Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols
Abstract
1. Introduction
2. Main Parameters Influencing Long-Term Durability of Polymer Electrolyte Membrane Fuel Cells (PEMFCs)
2.1. Degradation of Different Components of PEMFC
2.1.1. Catalyst Layer
Structure and Role
Degradation Phenomena and Mechanisms
- a
- Catalyst degradation
- b
- Carbon support corrosion
2.1.2. Membrane Degradation
Structure and Role
Degradation Phenomena and Mechanisms
2.1.3. Gas Diffusion Layers (GDLs)
Structure and Role
Degradation Phenomena and Mechanisms
2.1.4. Bipolar Plate
Structure and Role
Degradation Phenomena and Mechanisms
- (1)
- Dissolution of plate materials leading to poisoning of membrane;
- (2)
- Formation of resistive surface layer resulting in high ohmic resistance;
- (3)
- Possible fracture and continuous deformation of BBPs due to mechanical stress caused by high compressive pressure when sealing the stack.
- a
- Carbon bipolar plate degradation
- b
- Metallic bipolar plate degradation
2.1.5. Sealing Gasket
Structure and Role
Degradation Phenomena and Mechanisms
2.2. Operational Effects on PEMFC Durability
2.2.1. Impurity Effects
2.2.2. Subfreezing Effects
2.2.3. Other Operating Conditions Effects
2.3. Water Management
2.3.1. Dehydration
2.3.2. Flooding
2.4. Thermal Management
3. Cell/Stack Durability Test Protocols
3.1. Durability Testing Protocols for Transport Applications
3.1.1. The Dynamic Stress Test Protocol (DST)
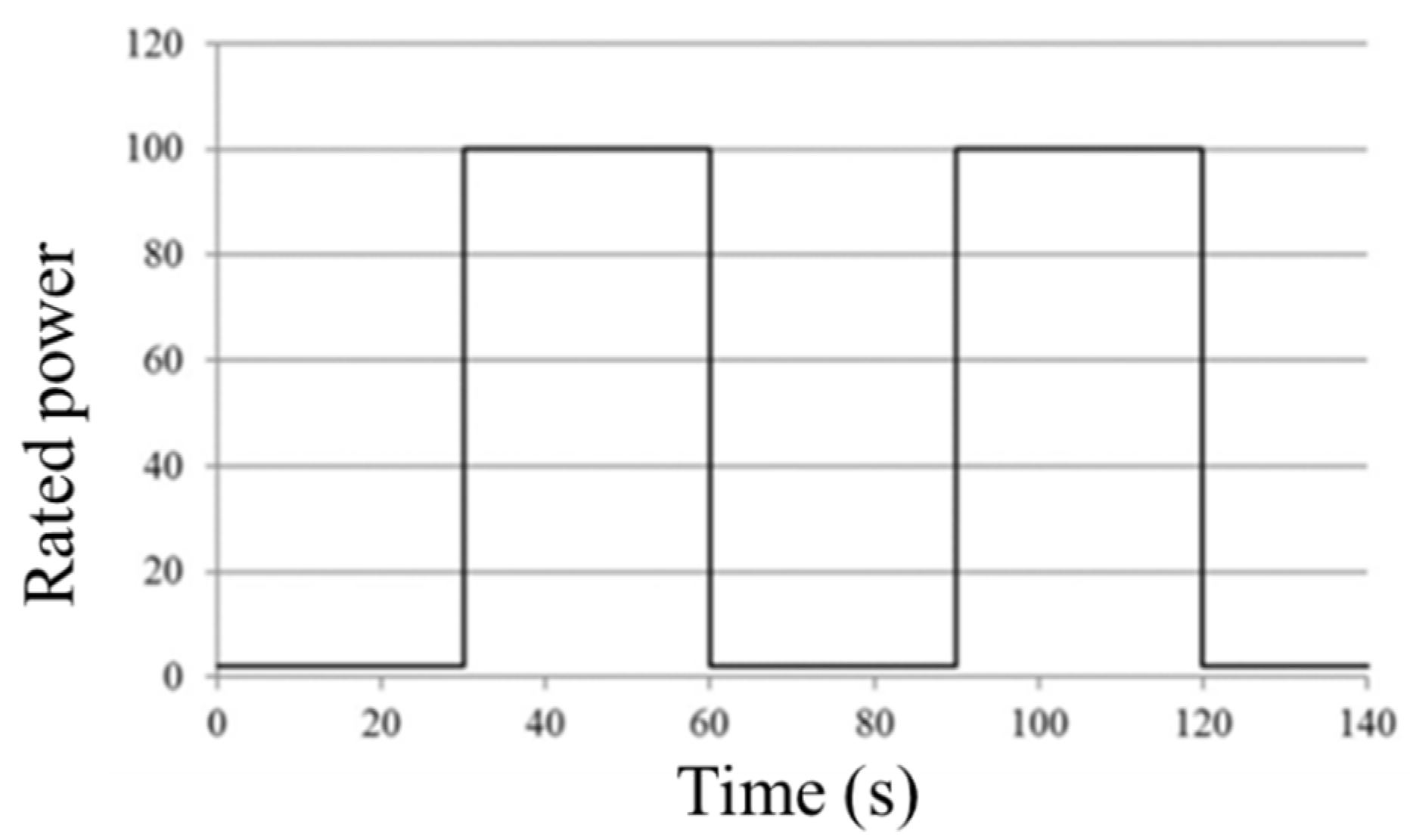
3.1.2. Fuel Cell Technical Team (FCTT)
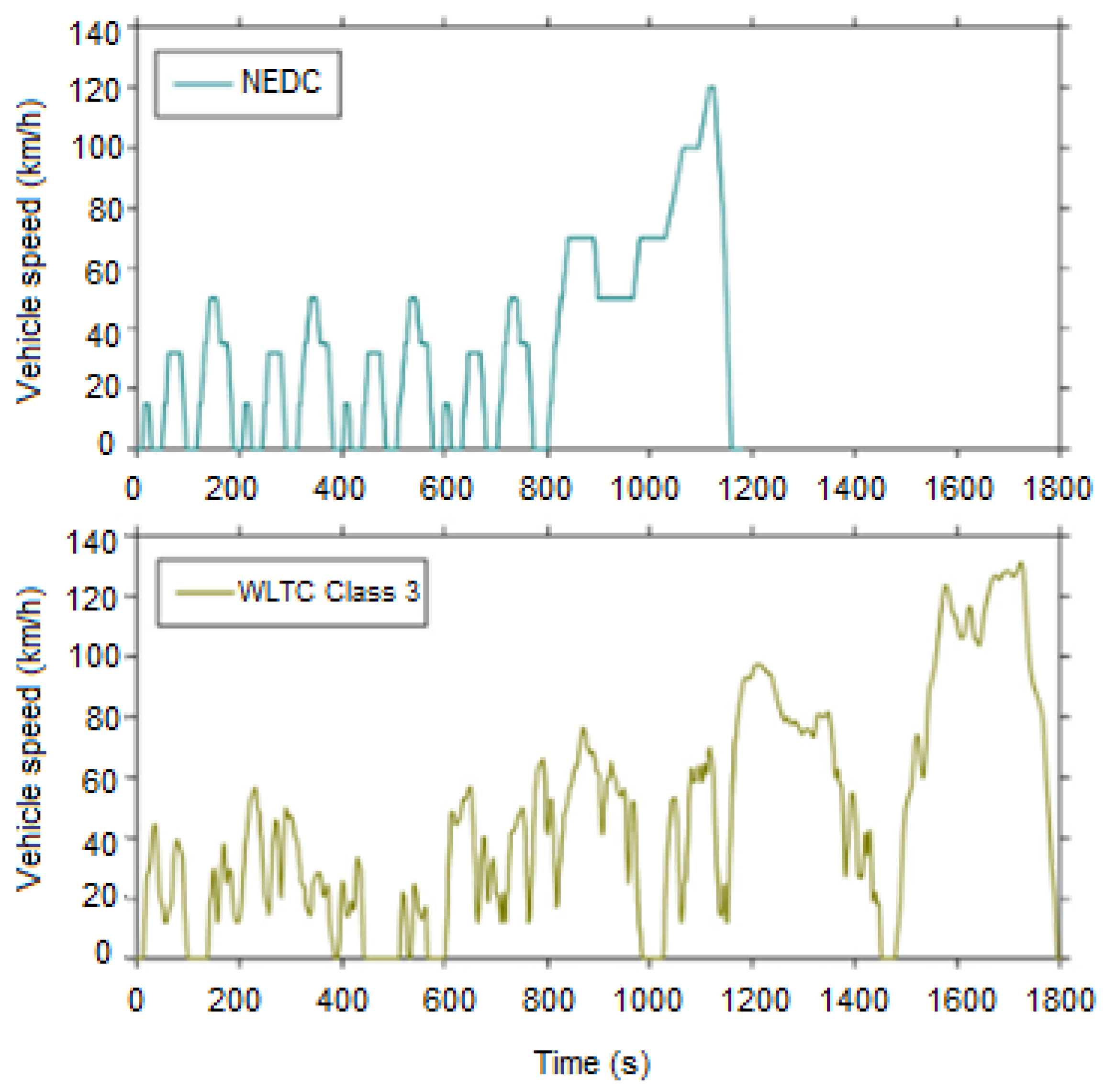
3.1.3. The New European Driving Cycle (NEDC)
3.1.4. Worldwide Harmonized Light Vehicle Test Procedure (WLTP)
3.1.5. The Fuel Cell Testing and Standardization Network (FCTESTNEST)
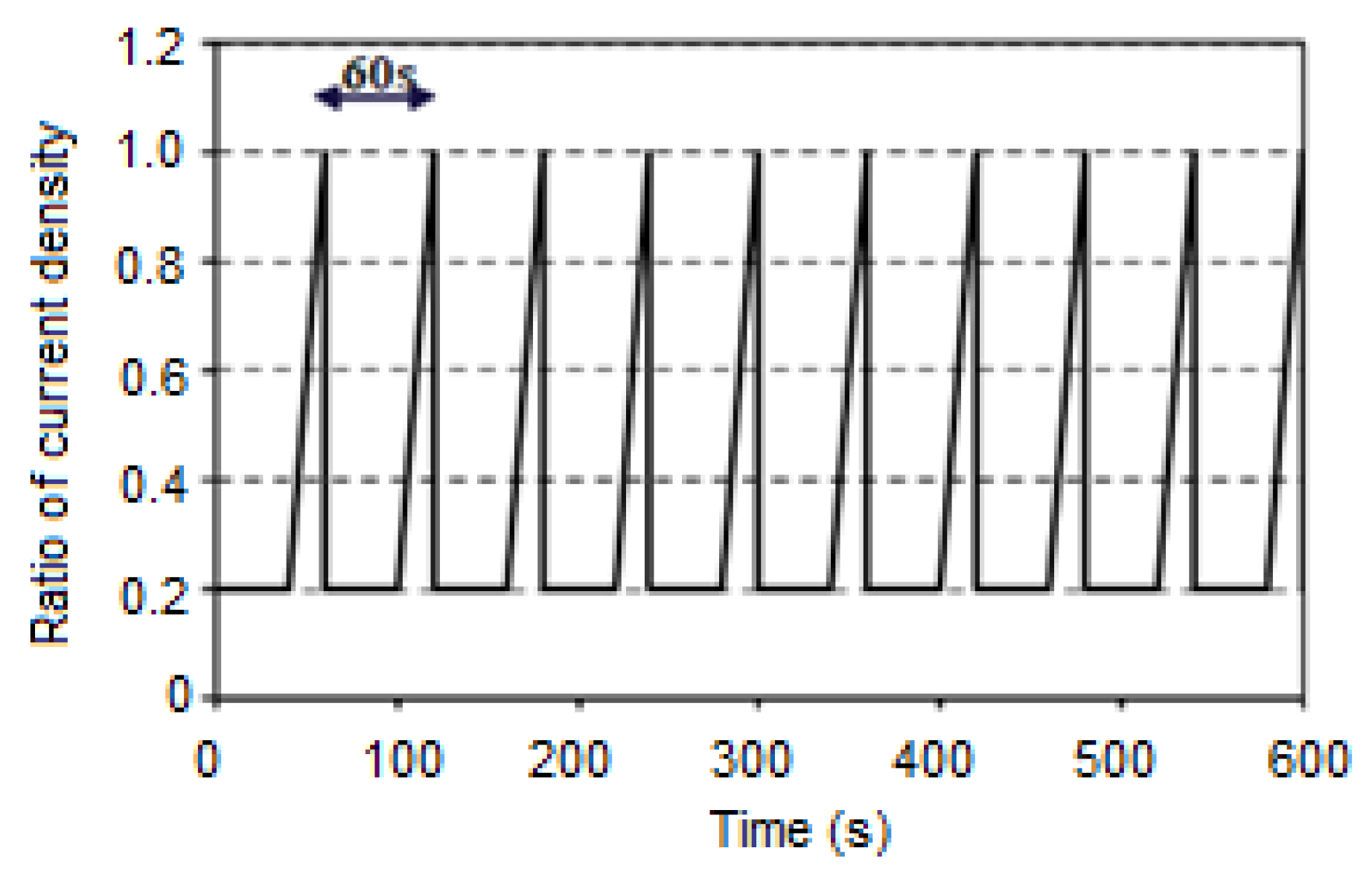
3.1.6. The Giantleap Protocol
3.1.7. The Japanese JC08 Cycle
3.1.8. Test Protocols in China
3.1.9. Summary
3.2. Test Protocols for Stationary Applications
3.2.1. Steady-State Durability Tests
3.2.2. Load Cycling Durability Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, X.Z.; Li, H.; Zhang, S.; Martin, J.; Wang, H. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Welch, P.M.; More, K. Polymer electrolyte membrane (PEM) fuel cell durability. In Materials Research Highlight, LALP-06-108; Los Alamos National Laboratory: Los Alamos, NM, USA, 2006. [Google Scholar]
- Chen, H.; Song, Z.; Zhao, X.; Zhang, T.; Pei, P.; Liang, C. A review of durability test protocols of the proton exchange membrane fuel cells for vehicle. Appl. Energy 2018, 224, 289–299. [Google Scholar] [CrossRef]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Stationary PEM Fuel Cells with Lifetimes beyond Five Years. Available online: https://cordis.europa.eu/project/id/256721 (accessed on 1 February 2021).
- Fuel Cells Section. In Multi-Year Research, Development, and Demonstartion Plan; U.S. Department of Energy: Washington, DC, USA, 2015; pp. 1–58.
- Fuel Cell Technical Team Roadmap. Available online: https://www.energy.gov/sites/prod/files/2017/11/f46/FCTT_Roadmap_Nov_2017_FINAL.pdf (accessed on 1 February 2021).
- Bloom, I.; Basco, J.K.; Walker, L.K.; Malkow, T.; DeMarco, G.; Saturnio, A.; Tsotridis, G. Fuel Cell Testing Protocols: An International Perspective, Joint ANL-JRC Scientific and Technical Report; Argonne National Laboratory: Argonne, IL, USA, 2013. [Google Scholar]
- U.S. Department of Transportation. Fuel Cells for Transportation Applications; Report No. FTA-TRI-IL-26-7006-2009.1; U.S. Department of Transportation: Washington, DC, USA, 2009.
- Wargo, E.A.; Dennison, C.R.; Kumbur, E.C. Durability of Polymer Electrolyte Fuel Cells: Status and Targets; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780123869364. [Google Scholar]
- Büchi, F.N.; Inaba, M.; Schmidt, T.J. Polymer Electrolyte Fuel Cell Durability; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780128114599. [Google Scholar]
- Fuel Cells and Hydrogen Joint Undertaking Programme Review Report; FCH-JU Programme Office: Brussels, Belgium, 2018; ISBN 9789292461362.
- Spernjak, D.; Fairweather, J.; Mukundan, R.; Rockward, T.; Borup, R.L. Influence of the microporous layer on carbon corrosion in the catalyst layer of a polymer electrolyte membrane fuel cell. J. Power Sources 2012, 214, 386–398. [Google Scholar] [CrossRef]
- Messing, M.; Kjeang, E. Empirical modeling of cathode electrode durability in polymer electrolyte fuel cells. J. Power Sources 2020, 451, 227750. [Google Scholar] [CrossRef]
- Kocha, S.S. Electrochemical Degradation: Electrocatalyst and Support Durability; Elsevier Inc.: Amsterdam, The Netherlands, 2011; ISBN 9780123869364. [Google Scholar]
- Jouin, M.; Gouriveau, R.; Hissel, D.; Péra, M.C.; Zerhouni, N. Degradations analysis and aging modeling for health assessment and prognostics of PEMFC. Reliab. Eng. Syst. Saf. 2016, 148, 78–95. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.Z.; Hin, J.N.C.; Wang, H.; Friedrich, K.A.; Schulze, M. A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells. J. Power Sources 2009, 194, 588–600. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Inbody, M.A. PEM fuel cell electrocatalyst durability measurements. J. Power Sources 2006, 163, 76–81. [Google Scholar] [CrossRef]
- Dhanushkodi, S.R.; Tam, M.; Kundu, S.; Fowler, M.W.; Pritzker, M.D. Carbon corrosion fingerprint development and de-convolution of performance loss according to degradation mechanism in PEM fuel cells. J. Power Sources 2013, 240, 114–121. [Google Scholar] [CrossRef]
- Sasaki, K.; Shao, M.; Adzic, R. Dissolution and stabilization of platinum in oxygen cathodes. Polym. Electrolyte Fuel Cell Durab. 2009, 7–27. [Google Scholar] [CrossRef]
- Wang, X.; Kumar, R.; Myers, D.J. Effect of voltage on platinum dissolution relevance to polymer electrolyte fuel cells. Electrochem. Solid-State Lett. 2006, 9, 225–227. [Google Scholar] [CrossRef]
- Ota, K.; Koizumi, Y. Platinum dissolution models and voltage cycling effects: Platinum dissolution in polymer electrolyte fuel cell (PEFC) and low-temperature fuel cells. Handb. Fuel Cells 2010, 1–7. [Google Scholar] [CrossRef]
- Harzer, G.S.; Schwämmlein, J.N.; Damjanović, A.M.; Ghosh, S.; Gasteiger, H.A. Cathode Loading Impact on Voltage Cycling Induced PEMFC Degradation: A Voltage Loss Analysis. J. Electrochem. Soc. 2018, 165, F3118–F3131. [Google Scholar] [CrossRef]
- Albarbar, A.; Alrweq, M. Proton Exchange Membrane Fuel Cells: Design, Modelling and Performance Assessment Techniques; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319707273. [Google Scholar]
