Primary Research of a New Zero-Liquid-Discharge Technology of Wet Flue Gas Desulfurization Wastewater by Low-Rank Heat from Flue Gas
Abstract
:1. Introduction
2. Concept of the New WFGD Wastewater Zero-Emission Technology
- (1)
- Low pH value about 4.5~6.0;
- (2)
- High Mg2+ (1000~5000 mg/L) and Ca2+ (2000~4000 mg/L) concentrations, which easily cause the material scale formation;
- (3)
- High concentration of the chloride ions, about 6000~20,000 mg/L, which easily cause the material corrosion and difficult to remove;
- (4)
- High concentration of the TDS (total dissolved solids) (10,000~40,000 mg/L);
- (5)
- The large amount of wastewater. For example, in a typical 600 MW boiler unit, the wastewater flow rate is about 5~10 ton/h; the wastewater composition varies with the coal type and the limestone type, especially for the Mg2+ and chloride ions.
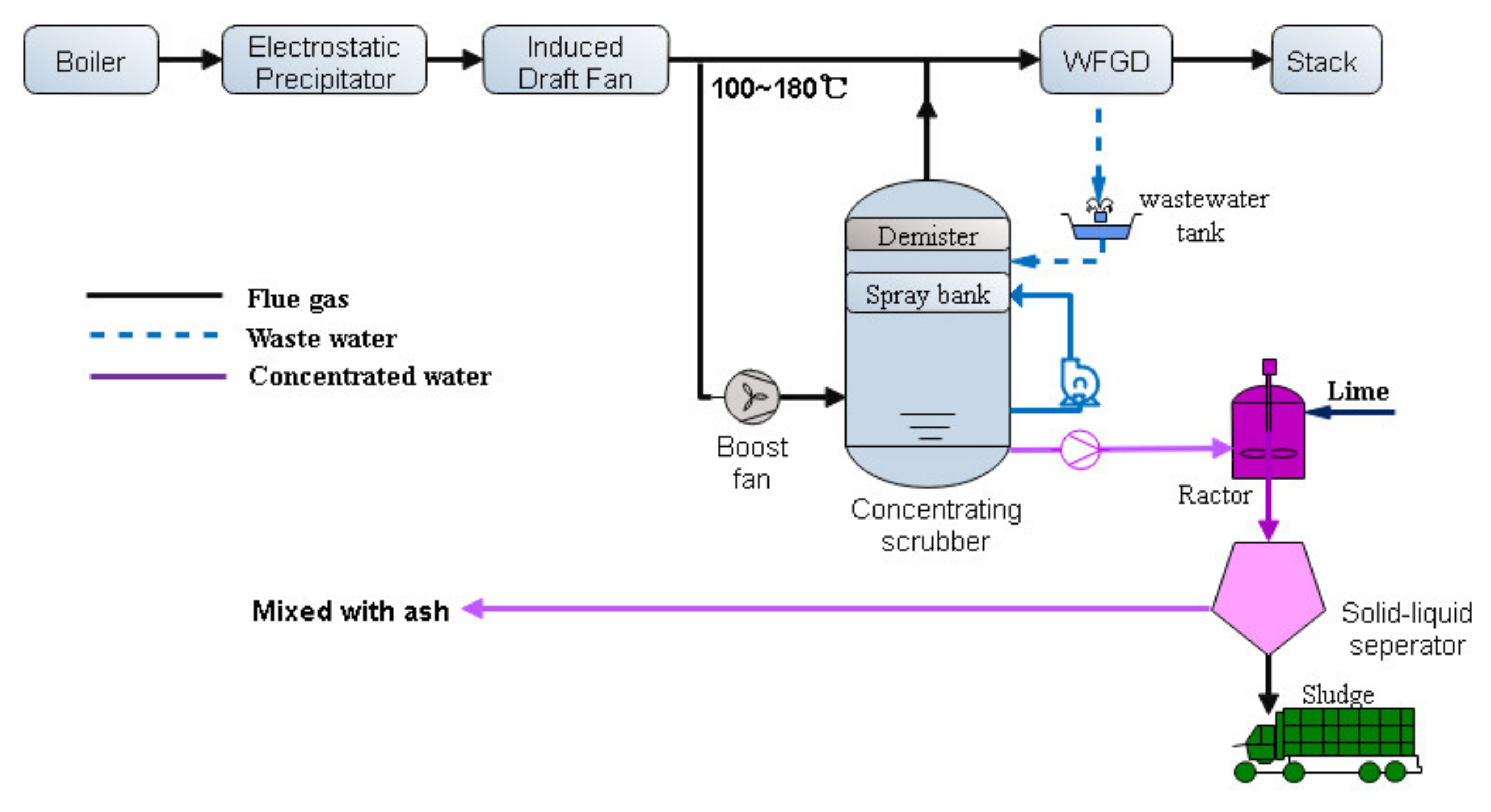
- (1)
- The preprocessing stage: The original WFGD wastewater goes into the cyclone and the flocculation basin to separate the liquid and the solids firstly to decrease the solids content. The filtered solution with solids concentration less than 1% can be used as the raw material of next concentrating process. The higher the solids concentration in the filtered solution, the higher the risk of the nozzle plugging in the concentrating scrubber in the next stage.
