Demonstration of a Top-Lit Updraft Based Pyrolytic Burner with Low Emission Operation and Automatic Process Control
Abstract
:1. Introduction
2. Objectives
3. Materials and Methods
3.1. Fuel
3.2. Experimental Set-Up
3.3. Combustion Test Procedure
4. Results
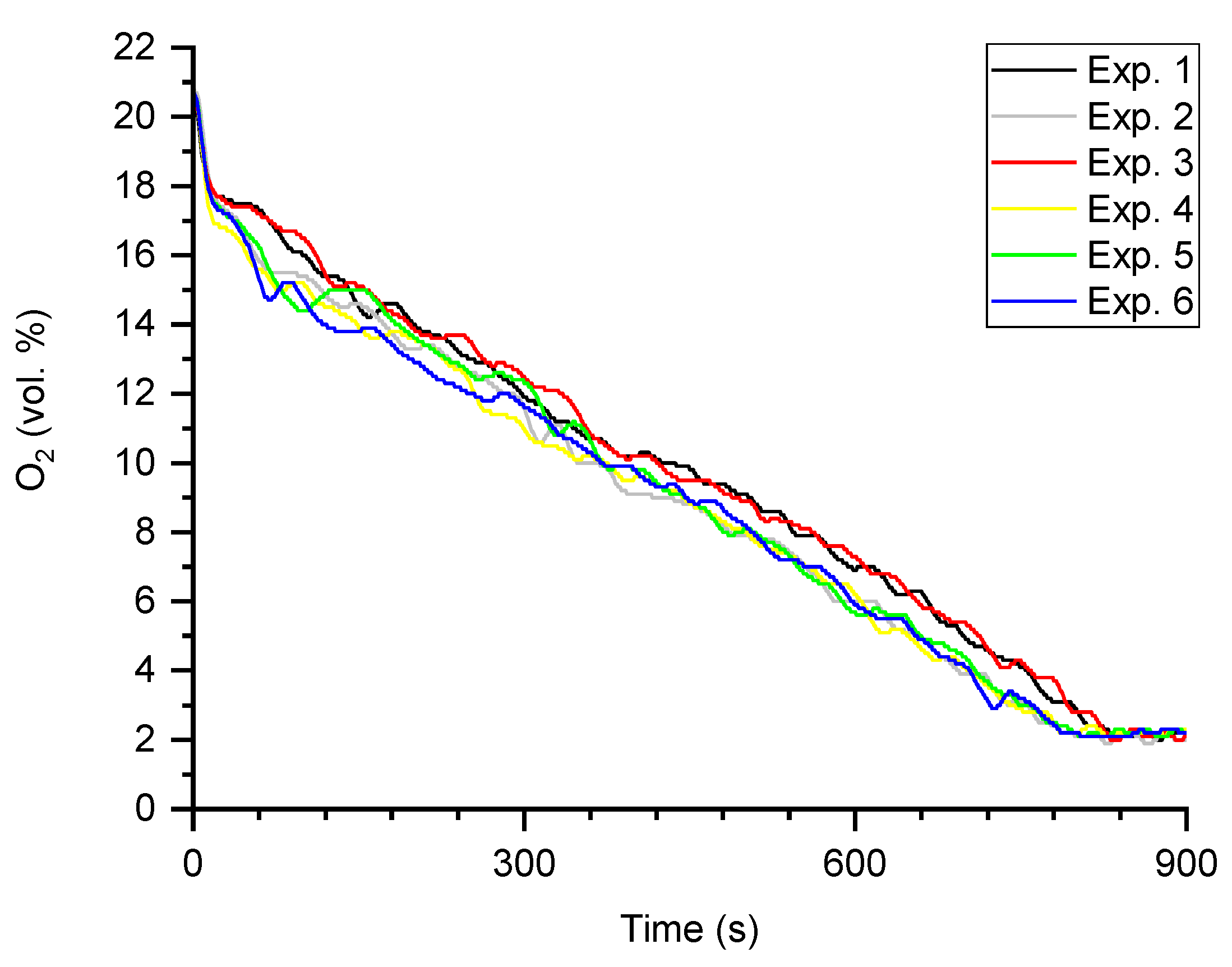
4.1. The Characteristics of the Overall Combustion Process
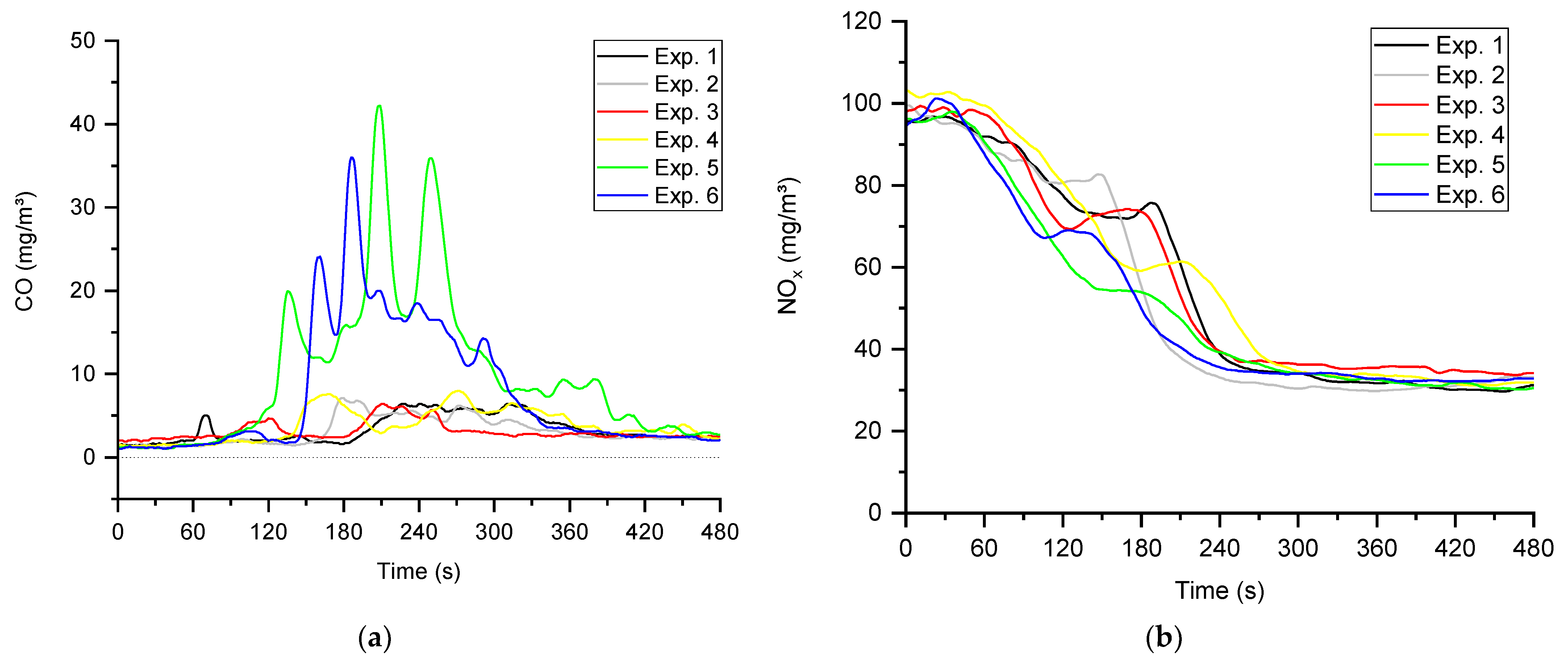
4.2. The Characteristics of the Transient Combustion Phases
4.2.1. Start-Up
4.2.2. Transition Phase
5. Discussion
6. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| n.d. | Not determined |
| PAH | Polyaromatic hydrocarbon |
| PM | Particulate matter |
| STP | Standard temperature and pressure |
| TLUD | Top-lit updraft |
| TPM | Total particulate matter |
| VOC | Volatile organic compounds |
References
- Dahlquist, E. Technologies for Converting Biomass to Useful Energy: Combustion, Gasification, Pyrolysis, Torrefaction and Fermentation; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2013. [Google Scholar]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Res. 2020, 27, 27491–27506. [Google Scholar] [CrossRef] [PubMed]
- Obaidullah, M.; Bram, S.; de Ruyck, J. Characteristics of Particle Mass Concentrations from small scale biomass combustion-a review. Small 2020, 5, 6. [Google Scholar]
- Jones, J.M.; Lea-Langton, A.R.; Ma, L.; Pourkashanian, M.; Williams, A. Pollutants Generated by the Combustion of Solid Biomass Fuels; Springer: London, UK, 2014; ISBN 978-1-4471-6437-1. [Google Scholar]
- Lenz, V.; Thrän, D.; Pfeiffer, D. Methods for Measuring Emissions of Particulate Matter from Solid Biomass Combustion: Series of the Funding Programme “Biomass Energy Use”; DBFZ Deutsches Biomasseforschungszentrum Gemeinnützige GmbH: Leipzig Germany, 2018; Volume 8. [Google Scholar]
- Gitau, K.; Mutune, J.; Sundberg, C.; Mendum, R.; Njenga, M. Factors influencing the adoption of biochar-producing gasifier cookstoves by households in rural Kenya. Energy Sustain. Dev. 2019, 52, 63–71. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. (Eds.) The Handbook of Biomass Combustion and Co-Firing-Earthscan; Earthscan Publishing: Abingdon, UK, 2008. [Google Scholar]
- Tryner, J. Combustion Phenomena in Biomass Gasifier Cookstoves. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2016. [Google Scholar]
- Toklu, E. Biomass energy potential and utilization in Turkey. Renew. Energy 2017, 107, 235–244. [Google Scholar] [CrossRef]
- Verma, V.; Bram, S.; De Ruyck, J. Small scale biomass heating systems: Standards, quality labelling and market driving factors—An EU outlook. Biomass Bioenergy 2009, 33, 1393–1402. [Google Scholar] [CrossRef]