- Andrew, L.; Dicks, D.A.J.R. Fuel Cell Systems Explained, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; ISBN 9788578110796. [Google Scholar]
- Chandesris, M.; Vincent, R.; Guetaz, L.; Roch, J.S.; Thoby, D.; Quinaud, M. Membrane degradation in PEM fuel cells: From experimental results to semi-empirical degradation laws. Int. J. Hydrogen Energy 2017, 42, 8139–8149. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Leung, J.; Lauritzen, M.V.; Kjeang, E. Isolated chemical degradation induced decay of mechanical membrane properties in fuel cells. Electrochim. Acta 2020, 352, 136489. [Google Scholar] [CrossRef]
- Danilczuk, M.; Lancucki, L.; Schlick, S.; Hamrock, S.J.; Haugen, G.M. In-depth profiling of degradation processes in a fuel cell: 2D spectral-spatial FTIR spectra of nafion membranes. ACS Macro Lett. 2012, 1, 280–285. [Google Scholar] [CrossRef]
- De Moor, G.; Bas, C.; Charvin, N.; Dillet, J.; Maranzana, G.; Lottin, O.; Caqué, N.; Rossinot, E.; Flandin, L. Perfluorosulfonic acid membrane degradation in the hydrogen inlet region: A macroscopic approach. Int. J. Hydrogen Energy 2016, 41, 483–496. [Google Scholar] [CrossRef]
- Liu, W.; Crum, M. Effective Testing Matrix for Studying Membrane Durability in PEM Fuel Cells: Part I. Chemical Durability. ECS Trans. 2006, 3, 531–540. [Google Scholar] [CrossRef]
- LaConti, A.B.; Hamdan, M.; McDonald, R.C. Mechanisms of membrane degradation. Handb. Fuel Cells 2010, 584, 1700–1712. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Liang, P.; Yi, P.; Lai, X. Mechanical degradation of proton exchange membrane along the MEA frame in proton exchange membrane fuel cells. Energy 2018, 165, 210–222. [Google Scholar] [CrossRef]
- Subianto, S.; Pica, M.; Casciola, M.; Cojocaru, P.; Merlo, L.; Hards, G.; Jones, D.J. Physical and chemical modification routes leading to improved mechanical properties of perfluorosulfonic acid membranes for PEM fuel cells. J. Power Sources 2013, 233, 216–230. [Google Scholar] [CrossRef]
- Schiraldi, D.A. Perfluorinated polymer electrolyte membrane durability. Polym. Rev. 2006, 46, 315–327. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.S.; Pinto, A.M.F.R. Simulation of membrane chemical degradation in a proton exchange membrane fuel cell by computational fluid dynamics. Int. J. Hydrogen Energy 2020, 6, 1106–1120. [Google Scholar] [CrossRef]
- Kundu, S.; Fowler, M.W.; Simon, L.C.; Abouatallah, R.; Beydokhti, N. Degradation analysis and modeling of reinforced catalyst coated membranes operated under OCV conditions. J. Power Sources 2008, 183, 619–628. [Google Scholar] [CrossRef]
- Oshiba, Y.; Kosaka, M.; Yamaguchi, T. Chemical durability of thin pore-filling membrane in open-circuit voltage hold test. Int. J. Hydrogen Energy 2019, 44, 28996–29001. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Zhang, S.; Ban, S.; Huang, C.; Wang, H.; Singara, V.; Fowler, M.; Schulze, M.; Haug, A.; Andreas Friedrich, K.; et al. Degradation of a PEM fuel cell stack with Nafion® membranes of different thicknesses. Part II: Ex situ diagnosis. J. Power Sources 2012, 205, 324–334. [Google Scholar] [CrossRef]
- Lim, C.; Ghassemzadeh, L.; Van Hove, F.; Lauritzen, M.; Kolodziej, J.; Wang, G.G.; Holdcroft, S.; Kjeang, E. Membrane degradation during combined chemical and mechanical accelerated stress testing of polymer electrolyte fuel cells. J. Power Sources 2014, 257, 102–110. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Wang, S.; Hao, S.; Li, J.; Mao, Z.; Mao, Z.; Ouyang, M.; Liu, Z. Degradation study of Membrane Electrode Assembly with PTFE/Nafion composite membrane utilizing accelerated stress technique. Int. J. Hydrogen Energy 2016, 41, 16212–16219. [Google Scholar] [CrossRef]
- Sun, X.; Shi, S.; Fu, Y.; Chen, J.; Lin, Q.; Hu, J.; Li, C.; Li, J.; Chen, X. Embrittlement induced fracture behavior and mechanisms of perfluorosulfonic-acid membranes after chemical degradation. J. Power Sources 2020, 453, 227893. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, M.; Shi, W.; Liu, W.; Liu, J.; Xing, D.; Yao, Y.; Hou, Z.; Ming, P.; Gu, J.; et al. The degradation study of Nafion/PTFE composite membrane in PEM fuel cell under accelerated stress tests. Int. J. Hydrogen Energy 2014, 39, 14381–14390. [Google Scholar] [CrossRef]
- Quiroga, M.A.; Malek, K.; Franco, A.A. A Multiparadigm Modeling Investigation of Membrane Chemical Degradation in PEM Fuel Cells. J. Electrochem. Soc. 2016, 163, F59–F70. [Google Scholar] [CrossRef]
- Ozden, A.; Shahgaldi, S.; Li, X.; Hamdullahpur, F. A review of gas diffusion layers for proton exchange membrane fuel cells—With a focus on characteristics, characterization techniques, materials and designs. Prog. Energy Combust. Sci. 2019, 74, 50–102. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- El-kharouf, A.; Pollet, B.G. Gas Diffusion Media and Their Degradation; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9780123869364. [Google Scholar]
- Lapicque, F.; Belhadj, M.; Bonnet, C.; Pauchet, J.; Thomas, Y. A critical review on gas diffusion micro and macroporous layers degradations for improved membrane fuel cell durability. J. Power Sources 2016, 336, 40–53. [Google Scholar] [CrossRef]
- Taherian, R. Applications of Polymer Based Composites: Gas Diffusion Layer of Pem Fuel Cells; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128125410. [Google Scholar]
- Yu, S.; Li, X.; Li, J.; Liu, S.; Lu, W.; Shao, Z.; Yi, B. Study on hydrophobicity degradation of gas diffusion layer in proton exchange membrane fuel cells. Energy Convers. Manag. 2013, 76, 301–306. [Google Scholar] [CrossRef]
- Toghyani, S.; Moradi Nafchi, F.; Afshari, E.; Hasanpour, K.; Baniasadi, E.; Atyabi, S.A. Thermal and electrochemical performance analysis of a proton exchange membrane fuel cell under assembly pressure on gas diffusion layer. Int. J. Hydrogen Energy 2018, 43, 4534–4545. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Bipolar Plate Durability and Challenges; Elsevier Inc: Amsterdam, The Netherlands, 2012; ISBN 9780123869364. [Google Scholar]
- Yuan, X.Z.; Wang, H.; Zhang, J.; Wilkinson, D.P. Bipolar plates for PEM fuel cells—From materials to processing. J. New Mater. Electrochem. Syst. 2005, 8, 257–267. [Google Scholar]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Shimpalee, S.; Lilavivat, V.; McCrabb, H.; Khunatorn, Y.; Lee, H.K.; Lee, W.K.; Weidner, J.W. Investigation of bipolar plate materials for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 13688–13696. [Google Scholar] [CrossRef]