- (2)
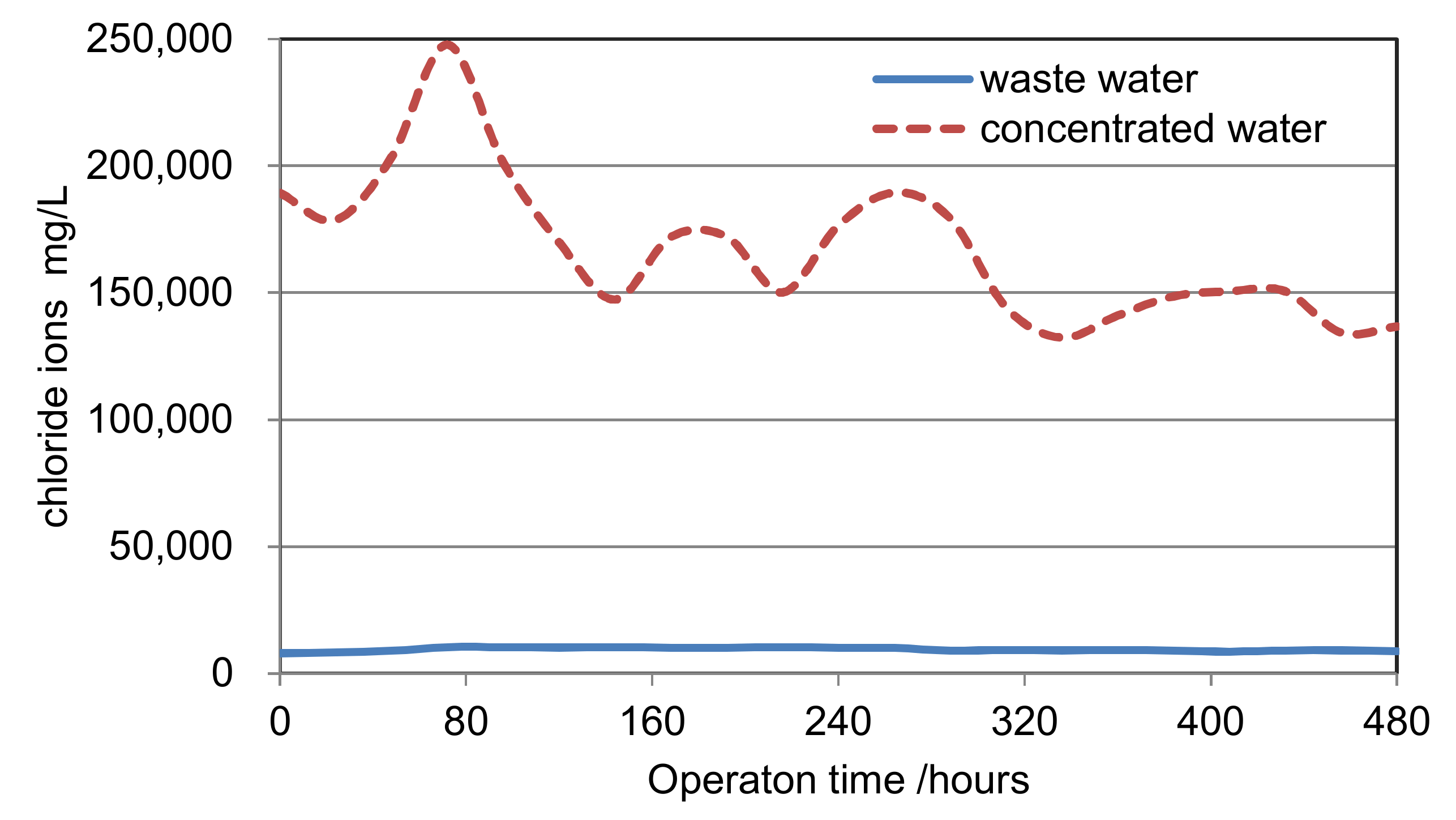
- The concentrating process: A concentrating scrubber is designed before WFGD in the concentrating process. The 120~180 °C flue gas before WFGD is the heat source of the concentrating process. The WFGD wastewater is cyclically sprayed downstream in the concentrating scrubber, and evaporated by the heat of the flue gas. A boost fan is used to overcome the resistance of the scrubber. In this way, the wastewater can be concentrated into the condensed slurry, which has the low pH value of 1~2 and the concentration of chloride ion about 150,000~200,000 mg/L. The concentration rate has a wide range of 0~25 due to the flexibility of scrubber. This process can greatly reduce wastewater discharge flow rate to next stage.