- Schmidl, C.; Luisser, M.; Padouvas, E.; Lasselsberger, L.; Rzaca, M.; Cruz, C.R.-S.; Handler, M.; Peng, G.; Bauer, H.; Puxbaum, H. Particulate and gaseous emissions from manually and automatically fired small scale combustion systems. Atmosph. Environ. 2011, 45, 7443–7454. [Google Scholar] [CrossRef]
- Boman, C.; Pettersson, E.; Westerholm, R.; Boström, D.; Nordin, A. Stove Performance and Emission Characteristics in Residential Wood Log and Pellet Combustion, Part 1: Pellet Stoves. Energy Fuels 2011, 25, 307–314. [Google Scholar] [CrossRef]
- Kirch, T.; Birzer, C.; van Eyk, P.; Medwell, P.R. Influence of Primary and Secondary Air Supply on Gaseous Emissions from a Small-Scale Staged Solid Biomass Fuel Combustor. Energy Fuels 2017, 32, 4212–4220. [Google Scholar] [CrossRef]
- Tissari, J.; Sippula, O.; Kouki, J.; Vuorio, K.; Jokiniemi, J. Fine Particle and Gas Emissions from the Combustion of Agricultural Fuels Fired in a 20 kW Burner. Energy Fuels 2008, 22, 2033–2042. [Google Scholar] [CrossRef]
- Bhattu, D.; Zotter, P.; Zhou, J.; Stefenelli, G.; Klein, F.; Bertrand, A.; Temime-Roussel, B.; Marchand, N.; Slowik, J.G.; Baltensperger, U.; et al. Effect of Stove Technology and Combustion Conditions on Gas and Particulate Emissions from Residential Biomass Combustion. Environ. Sci. Technol. 2019, 53, 2209–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberg, H.; Sippula, O.; Tissari, J.; Jokiniemi, J. Effects of Air Staging and Load on Fine-Particle and Gaseous Emissions from a Small-Scale Pellet Boiler. Energy Fuels 2011, 25, 4952–4960. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Fine Particulate Emissions from Modern Austrian Small-Scale Biomass Combustion Plants. In Proceedings of the 15th European Biomass Conference and Exhibition, Berlin, Germany, 7–11 May 2007; pp. 1546–1557. [Google Scholar]
- Jetter, J.; Zhao, Y.; Smith, K.R.; Khan, B.; Yelverton, T.; DeCarlo, P.; Hays, M.D. Pollutant Emissions and Energy Efficiency under Controlled Conditions for Household Biomass Cookstoves and Implications for Metrics Useful in Setting International Test Standards. Environ. Sci. Technol. 2012, 46, 10827–10834. [Google Scholar] [CrossRef] [PubMed]
- Jetter, J.J.; Kariher, P. Solid-fuel household cook stoves: Characterization of performance and emissions. Biomass Bioenergy 2009, 33, 294–305. [Google Scholar] [CrossRef]
- MacCarty, N.; Still, D.; Ogle, D. Fuel use and emissions performance of fifty cooking stoves in the laboratory and related benchmarks of performance. Energy Sustain. Dev. 2010, 14, 161–171. [Google Scholar] [CrossRef]