- Cunningham, B.D.; Huang, J.; Baird, D.G. Review of materials and processing methods used in the production of bipolar plates for fuel cells. Int. Mater. Rev. 2007, 52, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of bipolar plates in PEM fuel cells: Flow-field designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Hartnig, C.; Schmidt, T.J. On a new degradation mode for high-temperature polymer electrolyte fuel cells: How bipolar plate degradation affects cell performance. Electrochim. Acta 2011, 56, 4237–4242. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A review of metallic bipolar plates for proton exchange membrane fuel cells: Materials and fabrication methods. Adv. Mater. Sci. Eng. 2012, 2012, 828070. [Google Scholar] [CrossRef]
- Kakati, B.K.; Ghosh, A.; Verma, A. Efficient composite bipolar plate reinforced with carbon fiber and graphene for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 9362–9369. [Google Scholar] [CrossRef]
- Adloo, A.; Sadeghi, M.; Masoomi, M.; Pazhooh, H.N. High performance polymeric bipolar plate based on polypropylene/graphite/graphene/nano-carbon black composites for PEM fuel cells. Renew. Energy 2016, 99, 867–874. [Google Scholar] [CrossRef]
- Yu, H.N.; Lim, J.W.; Kim, M.K.; Lee, D.G. Plasma treatment of the carbon fiber bipolar plate for PEM fuel cell. Compos. Struct. 2012, 94, 1911–1918. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Jo, A.; Lee, K.; Ju, H. Development of ultralight and thin bipolar plates using epoxy-carbon fiber prepregs and graphite composites. Int. J. Hydrogen Energy 2017, 42, 1691–1697. [Google Scholar] [CrossRef]
- Kakati, B.K.; Sathiyamoorthy, D.; Verma, A. Electrochemical and mechanical behavior of carbon composite bipolar plate for fuel cell. Int. J. Hydrogen Energy 2010, 35, 4185–4194. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Bi, F.; Hou, K.; Yi, P.; Peng, L.; Lai, X. Mechanisms of growth, properties and degradation of amorphous carbon films by closed field unbalanced magnetron sputtering on stainless steel bipolar plates for PEMFCs. Appl. Surf. Sci. 2017, 422, 921–931. [Google Scholar] [CrossRef]
- Pilinski, N.; Käding, C.; Dushina, A.; Hickmann, T.; Dyck, A.; Wagner, P. Investigation of corrosion methods for bipolar plates for high temperature polymer electrolyte membrane fuel cell application. Energies 2020, 13, 235. [Google Scholar] [CrossRef]
- Yan, W.M.; Chen, C.Y.; Liang, C.H. Comparison of performance degradation of high temperature PEM fuel cells with different bipolar plates. Energy 2019, 186, 115836. [Google Scholar] [CrossRef]
- Penga, Ž.; Radica, G.; Barbir, F.; Eckert, P. Giantleap Deliverable D1.3: Degradation Mechanisms in Automotive Fuel Cell Systems; FCH-JU Programme Office: Brussels, Belgium, 2017; p. 46. [Google Scholar]
- Gatto, I.; Urbani, F.; Giacoppo, G.; Barbera, O.; Passalacqua, E. Influence of the bolt torque on PEFC performance with different gasket materials. Int. J. Hydrogen Energy 2011, 36, 13043–13050. [Google Scholar] [CrossRef]
- Qiu, D.; Liang, P.; Peng, L.; Yi, P.; Lai, X.; Ni, J. Material behavior of rubber sealing for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 5465–5473. [Google Scholar] [CrossRef]
- Ye, D.H.; Zhan, Z.G. A review on the sealing structures of membrane electrode assembly of proton exchange membrane fuel cells. J. Power Sources 2013, 231, 285–292. [Google Scholar] [CrossRef]
- Bieringer, R.; Adler, M.; Geiss, S.; Viol, M. Gaskets: Important durability issues. In Polymer Electrolyte Fuel Cell Durability; Springer: New York, NY, USA, 2009; pp. 271–281. [Google Scholar] [CrossRef]
- Jake, S.; Jiang, K. Elastomer Application in Microsystem and Microfluidics. Adv. Elastomers Technol. Prop. Appl. 2012. [Google Scholar] [CrossRef][Green Version]
- Tan, J.; Chao, Y.J.; Van Zee, J.W.; Lee, W.K. Degradation of elastomeric gasket materials in PEM fuel cells. Mater. Sci. Eng. A 2007, 445–446, 669–675. [Google Scholar] [CrossRef]
- Li, G.; Tan, J.; Gong, J. Degradation of the elastomeric gasket material in a simulated and four accelerated proton exchange membrane fuel cell environments. J. Power Sources 2012, 205, 244–251. [Google Scholar] [CrossRef]
- Husar, A.; Serra, M.; Kunusch, C. Description of gasket failure in a 7 cell PEMFC stack. J. Power Sources 2007, 169, 85–91. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.J.; Yang, M.; Lee, W.K.; Van Zee, J.W. Chemical and mechanical stability of a Silicone gasket material exposed to PEM fuel cell environment. Int. J. Hydrogen Energy 2011, 36, 1846–1852. [Google Scholar] [CrossRef]
- Li, G.; Tan, J.; Gong, J. Chemical aging of the silicone rubber in a simulated and three accelerated proton exchange membrane fuel cell environments. J. Power Sources 2012, 217, 175–183. [Google Scholar] [CrossRef]
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Sealing force prediction of elastomeric seal material for PEM fuel cell under temperature cycling. Int. J. Hydrogen Energy 2014, 39, 1430–1438. [Google Scholar] [CrossRef]
- Yang, D.; Ma, J.; Zhang, Q.; Li, B.; Ming, P.; Zhang, C. Accelerated Test of Silicone Rubbers Exposing to PEMFC environment. Prog. Nat. Sci. Mater. Int. 2020, 30, 882–889. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A.; De Marco, G.; Malkow, T. EU Harmonised Test Protocols for PEMFC MEA Testing in Single Cell Configuration for Automotive Applications; JRC Science for Policy Report; Join Research Centre: Petten, The Netherlands, 2015; ISBN 978-92-79-54132-2. [Google Scholar]
- Zhao, Y.; Mao, Y.; Zhang, W.; Tang, Y.; Wang, P. Reviews on the effects of contaminations and research methodologies for PEMFC. Int. J. Hydrogen Energy 2020, 45, 23174–23200. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739–756. [Google Scholar] [CrossRef]
- Jing, F.; Hou, M.; Shi, W.; Fu, J.; Yu, H.; Ming, P.; Yi, B. The effect of ambient contamination on PEMFC performance. J. Power Sources 2007, 166, 172–176. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Qian, W.; Zhang, S.; Wessel, S.; Cheng, T.T.H.; Shen, J.; Wu, S. Chloride contamination effects on proton exchange membrane fuel cell performance and durability. J. Power Sources 2011, 196, 6249–6255. [Google Scholar] [CrossRef]
- Amamou, A.A.; Kelouwani, S.; Boulon, L.; Agbossou, K. A Comprehensive Review of Solutions and Strategies for Cold Start of Automotive Proton Exchange Membrane Fuel Cells. IEEE Access 2016, 4, 4989–5002. [Google Scholar] [CrossRef]
- Electron Microscope Analysis of Low Temperature Damage in PEM Fuel Cells. Available online: http://www2.optics.rochester.edu/workgroups/cml/opt307/spr05/eric/ (accessed on 1 February 2021).
