Chien, Y.-C.; Dunn-Rankin, D.; Sun, W.T.; et al. Stages in dynamics of hydrate formation and consequences for design of experiments for hydrate formation in sediments. Energies 2019, 12, 3399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wu, Q.B.; Jiang, G.L.; Zhan, J.; Wang, Y.M. Water transfer characteristics in the vertical direction during methane hydrate formation and dissociation processes inside non-saturated media. J. Nat. Gas Chem. 2010, 19, 139–145. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.B.; Mu, C.C.; Chen, X.P. Nucleation mechanisms of CO2 hydrate reflected by gas solubility. Sci. Rep. 2018, 8, 10441. [Google Scholar] [CrossRef]
- Guo, G.J.; Rodger, P.M. Solubility of aqueous methane under metastable conditions: Implications for gas hydrate nucleation. J. Phys. Chem. B. 2013, 117, 6498–6504. [Google Scholar] [CrossRef] [PubMed]
- Linga, P.; Daraboina, N.; Ripmeester, J.A.; Englezos, P. Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem. Eng. Sci. 2012, 68, 617–623. [Google Scholar] [CrossRef]
- Guenther, A.; Khan, S.A.; Thalmann, M.; Trachsel, F.; Jensen, K.F. Transport and reaction in microscale segmented gas–liquid flow. Lab Chip. 2004, 4, 278–286. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, S.; Zhang, P.; Chen, W.; Wu, Q.; Zhan, J.; Wang, Y. Promoted Disappearance of CO2 Hydrate Self-Preservation Effect by Surfactant SDS. Energies 2021, 14, 3909. https://doi.org/10.3390/en14133909
Chen X, Li S, Zhang P, Chen W, Wu Q, Zhan J, Wang Y. Promoted Disappearance of CO2 Hydrate Self-Preservation Effect by Surfactant SDS. Energies. 2021; 14(13):3909. https://doi.org/10.3390/en14133909
Chicago/Turabian StyleChen, Xueping, Shuaijun Li, Peng Zhang, Wenting Chen, Qingbai Wu, Jing Zhan, and Yingmei Wang. 2021. "Promoted Disappearance of CO2 Hydrate Self-Preservation Effect by Surfactant SDS" Energies 14, no. 13: 3909. https://doi.org/10.3390/en14133909
APA StyleChen, X., Li, S., Zhang, P., Chen, W., Wu, Q., Zhan, J., & Wang, Y. (2021). Promoted Disappearance of CO2 Hydrate Self-Preservation Effect by Surfactant SDS. Energies, 14(13), 3909. https://doi.org/10.3390/en14133909