- Roth, C. Micro-Gasification: Cooking with Gas from Dry Biomass: An Introduction to Concepts and Applications of Wood-Gas. Burning Technologies for Cooking, 2nd ed.; GIZ-Deutsche Gesellschaft für Internationale: Bonn, Germany, 2013. [Google Scholar]
- Tryner, J.; Tillotson, J.W.; Baumgardner, M.E.; Mohr, J.T.; Defoort, M.W.; Marchese, A.J. The Effects of Air Flow Rates, Secondary Air Inlet Geometry, Fuel Type, and Operating Mode on the Performance of Gasifier Cookstoves. Environ. Sci. Technol. 2016, 50, 9754–9763. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Defoort, M.; Gao, X.; Tryner, J.; Dryer, F.L.; Haas, F.; Lorenz, N. Achieving Tier 4 Emissions in Biomass Cookstoves; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2018. [Google Scholar]
- Scholz, S.B.; Sembres, T.; Roberts, K.; Whitman, T.; Wilson, K.; Lehmann, J. Biochar Systems for Smallholders in Developing Countries: Leveraging Current Knowledge and Exploring Future Potential for Climate-Smart Agriculture; International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2014. [Google Scholar]
- ISO 17225-2:2014. Solid Biofuels—Fuel Specifications and Classes: Part. 2: Graded Wood Pellets; International Organization for Standardization: London, UK, 2014. [Google Scholar]
- EN 15267-1:2009. Air Quality—Certification of Automated Measuring Systems: Part. 1: General Principles; Beuth: Berlin, Germany, 2009. [Google Scholar]
- EN 15267-2:2009. Air Quality—Certification of Automated Measuring Systems: Part. 2: Initial Assessment of the AMS Manufacturer’s Quality Management System and Post Certification Surveillance for the Manufacturing Process; Beuth: Berlin, Germany, 2009. [Google Scholar]
- EN 15267-4:2017. Air Quality—Certification of Automated Measuring Systems: Part. 4: Performance Criteria and Test. Procedures for Automated Measuring Systems for Periodic Measurements of Emissions from Stationary Sources; Beuth: Berlin, Germany, 2017. [Google Scholar]
- ISO 14175:2008. Welding Consumables—Gases and Gas. Mixtures for Fusion Welding and Allied Processes; International Organization for Standardization: London, UK, 2008. [Google Scholar]
- EN 13284-1:2017. Stationary Source Emissions—Determination of Low Range Mass Concentration of Dust: Part. 1: Manual Gravimetric Method; Beuth: Berlin, Germany, 2017. [Google Scholar]
- Gothe, P.; GmbH. Datenblatt/Data Sheet QUARZ-MIKROFASERFILTER: MK 360. Available online: https://www.paulgothe.com/. (accessed on 23 June 2021).