- Yan, Q.; Toghiani, H.; Lee, Y.W.; Liang, K.; Causey, H. Effect of sub-freezing temperatures on a PEM fuel cell performance, startup and fuel cell components. J. Power Sources 2006, 160, 1242–1250. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Pei, P.; Chen, H. Main factors affecting the lifetime of Proton Exchange Membrane fuel cells in vehicle applications: A review. Appl. Energy 2014, 125, 60–75. [Google Scholar] [CrossRef]
- Lin, R.; Cui, X.; Shan, J.; Técher, L.; Xiong, F.; Zhang, Q. Investigating the effect of start-up and shut-down cycles on the performance of the proton exchange membrane fuel cell by segmented cell technology. Int. J. Hydrogen Energy 2015, 40, 14952–14962. [Google Scholar] [CrossRef]
- Sorrentino, A.; Sundmacher, K.; Vidakovic-Koch, T. Polymer Electrolyte Fuel Cell Degradation Mechanisms and Their Diagnosis by Frequency Response Analysis Methods: A Review. Energies 2020, 13, 5825. [Google Scholar] [CrossRef]
- Chang, Y.; Qin, Y.; Yin, Y.; Zhang, J.; Li, X. Humidification strategy for polymer electrolyte membrane fuel cells—A review. Appl. Energy 2018, 230, 643–662. [Google Scholar] [CrossRef]
- Sanchez, D.G.; Ruiu, T.; Biswas, I.; Schulze, M.; Helmly, S.; Friedrich, K.A. Local impact of humidification on degradation in polymer electrolyte fuel cells. J. Power Sources 2017, 352, 42–55. [Google Scholar] [CrossRef]
- Vengatesan, S.; Kim, H.J.; Lee, S.Y.; Cho, E.A.; Ha, H.Y.; Oh, I.H.; Lim, T.H. Operation of a proton exchange membrane fuel cell under non-humidified conditions using a membrane-electrode assemblies with composite membrane and electrode. J. Power Sources 2007, 167, 325–329. [Google Scholar] [CrossRef]
- Le Canut, J.-M.; Abouatallah, R.M.; Harrington, D.A. Detection of Membrane Drying, Fuel Cell Flooding, and Anode Catalyst Poisoning on PEMFC Stacks by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2006, 153, A857. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Hu, M.; Cheng, X.; He, K.; Mao, L. A Comparative Study of Using Polarization Curve Models in Proton Exchange Membrane Fuel Cell Degradation Analysis. Energies 2020, 13, 3759. [Google Scholar] [CrossRef]
- Authayanun, S.; Pothong, W.; Ngamsai, K.; Patniboon, A.; Arpornwichanop, A. Effect of water transport on the electrical performance of PEM fuel cell. Energy Procedia 2014, 61, 1553–1556. [Google Scholar] [CrossRef]
- Knights, S.D.; Colbow, K.M.; St-Pierre, J.; Wilkinson, D.P. Aging mechanisms and lifetime of PEFC and DMFC. J. Power Sources 2004, 127, 127–134. [Google Scholar] [CrossRef]
- Ji, M.; Wei, Z. A review of water management in polymer electrolyte membrane fuel cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef]
- Bhattacharya, P.K. Water flooding in the proton exchange membrane fuel cell. Directions 2015, 15, 24–33. [Google Scholar]
- Nguyen, T.V.; White, R.E. A Water and Heat Management Model for Proton-Exchange-Membrane Fuel Cells. J. Electrochem. Soc. 1993, 140, 2178–2186. [Google Scholar] [CrossRef]
- Thangavelautham, J. Degradation in PEM Fuel Cells and Mitigation Strategies Using System Design and Control. Prot. Exch. Membr. Fuel Cell 2018. [Google Scholar] [CrossRef]
- He, W.; Lin, G.; Van Nguyen, T. Diagnostic Tool to Detect Electrode Flooding in Proton-Exchange Membrane Fuel Cells. AIChE J. 2003, 49, 3221–3228. [Google Scholar] [CrossRef]
- Wang, X.R.; Ma, Y.; Gao, J.; Li, T.; Jiang, G.Z.; Sun, Z.Y. Review on water management methods for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Hussaini, I.S.; Wang, C.Y. Visualization and quantification of cathode channel flooding in PEM fuel cells. J. Power Sources 2009, 187, 444–451. [Google Scholar] [CrossRef]
- Kim, M.; Jung, N.; Eom, K.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Kim, H.J.; Hong, B.K.; Cho, E. Effects of anode flooding on the performance degradation of polymer electrolyte membrane fuel cells. J. Power Sources 2014, 266, 332–340. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Ye, F.; Ma, C.F. Water flooding and pressure drop characteristics in flow channels of proton exchange membrane fuel cells. Electrochim. Acta 2007, 52, 3607–3614. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, S.; Wei, P.; Pei, P.; Yao, S.; Sun, H. Lowering the Pressure Drop and Cost of a Proton Exchange Membrane Fuel Cell by Flow Field Plate Design. Energy Fuels 2020, 34, 8857–8863. [Google Scholar] [CrossRef]
- Mishler, J.; Wang, Y.; Lujan, R.; Mukundan, R.; Borup, R.L. An Experimental Study of Polymer Electrolyte Fuel Cell Operation at Sub-Freezing Temperatures. J. Electrochem. Soc. 2013, 160, F514–F521. [Google Scholar] [CrossRef]
- Macauley, N.; Lujan, R.W.; Spernjak, D.; Hussey, D.S.; Jacobson, D.L.; More, K.; Borup, R.L.; Mukundan, R. Durability of Polymer Electrolyte Membrane Fuel Cells Operated at Subfreezing Temperatures. J. Electrochem. Soc. 2016, 163, F1317–F1329. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.; Hu, Y.; Chen, H.; Ma, Y. Challenges and developments of automotive fuel cell hybrid power system and control. Sci. China Inf. Sci. 2019, 62, 1–25. [Google Scholar] [CrossRef]
- Barique, M.A.; Tsuchida, E.; Ohira, A.; Tashiro, K. Effect of Elevated Temperatures on the States of Water and Their Correlation with the Proton Conductivity of Nafion. ACS Omega 2017, 3, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Bargal, M.H.S.; Abdelkareem, M.A.A.; Tao, Q.; Li, J.; Shi, J.; Wang, Y. Liquid cooling techniques in proton exchange membrane fuel cell stacks: A detailed survey. Alexandria Eng. J. 2020, 59, 635–655. [Google Scholar] [CrossRef]
- Jafri, N.H.; Gupta, S. An overview of Fuel Cells application in transportation. In Proceedings of the 2016 IEEE Transporation Electrification Conference and Expo, Asia-Pacific (ITEC), Busan, Korea, 1–4 June 2016; pp. 129–133. [Google Scholar] [CrossRef]
- Bloom, I.; Walker, L.K.; Basco, J.K.; Malkow, T.; Saturnio, A.; De Marco, G.; Tsotridis, G. A comparison of Fuel Cell Testing protocols—A case study: Protocols used by the U.S. Department of Energy, European Union, International Electrotechnical Commission/Fuel Cell Testing and Standardization Network, and Fuel Cell Technical Team. J. Power Sources 2013, 243, 451–457. [Google Scholar] [CrossRef]
- Fabrice MICOUD, S.R. (CEA) STACKTEST: WP5: International Standard Status Report; Fuel Cells and Hydrogen Joint Undertaking (FCH JU): Zentrum für Sonnenenergie- und Wasserstoff-Forschung Baden-Württemberg (ZSW): Ulm, Germany, 2013. [Google Scholar]
- Han, J.; Han, J.; Yu, S. Experimental analysis of performance degradation of 3-cell PEMFC stack under dynamic load cycle. Int. J. Hydrogen Energy 2020, 45, 13045–13054. [Google Scholar] [CrossRef]
- The Challenge for AUTOMAKERS—A More Stringent Test Procedure for EVs and HEVs. Available online: https://www.silicon-mobility.com/the-challenge-for-automakers-a-more-stringent-test-procedure-for-evs-and-hevs/ (accessed on 1 February 2021).
- WLTP: How It Will Change Road Tax and VRT in Ireland. Available online: https://www.irishevowners.ie/useful-info/wltp-how-it-will-change-road-tax-and-vrt-in-ireland/ (accessed on 1 February 2021).
- WLTP Explained: What Is WLTP and How Does It Work? Available online: https://www.nationwidevehiclecontracts.co.uk/guides/ask-nvc/wltp-explained (accessed on 1 February 2021).