- (3)
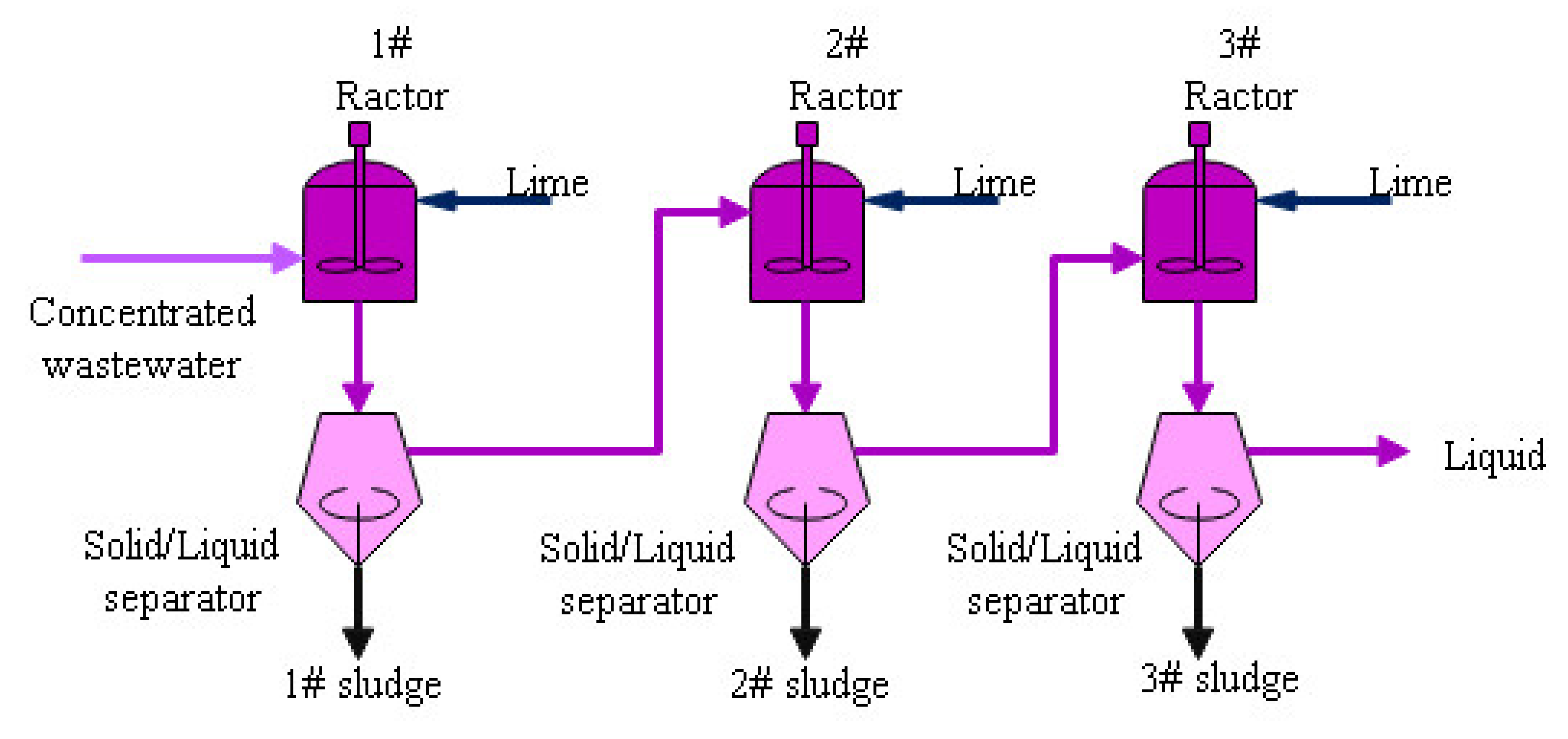
- The chemical dosing process: The concentrated water is neutralized by adding the Ca(OH)2 to raise the pH value. In this process, most Ca2+, Mg2+ and SO42− in the water converted into Mg(OH)2 and CaSO4. A filter press is used to separate the liquid and the sludge. The final filtered liquor is mixed with the ash collected by the ESP to reach zero liquid discharge. The sludge can be mixed with coal or just trucked out of the power plant to third-party disposal vendor.