- Sippula, O.; Hokkinen, J.; Puustinen, H.; Yli-Pirilä, P.; Jokiniemi, J. Comparison of particle emissions from small heavy fuel oil and wood-fired boilers. Atmosph. Environ. 2009, 43, 4855–4864. [Google Scholar] [CrossRef]
- Kaivosoja, T.; Jalava, P.; Lamberg, H.; Virén, A.; Tapanainen, M.; Torvela, T.; Tapper, U.; Sippula, O.; Tissari, J.; Hillamo, R.; et al. Comparison of emissions and toxicological properties of fine particles from wood and oil boilers in small (20–25 kW) and medium (5–10 MW) scale. Atmosph. Environ. 2013, 77, 193–201. [Google Scholar] [CrossRef]
- Motyl, P.; Król, D.; Poskrobko, S.; Juszczak, M. Numerical Modelling and Experimental Verification of the Low-Emission Biomass Combustion Process in a Domestic Boiler with Flue Gas Flow around the Combustion Chamber. Energies 2020, 13, 5837. [Google Scholar] [CrossRef]
- Baumgarten, B.; Grammer, P.; Ehard, F.; Winkel, O.; Vogt, U.; Baumbach, G.; Scheffknecht, G.; Thorwarth, H. Novel metal mesh filter using water-based regeneration for small-scale biomass boilers. Biomass Convers. Biorefin. 2020, 636, 1–13. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Mandl, C.; Kerschbaum, M.; Svetlik, T. Strategies and technologies towards zero emission biomass combustion by primary measures. Energ. Proc. 2017, 120, 681–688. [Google Scholar] [CrossRef]