- WLTP—Worldwide Harmonized Light Vehicles Test Procedure. Available online: https://www.vda.de/en/topics/environment-and-climate/exhaust-emissions/wltp-worldwide-harmonized-light-vehicles-test-procedure.html (accessed on 1 February 2021).
- International: Light-Duty: Worldwide Harmonized Light Vehicles Test Procedure (WLTP). Available online: https://www.transportpolicy.net/standard/international-light-duty-worldwide-harmonized-light-vehicles-test-procedure-wltp/ (accessed on 1 February 2021).
- ICCT. Policy Update World-Harmonized Light-Duty Vehicles Test Procedure; International Council on Clean Transportation: Washington, DC, USA, 2013. [Google Scholar]
- Worldwide Emission Standards and Related Regulations—Passenger Cars/Light and Medium Duty Vehicles, Powertrain; CPT Group GmbH: Regensburg, Germany, 2019.
- Tsokolis, D.; Tsiakmakis, S.; Dimaratos, A.; Fontaras, G.; Pistikopoulos, P.; Ciuffo, B.; Samaras, Z. Fuel consumption and CO2 emissions of passenger cars over the New Worldwide Harmonized Test Protocol. Appl. Energy 2016, 179, 1152–1165. [Google Scholar] [CrossRef]
- WLTP and NEDC. Part II: Comparing the Measurements. Available online: https://www.daimler.com/sustainability/climate/wltp/wltp-part-2.html (accessed on 1 February 2021).
- Cycle WLTP: Ce qui Change Pour Les Voitures Électriques et Thermiques. Available online: https://www.automobile-propre.com/cycle-wltp-ce-qui-change-pour-les-voitures-electriques-et-thermiques/ (accessed on 1 February 2021).
- FCTESTNET Test Module TM PEFC SC 5-7, Version 30 04; JRC Scientific and Technical Publications Office of the European Union: Luxembourg, 2010.
- Bloom, I.; Walker, L.; Basco, J.; Malkow, T.; De Marco, G.; Tsotridis, G. A Comparison of Fuel Cell Test Protocols. ECS Trans. 2011, 30, 227–235. [Google Scholar] [CrossRef]
- Barnett, A.O. Giantleap: Protocols for Experiments and Validation Activities, Fuel Cells and Hydrogen Joint Undertaking; Fuel Cell and Hydrogen Joint Undertaking: Brussels, Belgium, 2017. [Google Scholar]
- Japanese JC08 Cycle. Available online: https://dieselnet.com/standards/cycles/jp_jc08.php (accessed on 1 February 2021).
- Japan JC08 Fuel Mode Interpretation: More Grounded Change. Available online: https://www.pcauto.com.cn/tech/384/3848379_1.html (accessed on 1 February 2021).
- Noto, H.; Kondo, M.; Otake, Y.; Kato, M. Development of fuel cell hybrid vehicle by Toyota-durability. SAE Tech. Pap. 2009. [Google Scholar] [CrossRef]
- Steen, A.V.D. Overview of Hydrogen and Fuel Cell Developments in China; MDPI: Basel, Switzerland, 2019. [Google Scholar]
- FCTESTNET Test Module PEFC SC 5-6, Version 30 04; JRC Scientific and Technical Reports; Publications Office of the European Union: Luxembourg, 2010.