3. The Pilot Plant Test to Verify the Concentrating Process
4. Small-Scale Experiment of the Chemical Dosing Process
5. Key Parameter of Demonstration Project of the Wastewater Treatment Process
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Peterhansen, J. Review of the direct sulfation reaction of limestone. Prog. Energy Combust. Sci. 2006, 32, 386–407. [Google Scholar] [CrossRef]
- Chowdhury, B.H. Emission Control Alternatives for Electric Utility Power Plants. Energy Sources 1996, 18, 393–406. [Google Scholar] [CrossRef]
- Karatepe, N. A Comparison of Flue Gas Desulfurization Processes. Energy Sources 2000, 22, 197–206. [Google Scholar] [CrossRef]
- Tomás-Alonso, F. A New Perspective about Recovering SO2 Offgas in Coal Power Plants: Energy Saving. Part III. Selection of the Best Methods. Energy Sources 2005, 27, 1051–1060. [Google Scholar] [CrossRef]
- Brogren, C.; Karlsson, H.T. The impact of the electrical potential gradient on limestone dissolution under wet flue gas desulfurization conditions. Chem. Eng. Sci. 1997, 52, 3101–3106. [Google Scholar] [CrossRef]
- Ersoy-Meriçboyu, A. Removal of Sulphur Dioxide from Flue Gases. Energy Sources 1999, 21, 611–619. [Google Scholar] [CrossRef]
- Hlincik, T.; Buryan, P. Evaluation of limestones for the purposes of desulphurisation during the fluid combustion of brown coal. Fuel 2013, 104, 208–215. [Google Scholar] [CrossRef]
- United States Enviromental Protection Agency. Steam Electric Power Generating Point Source Category: Final Detailed Study Report; Environmental Protection Agency: Washington, DC, USA, 2009.
- Higgins, T.E.; Sandy, T.; Givens, S.W. Flue Gas Desulfurization Wastewater Treatment Primer. Power 2009, 153, 34. [Google Scholar]
- Ma, S.; Chai, J.; Chen, G.; Yu, W.; Zhu, S. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar]
- Dong, X.F.; Shen, G.F.; Zhou, X.X.; Guo, J.J.; Zhang, X.Y.; Wu, W.L. The Problems and Suggestions of the Desulfurization Wastewater Treatment System during the Operation. Adv. Mater. Res. 2012, 599, 521–524. [Google Scholar] [CrossRef]
- Han, F.C.; Wang, X.; Zhang, R.; Yang, L.; Wang, W.F. Optimized Reconstruction of Limestone-Gypsum Wet Flue Gas Desulfurization Wastewater Treatment Process. China Water Wastewater 2016, 32, 99–102. [Google Scholar]
- Kang, M.; Deng, J.; Chen, D.; Pan, L. Analysis on the Feasibility of Desulfurization Wastewater Evaporation Treatment in Flue Gas Duct Without Pollution Discharge. J. Civ. Archit. Environ. Eng. 2013, 35, 238–240. [Google Scholar]
- Wang, R.D.; Ye-Ran, L.I.; Guo-Quan, G.U.; Feng-Jun, X.U. FGD Wastewater Treatment Using Improved Chemical Precipitation in Jingmen Power Plant Pollut. Control Technol. 2012, 25, 13–15. [Google Scholar]
- Fu, J.; Hu, N.; Yang, Z.; Wang, L. Experimental study on zero liquid discharge (ZLD) of FGD wastewater from a coal-fired power plant by flue gas exhausted heat. J. Water Process. Eng. 2018, 26, 100–107. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G. Advances in membrane technologies for the treatment of liquid radioactive waste. Desalination 2013, 321, 119–130. [Google Scholar] [CrossRef]