- BImSchV. Erste Verordnung zur Durchführung des BundesImmissionsschutzgesetzes (Verordnung über Kleine und Mittlere Feuerungsanlagen—1. BImSchV). 2010. Available online: https://www.gesetze-im-internet.de/bimschv_1_2010/1._BImSchV.pdf (accessed on 22 October 2020).
- Blauer Engel. Holzpelletöfen. DE-UZ 111 February 2016, Version 2. Available online: https://produktinfo.blauer-engel.de/uploads/criteriafile/de/DE-UZ%20111-201602-de%20Kriterien-2020-01-07.pdf (accessed on 23 June 2021).
- Blauer Engel. Holzpellet—und Holzhackschnitzelheizlessel. DE-UZ 112, February2016, Version 2. Available online: https://produktinfo.blauer-engel.de/uploads/criteriafile/de/DE-UZ%20112-201602-de%20Kriterien-2020-01-03.pdf (accessed on 23 June 2021).
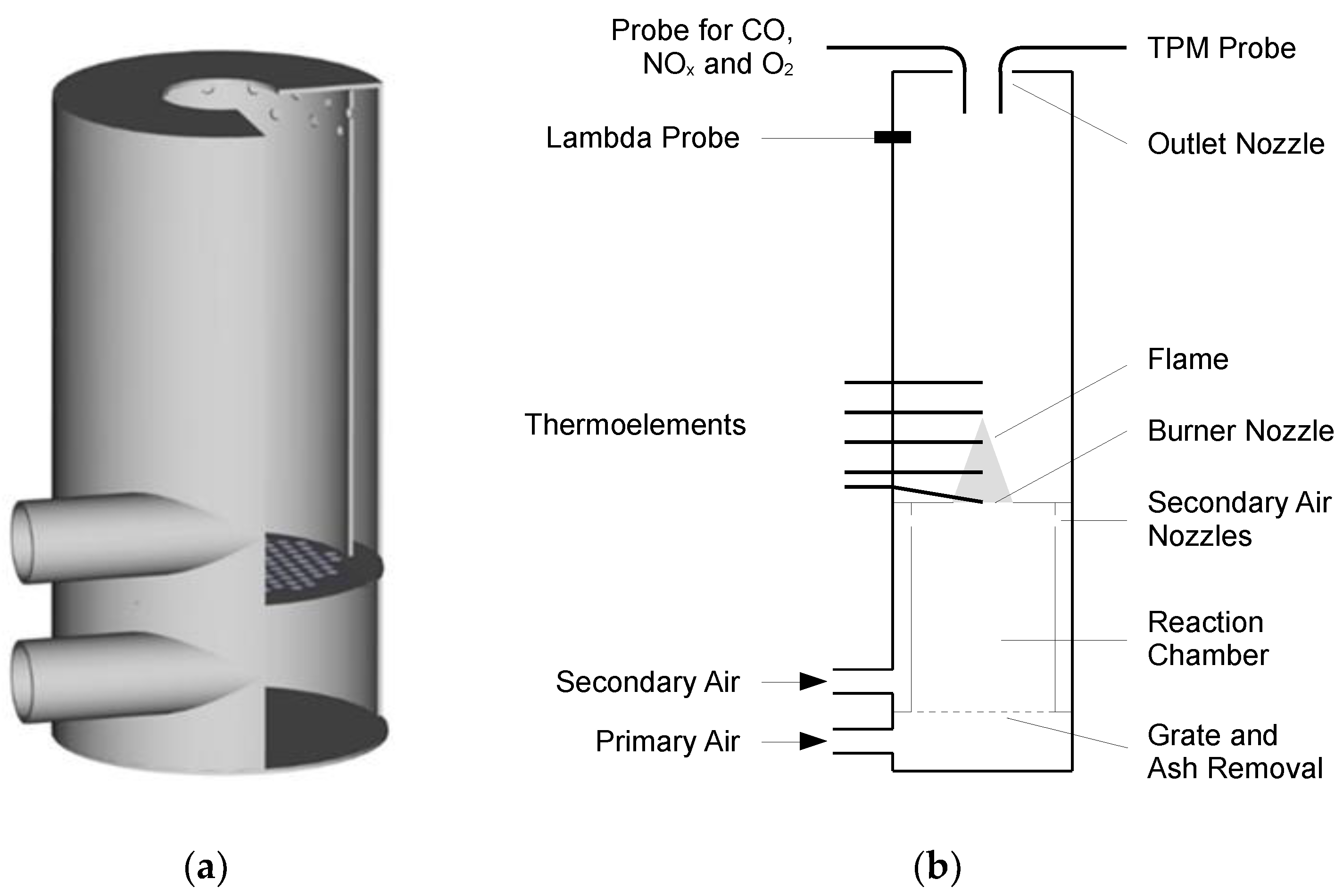
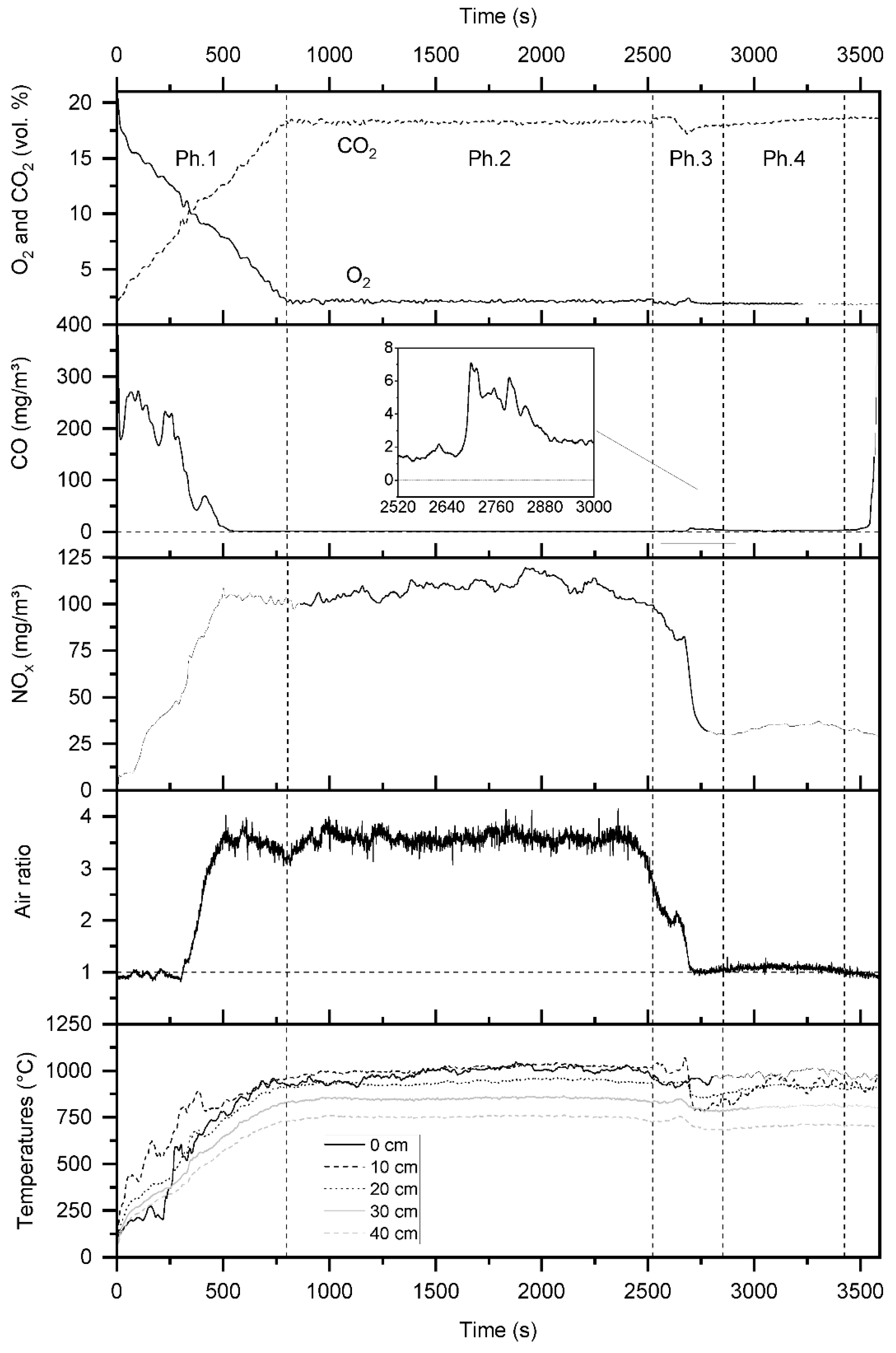
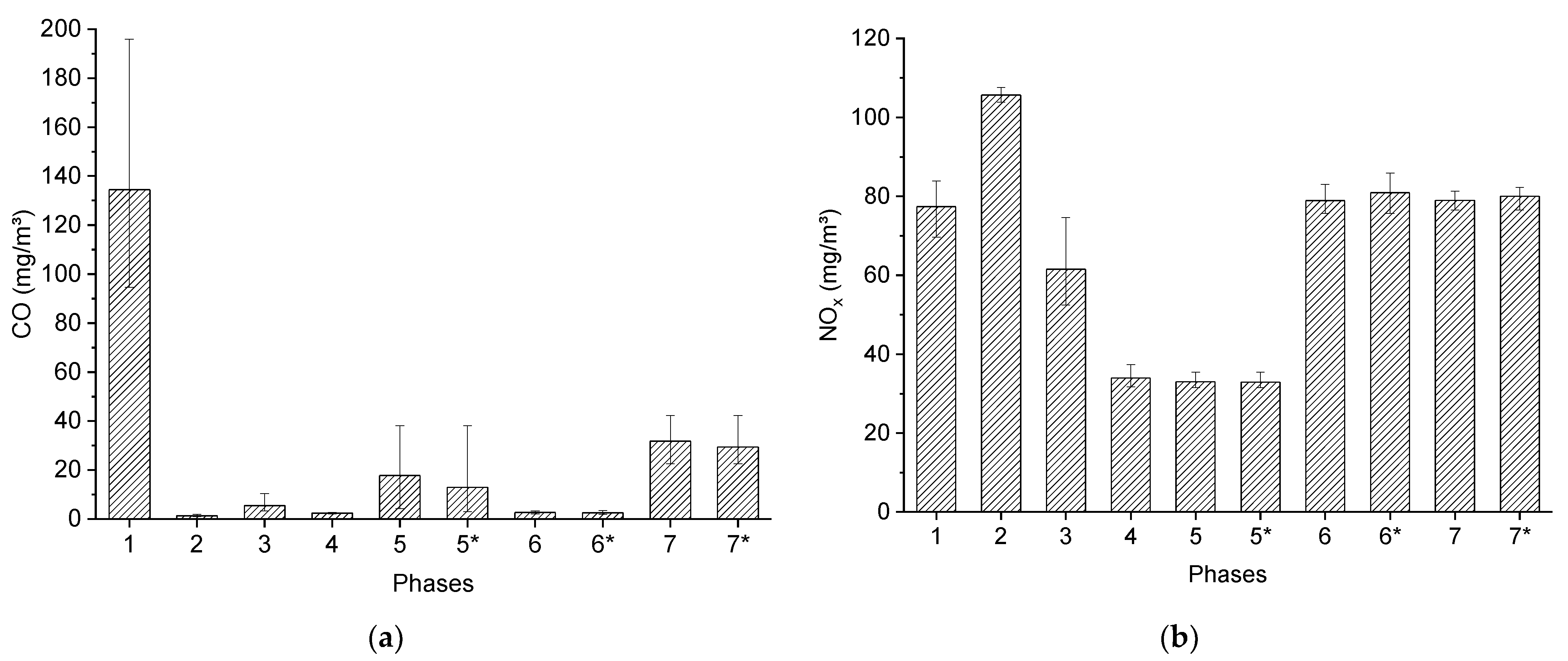

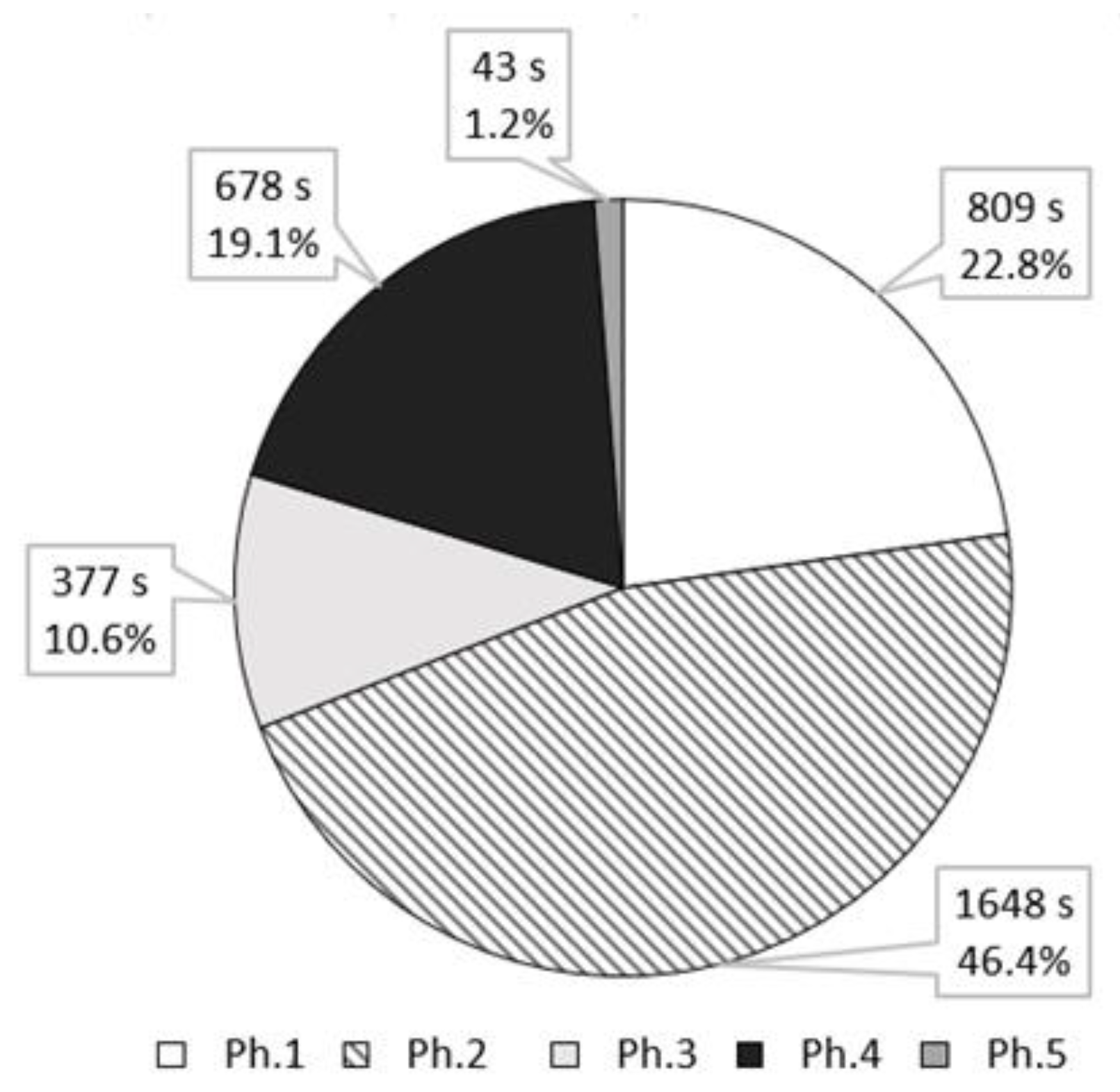

| Parameter | Value |
|---|---|
| Moisture (as received, wt.%) | 6.2 |
| Ash (550 °C) (dry, wt.%) | 0.31 |
| Proximate analysis (dry, wt.%) | - |
| C | 51.0 |
| H | 6.18 |
| N | 0.05 |
| S | 0.06 |
| O (by difference) | 42.4 |
| Lower heating value (MJ/kg) | 19.0 |
| Designation | Type | Model |
|---|---|---|
| Primary Air Fan | Actuator | Sanyo Denki 9GAX0412P3S001 |
| Secondary Air Fan | Actuator | Sanyo Denki 9GAX0412P3S001 |
| Primary Air—Air Mass Sensor | Sensor | Honeywell AWM720P1 |