- Hunger, J.; Mitzel, J.; Berg-Nygaard, F.; Jungmann, T.; Harms, C.; Rosini, S.; Jörissen, L. STACK-TEST: FCH-GA 303445 Final Report I: Development of PEM Fuel Cell Stack Reference Test Procedures for Industry; Zentrum für Sonnenenergie- und Wasserstoff-Forschung Baden-Württemberg (ZSW): Ulm, Germany, 2015. [Google Scholar]
- Xu, L.; Reimer, U.; Li, J.; Huang, H.; Hu, Z.; Jiang, H.; Janßen, H.; Ouyang, M.; Lehnert, W. Design of durability test protocol for vehicular fuel cell systems operated in power-follow mode based on statistical results of on-road data. J. Power Sources 2018, 377, 59–69. [Google Scholar] [CrossRef]
- Rosini, S.; Micoud, F.; Araya, S.S.; Veltze, S.; Nygaard, F.; Hunger, J. Test Module D-01: Constant Load Durability; Zentrum für Sonnenenergie- und Wasserstoff-Forschung Baden-Württemberg (ZSW): Ulm, Germany, 2015. [Google Scholar]
- Topal, L.; Harms, C.; Kabza, A.; Hunger, J. Test Module D-02: Load Cycling Durability; Zentrum für Sonnenenergie- und Wasserstoff-Forschung Baden-Württemberg (ZSW): Ulm, Germany, 2015. [Google Scholar]
- Harms, C.; Topal, L.; Veltzé, S.; Kabza, A.; Hunger, J.; Nygaard, F. Test Program D-01: Durability; Zentrum für Sonnenenergie- und Wasserstoff-Forschung Baden-Württemberg (ZSW): Ulm, Germany, 2015. [Google Scholar]

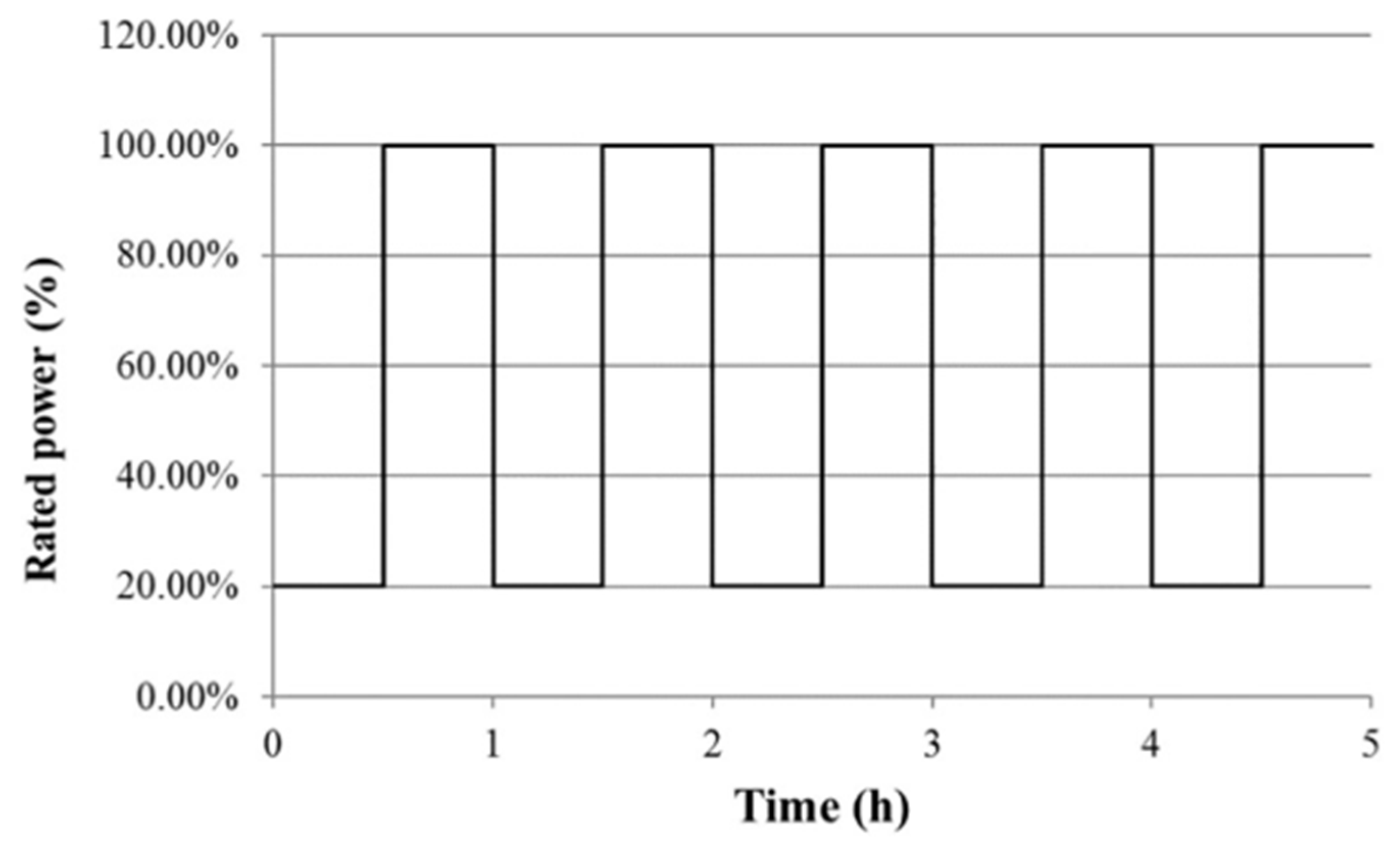
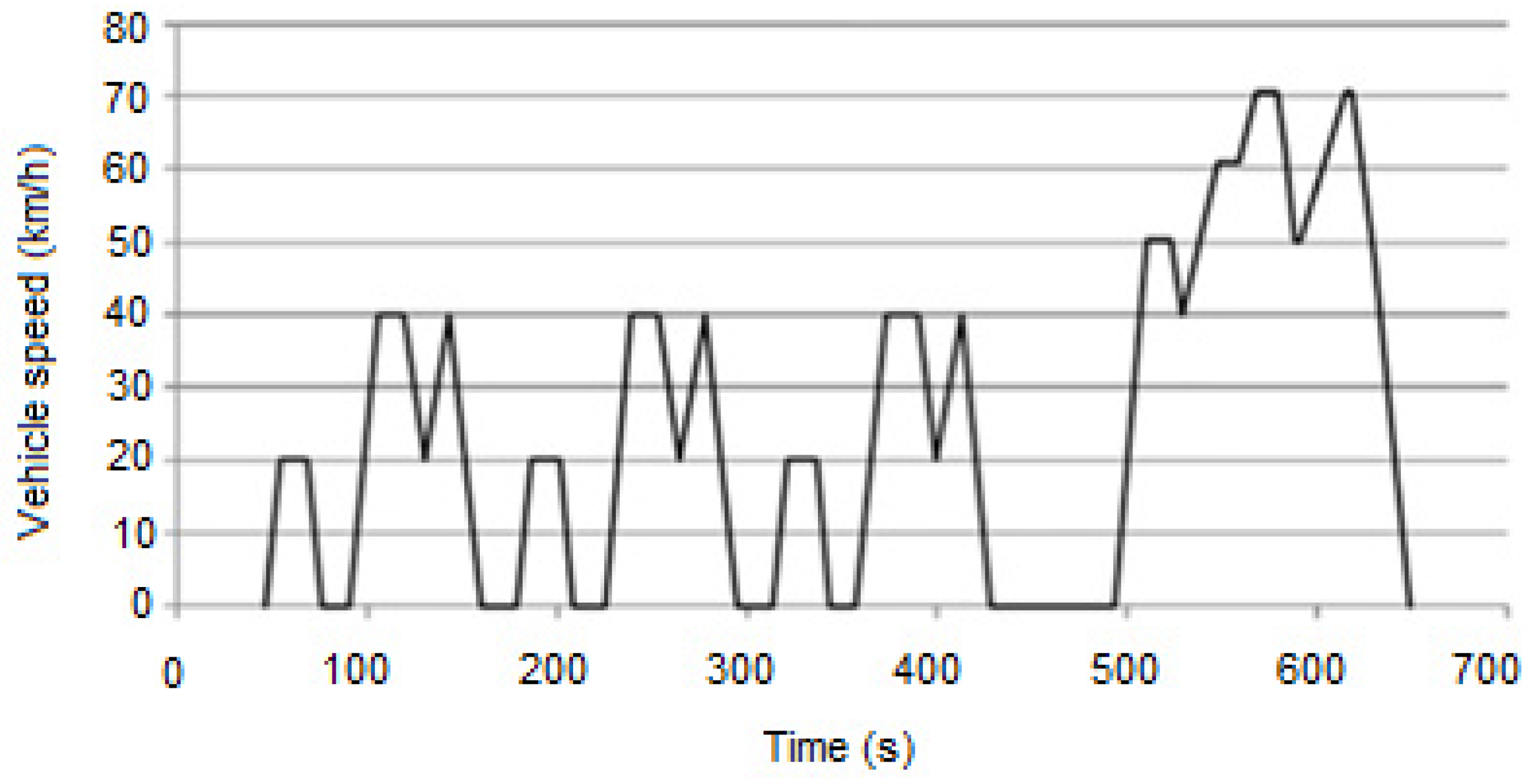
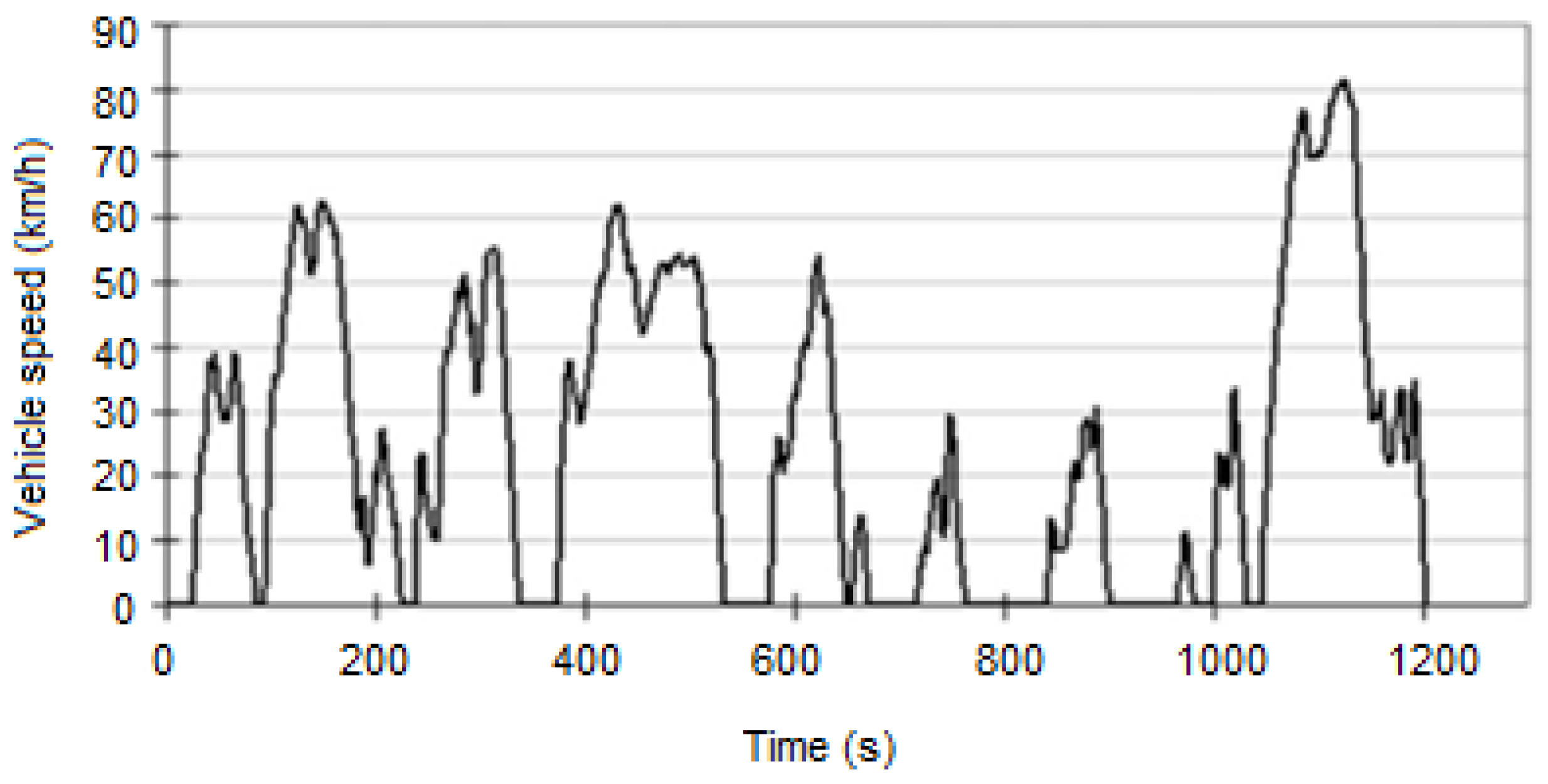
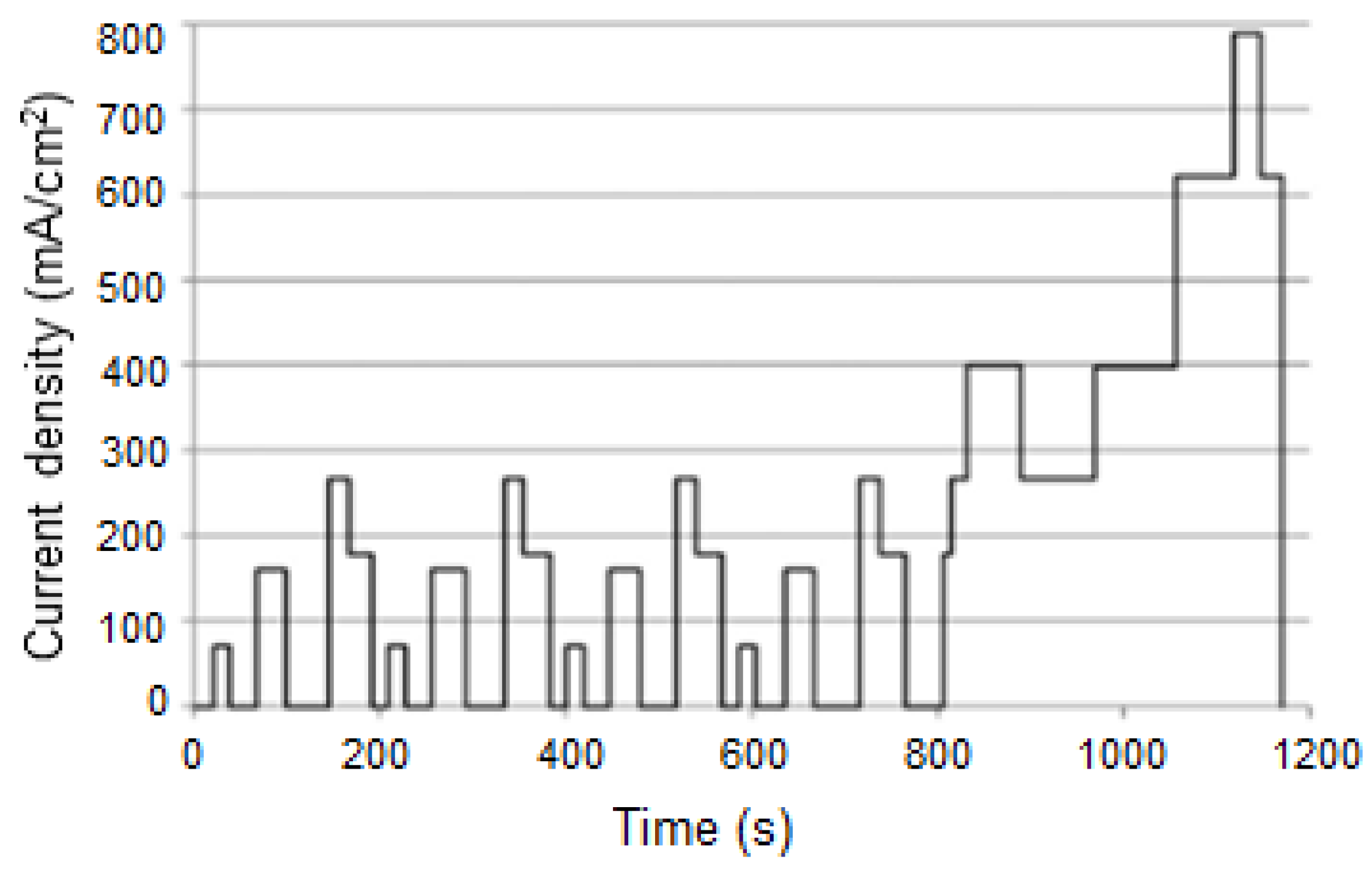
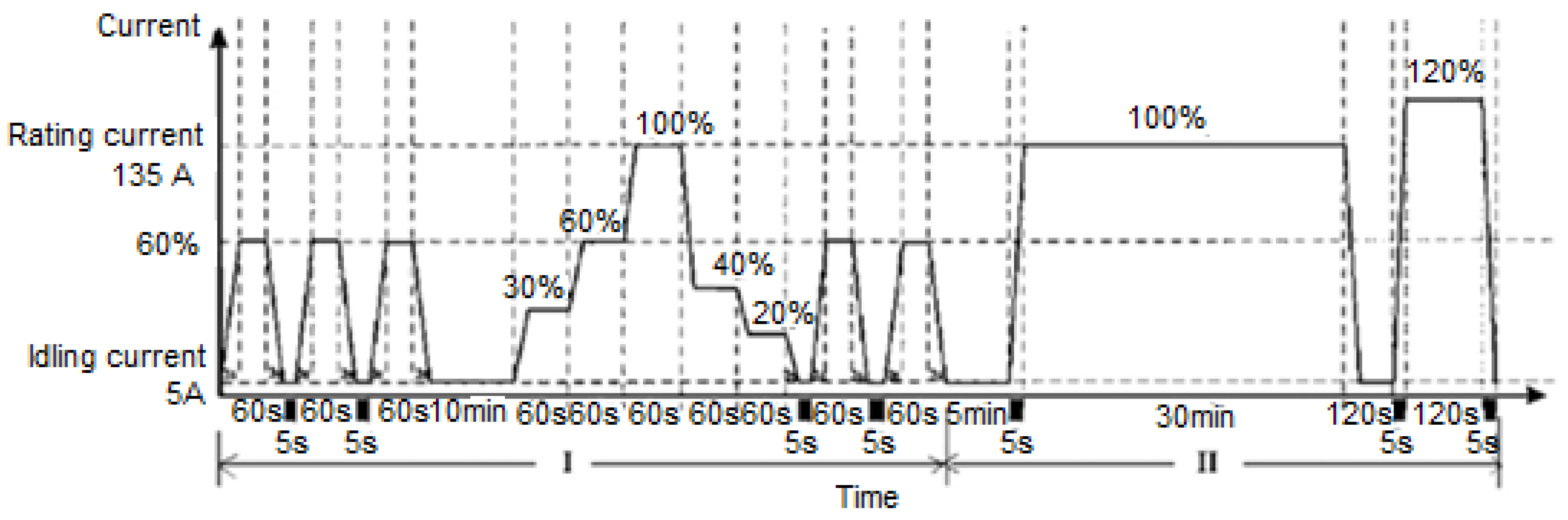
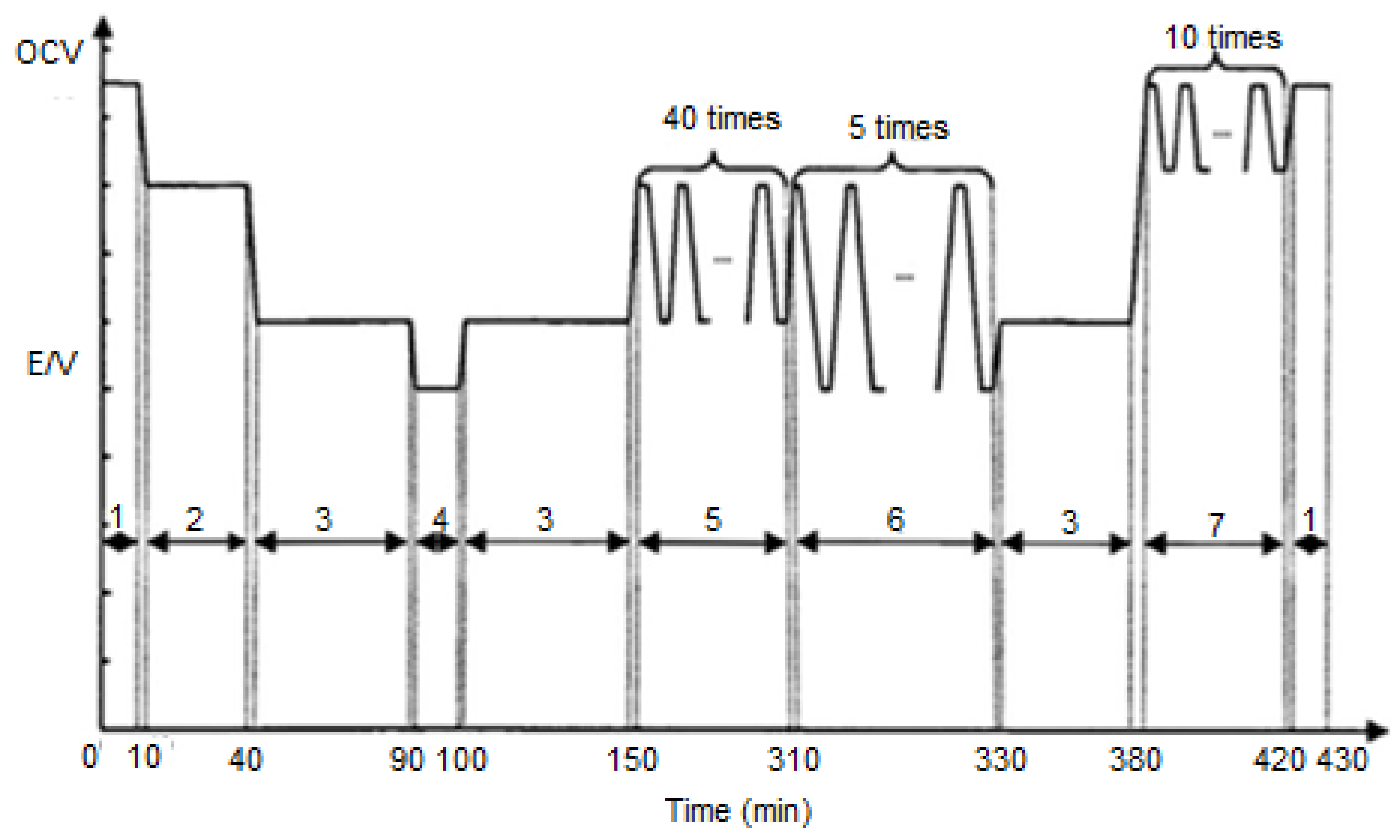

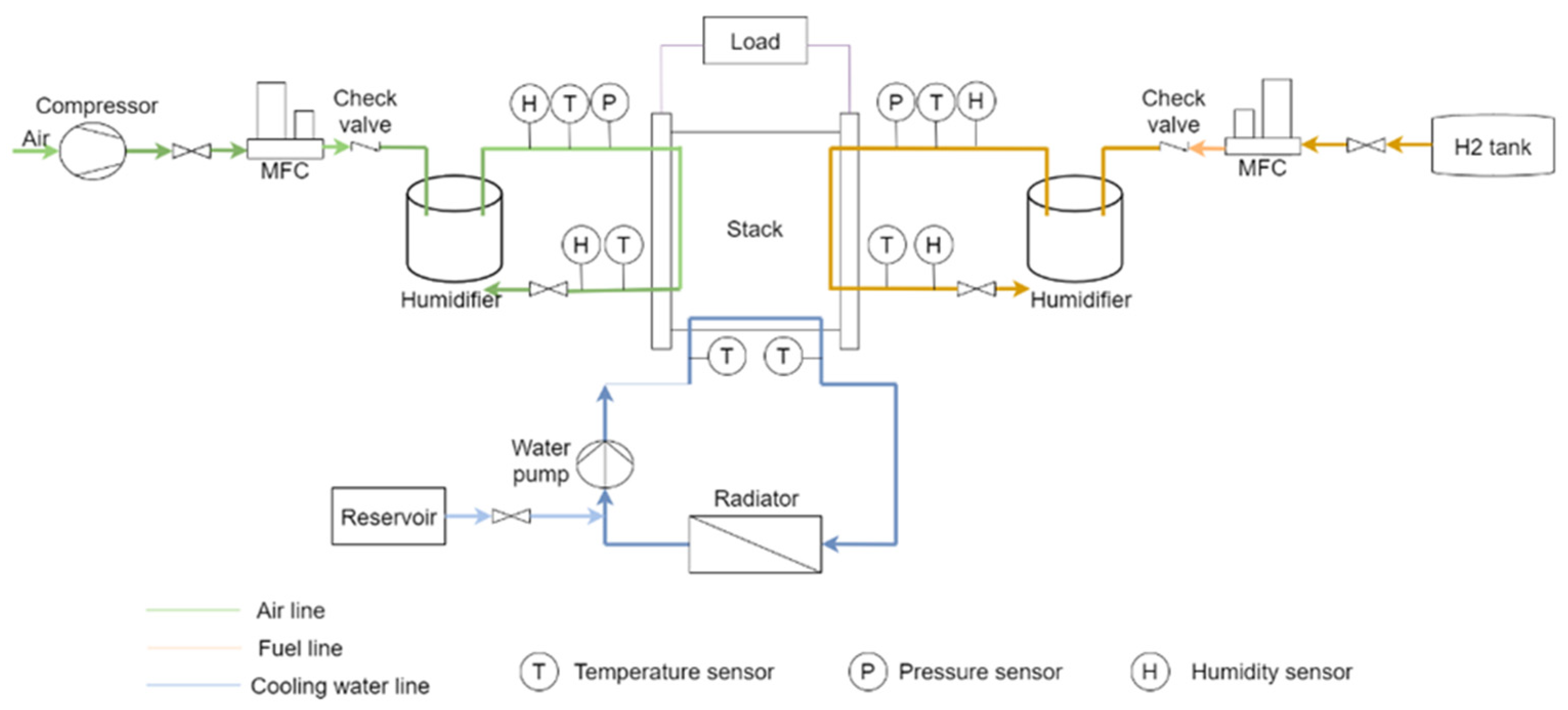

| Component | Failure Modes | Causes |
|---|---|---|
| Membrane | Mechanical degradation | Mechanical stress due to non-uniform pressing and swelling Penetration of the catalyst and seal material traces and foreign particles |
| Chemical degradation | Radical attack Contaminations | |
| Conductivity loss | Ionic contaminations | |
| Catalyst/catalyst layer CLs | Activation losses | Sintering or de-alloying of electrocatalyst |
| Conductivity loss | Corrosion of catalyst support | |
| Loss of reformate tolerance | Dissolution of alloying elements and contamination | |
| Decrease in mass transport rate of reactants | Mechanical stress | |
| Decrease in water management ability | Changing in hydrophobicity of materials | |
| GDL | Decrease in mass transport | Mechanical stress, compression Degradation of backing material |
| Decrease in water management ability | Mechanical stress Change in hydrophobicity of materials | |
| Thinning | Corrosion | |
| Bipolar plate | Conductivity loss | Corrosion, oxidation |
| Fracture/deformation | Mechanical stress | |
| Sealing gasket | Mechanical failure and brittleness | Deformation, compression, and chemical reaction |