| Secondary Air—Air Mass Sensor | Sensor | Honeywell AWM720P1 |
| Wideband Lambda Sensor | Sensor | Bosch LSU 4.2 |
| Gas analyzer | Measuring Device | Horiba PG-350E * |
| Particle Measurement | Measuring Device | Paul Gothe GmbH particle measuring system ITES |
| Ph. 1 | Ph. 2 | Ph. 3 | Ph. 4 | Ph. 5 | Ph. 6 | Ph. 7 | |
|---|---|---|---|---|---|---|---|
| O2 (vol. %) | |||||||
| Exp. 1 | 10.2 | 2.1 | 2.0 | 1.9 | 1.9 * | 2.0 * | 4.0 * |
| Exp. 2 | 9.7 | 2.1 | 2.0 | 1.9 | 1.9 * | 2.1 * | 3.8 * |
| Exp. 3 | 10.3 | 2.1 | 2.0 | 1.9 | 1.9 | 2.0 | 4.0 |
| Exp. 4 | 9.7 | 2.2 | 2.0 | 1.9 | 2.0 | 2.1 | 3.8 |
| Exp. 5 | 9.8 | 2.2 | 2.1 | 2.0 | 2.0 | 2.1 | 3.7 |
| Exp. 6 | 9.7 | 2.2 | 2.1 | 2.0 | 2.0 | 2.1 | 3.9 |
| CO (mg/m3) | |||||||
| Exp. 1 | 94.6 | 1.4 | 3.5 | 2.5 | 3.0 * | 2.0 * | 24.9 * |
| Exp. 2 | 99.0 | 1.2 | 3.3 | 2.4 | 3.6 * | 1.8 * | 24.4 * |
| Exp. 3 | 117.1 | 1.9 | 3.4 | 2.7 | 23.4 | 2.7 | 22.5 |
| Exp. 4 | 156.3 | 1.3 | 3.9 | 2.2 | 4.3 | 2.0 | 26.6 |
| Exp. 5 | 195.8 | 1.1 | 10.4 | 2.3 | 5.1 | 2.8 | 42.3 |
| Exp. 6 | 143.5 | 0.9 | 8.7 | 2.2 | 38.1 | 3.4 | 35.8 |
| NOx (mg/m3) | |||||||
| Exp. 1 | 77.4 | 107.2 | 65.5 | 31.7 | 32.6 * | 83.9 * | 82.3 * |
| Exp. 2 | 69.7 | 107.6 | 62.1 | 34.2 | 32.6 * | 85.8 * | 82.1 * |
| Exp. 3 | 75.5 | 105.7 | 74.6 | 37.3 | 35.5 | 82.9 | 81.2 |
| Exp. 4 | 78.5 | 103.9 | 58.4 | 33.9 | 31.8 | 78.6 | 78.6 |
| Exp. 5 | 79.5 | 104.4 | 52.5 | 34.0 | 33.3 | 75.7 | 76.5 |
| Exp. 6 | 83.9 | 105.3 | 56.1 | 32.7 | 31.5 | 78.2 | 79.5 |
| TPM (mg/m3) | |||||||
| Exp. 1 | 5.0 | 1.7 | n.d. | 7.2 | n.d. | n.d. | n.d. |
| Exp. 2 | 6.6 | 2.0 | n.d. | 6.7 | n.d. | n.d. | n.d. |
| Exp. 3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.6 |
| Exp. 4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.4 |
| Exp. 5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.9 |
| Exp. 6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.3 |
| Time (s) | |||||||
| Exp. 1 | 839 | 1614 | 372 | 534 | 33 * | 2553 * | 3392 * |
| Exp. 2 | 799 | 1722 | 333 | 556 | 14 * | 2625 * | 3424 * |
| Exp. 3 | 837 | 1677 | 260 | 734 | 53 | 2724 | 3561 |
| Exp. 4 | 789 | 1628 | 463 | 668 | 43 | 2802 | 3591 |
| Exp. 5 | 803 | 1729 | 447 | 937 | 6 | 3119 | 3922 |
| Exp. 6 | 793 | 1524 | 394 | 645 | 71 | 2634 | 3427 |
| Ph. 2 | Ph. 4 | Ph. 6 | Ph. 7 | ||
|---|---|---|---|---|---|
| CO | Mean | 1.3 | 2.4 | 2.5 * | 29.4 * |
| Max. | 1.9 | 2.7 | 3.4 | 42.3 | |
| NOx | Mean | 105.7 | 34.0 | 80.9 * | 80.0 * |
| Max. | 107.6 | 37.3 | 85.8 * | 82.3 * | |
| TPM | Mean | 1.9 | 7.0 | n.d. | 3.6 |
| Max. | 2.0 | 7.2 | n.d. | 3.9 |
| Appliance Type | Fuel | Nominal Heat Output | PM (mg/m3) | CO (mg/m3) | NOx (mg/m3) | Ref. O2 Content (vol. %) |