| Sources | Potential Impurities | |
|---|---|---|
| Hydrogen fuel impurities | Crude oil | CO, NH3, H2S, HCN, hydrocarbons |
| Natural gas | CO, NH3, H2S, HCN, hydrocarbons | |
| Methanol | CO, odorants, alcohols | |
| Biomass | Cations, aldehydes, alcohols, formic acid, NH3, H2S, HCN | |
| Water electrolysis | Anions, cations | |
| Air impurities | Fuel combustion pollution | SOx, NOx, hydrocarbons, soot, and particulates |
| Ambient air, farming | NH3 | |
| Natural sources | Ocean salts, dust | |
| Others | Deicers | NaCl, CaCl2 |
| Fuel cell corrosion products | Cations, anions |
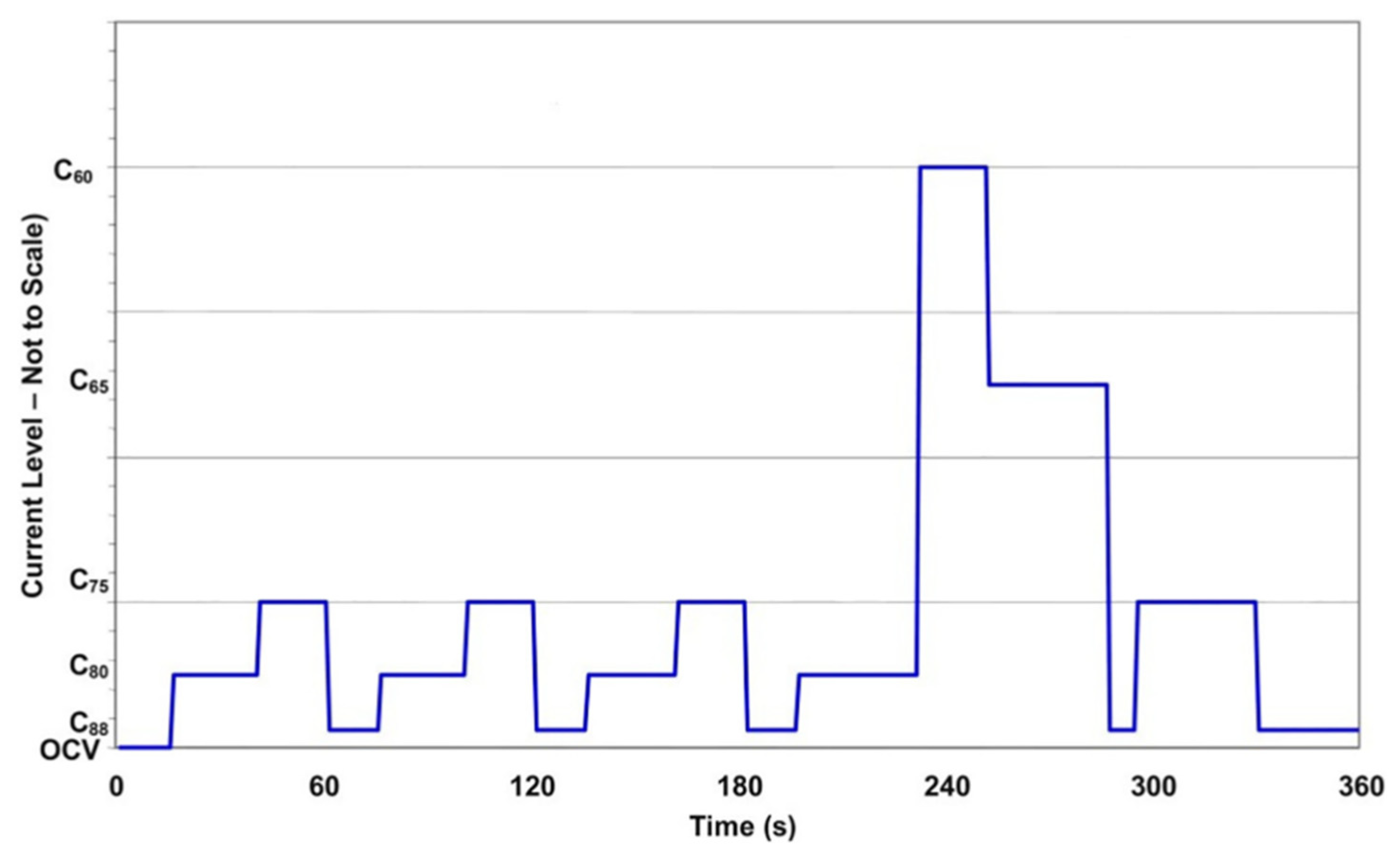
| Step | Duration (s) | Cxx | Step | Duration (s) | Cxx |
|---|---|---|---|---|---|
| 1 | 15 | OCV | 9 | 20 | C75 |
| 2 | 25 | C80 | 10 | 15 | C88 |
| 3 | 20 | C75 | 11 | 35 | C80 |
| 4 | 15 | C88 | 12 | 20 | C60 |
| 5 | 24 | C80 | 13 | 35 | C65 |
| 6 | 20 | C75 | 14 | 8 | C88 |
| 7 | 15 | C88 | 15 | 35 | C75 |
| 8 | 25 | C80 | 16 | 40 | C88 |
| Parameter | NEDC | WLTP (Class 3) | Difference | Note |
|---|---|---|---|---|
| Starting temperature | Cold | Cold | ||
| Test cycle | Single test cycle | Dynamic cycle more representative of real driving | ||
| Cycle time | 1180 s | 1800 s | +35% | |
| Idle time | 23.7% | 13% | −45% | Less effect of engine start/stop |
| Cruise time | 38% | 4% | −90% | Less stationary operation |
| Acceleration | 20% | 44% | +117% | More acceleration |
| Deceleration | 14% | 40% | +179% | More deceleration |
| Cycle distance | 11 km | 23.25 km | ||
| Driving phases | Two phases, 66% urban and 34% non-urban driving | Four dynamic phases with more emphasis on high performance, 52% urban and 48% non-urban driving | Two more | |
| Average speed | 34 km/h | 46.5 km/h | +53% | |
| Maximum speed | 120 km/h | 131 km/h | +4% |
| Parameters | Unit | Ref of NEDC | Giantleap Conditions at Low Current Density | Giantleap Conditions at High Current Density | |
|---|---|---|---|---|---|
| Nominal cell operating temperature | °C | 80 | 73 | 75 | |
| Anode | Fuel gas inlet temperature | °C | 85 | 73 | 75 |
| Fuel gas inlet humidity | % RH | 50% (DPT 64 °C @ 80 °C) | 45 | 45 | |
| Fuel gas inlet pressure (absolute) | kPa | 250 | 140 | 190 | |
| Fuel stoichiometry | 1.3 | 1.5 | 2.5 | ||
| Cathode | Oxidation gas inlet temperature | °C | 85 | 73 | 75 |
| Oxidation gas inlet humidity | % RH | 30 (DPT 53 °C @ 80 °C) | 75 | 75 | |
| Oxidation gas inlet pressure (absolute) | kPa | 230 | 130 | 180 | |
| Air stoichiometry | 1.5 | 1.7 | 1.7 | ||
| Minimum current density for stoichiometry operation | A/cm2 | 0.2 | 0.2 | 0.2 |
| Operating Condition | Tongji University | Tsinghua University | Wuhan University of Technology | Dalian Institute of Chemical Physics |