|---|---|---|---|---|---|---|
| Blue Angel Boiler | Wood pellets | <500 kWth | 15 | 150 | 150 | 13 |
| Wood chips | <500 kWth | 15 | 165 | 150 | 13 | |
| Blue Angel Stoves | Wood pellet | <15 kWth | 15 | 160 a/250 b | 150 a/200 b | 13 |
| 1. BlmSchV small-medium sized plants | Wood including bark (logs, wood chips), sawdust as well as pressings made of natural wood (briquettes, pellets) | >4 kWth | 20 | 400 | - | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, D.; Mutlu, Ö.Ç. Demonstration of a Top-Lit Updraft Based Pyrolytic Burner with Low Emission Operation and Automatic Process Control. Energies 2021, 14, 3913. https://doi.org/10.3390/en14133913
Krüger D, Mutlu ÖÇ. Demonstration of a Top-Lit Updraft Based Pyrolytic Burner with Low Emission Operation and Automatic Process Control. Energies. 2021; 14(13):3913. https://doi.org/10.3390/en14133913
Chicago/Turabian StyleKrüger, Dennis, and Özge Çepelioğullar Mutlu. 2021. "Demonstration of a Top-Lit Updraft Based Pyrolytic Burner with Low Emission Operation and Automatic Process Control" Energies 14, no. 13: 3913. https://doi.org/10.3390/en14133913
APA StyleKrüger, D., & Mutlu, Ö. Ç. (2021). Demonstration of a Top-Lit Updraft Based Pyrolytic Burner with Low Emission Operation and Automatic Process Control. Energies, 14(13), 3913. https://doi.org/10.3390/en14133913