|---|---|---|---|---|
| OCV | 7.3% of T | 4.6% of T | ||
| Idling | 38.9% of T | 21.1% of T | ||
| Partial power | 80%P is 6.2% of T 70%P is 8.4% of T 90%P is 0.8% of T | 20%P is 3.5% of T 40%P is 3.3% of T 60%P is 3.3% of T 80%P is 3.3% of T | 40%P is 8.4% of T 60%P is 26.3% of T 80%P is 10.5% of T | |
| Full power | 22.5% of T | 28.2% of T | 34.9% of T | 31.6% of T |
| Overload | 5.1% of T | 1.8% of T | 2.3% of T | 2.1% of T |
| Dynamic load | OCV to 15%P, cycling four times, 8.7% of T OCV to 32%P, cycling four times, 17.4% of T OCV to 50%P to 32%P, cycling four times, 23.6% of T | Idling to full power, cycling 20 times, 17.7% of T | Idling to full power, cycling 40 times, 37.2% of T Idling to overload, cycling five times, 4.7% of T OCV to idling, cycling ten times, 9.3% of T |
| Protocol | Vehicle Types | ||
|---|---|---|---|
| Full Power Cars | Hybrid Cars | City Bus | |
| Dynamic Stress Test (DST) | ✓ | ✓ | |
| FCTT | ✓ | ✓ | ✓ |
| NEDC | ✓ | ✓ | |
| WLTP | ✓ | ✓ | |
| FCTESTNET | ✓ | ✓ | ✓ |
| Giantleap | ✓ | ||
| JC08 | ✓ | ✓ | |
| Tongji University | ✓ | ✓ | |
| Tsinghua University | ✓ | ||
| Wuhan University of Technology | ✓ | ✓ | |
| Dalian Institute of Chemical Physics | ✓ | ✓ | |
| Parameter | Stationary Application | |
|---|---|---|
| Combined Heat and Power (CHP) | Backup | |
| Stack temperature (Coolant inlet) | 70 °C | 65 °C |
| Reactant inlet temperature | 75 °C | 70 °C |
| Fuel stoichiometry | 1.2 | 1.25 |
| Oxidation stoichiometry | 2.0 | 2.0 |
| Fuel relative humidity | 80% | 40% |
| Dew point temperature fuel | 69.5 °C | 45.5 °C |
| Oxidant relative humidity | 80% | 40% |
| Dew point temperature oxidation | 65 °C | 45.5 °C |
| Fuel outlet pressure | Ambient | 120 kPaabs |
| Oxidant outlet pressure | Ambient | Ambient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.-M.; Le, D.D. Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols. Energies 2021, 14, 4048. https://doi.org/10.3390/en14134048
Nguyen HL, Han J, Nguyen XL, Yu S, Goo Y-M, Le DD. Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols. Energies. 2021; 14(13):4048. https://doi.org/10.3390/en14134048
Chicago/Turabian StyleNguyen, Huu Linh, Jeasu Han, Xuan Linh Nguyen, Sangseok Yu, Young-Mo Goo, and Duc Dung Le. 2021. "Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols" Energies 14, no. 13: 4048. https://doi.org/10.3390/en14134048
APA StyleNguyen, H. L., Han, J., Nguyen, X. L., Yu, S., Goo, Y.-M., & Le, D. D. (2021). Review of the Durability of Polymer Electrolyte Membrane Fuel Cell in Long-Term Operation: Main Influencing Parameters and Testing Protocols. Energies, 14(13), 4048. https://doi.org/10.3390/en14134048