- Chunsong, Y.E.; Luo, S.; Zhang, X.; Xia, M.; Huang, J. Key problems and developing trend of zero discharge technology of desulfurization wastewater. Therm. Power Gener. 2016, 45, 105–108. [Google Scholar]
- Liu, H.Y.; Xia, H.X.; Jiang, C.Y.; Xiao-Bing, G.U.; Ye-Hong, L.I. Research Advances in Wet Flue Gas Desulfurization Wastewater Treament Technology in Coal-Fired Power Plant. Environ. Eng. 2016, 34, 31–35. [Google Scholar]
- Couto, R.S.D.P.; Oliveira, A.F.; Guarino, A.W.S.; Perez, D.V.; Marques, M.R.D.C. Removal of ammonia nitrogen from distilled old landfill leachate by adsorption on raw and modified aluminosilicate. Environ. Technol. 2016, 38, 816–826. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.; Li, Z. Effect of pH on elemental sulfur conversion and microbial communities by autotrophic simultaneous desulfurization and denitrification. Environ. Technol. 2016, 37, 3014–3023. [Google Scholar] [CrossRef]
- Yin, Z.; Xie, L.; Khanal, S.K.; Zhou, Q. Interaction of organic carbon, reduced sulphur and nitrate in anaerobic baffled reactor for fresh leachate treatment. Environ. Technol. 2016, 37, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Determination of Reaction Conditions for Magnesium Oxide Synthesis from Brine. J. Salt Sci. Chem. Ind. 2000, 29, 8–10. [Google Scholar]
- Cassie, W.; Vimal, B.; Carloalberto, P.; Krishan, R.; Seth, D.B.; Venugopal, M. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar]
- Ken, R. Reaction Engineering of Direct Coal Liquefaction. Energies 2013, 8, 976–1006. [Google Scholar]
- Tańczuk, M.; Masiukiewicz, M.; Anweiler, S.; Junga, R. Technical Aspects and Energy Effects of Waste Heat Recovery from District Heating Boiler Slag. Energies 2018, 11, 796. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ren, Y.; Wang, Z.; Hang, Z.; Luo, Y. Simulation and Economic Research of Circulating Cooling Water Waste Heat and Water Resource Recovery System. Energies 2021, 14, 2496. [Google Scholar] [CrossRef]
- Shaw, W.A. Fundamentals of Zero Liquid Discharge System Design. Power 2011, 155, 56–58. [Google Scholar]

| Item | Unit | Inlet | Outlet | |
|---|---|---|---|---|
| 1 | Flow rate | NM3/h | 28,804 | 30,510 |
| 2 | Temperature | °C | 130~150 | 55~60 |
| 3 | Gauge Pressure | KPa | 2.0 | 1.0 |
| 4 | H2O | Vol. % | 8 | 13.15 |
| 5 | O2 | Vol. % | 5.8 | 5.8 |
| 6 | SO2 (wet basis) | ppm | 2010 | 2000 |
| 7 | HCl (wet basis) | ppm | 50 | 5 |
| 8 | Dust (wet basis) | mg/Nm3 | 30 | 20 |
| Item | Unit | Waste Water | Concentrated Water | Concentration Rate | |
|---|---|---|---|---|---|
| 1 | Liquid flow rate | m3/h | 1.5 | 0.075 | 20 |
| 2 | Solid content | % | 0.8% | 27.3% | / |
| 3 | Density | Kg/m3 | 1.02 | 1.29 | / |
| 4 | pH value | -- | 5.6 | 0.24 | / |
| 5 | Ca2+ | mg/L | 658 | 1202 | 1.83 |
| 6 | Mg2+ | mg/L | 4225 | 48,195 | 11.41 |
| 7 | Na+ | mg/L | 166 | 3296 | 19.86 |
| 8 | K+ | mg/L | 32 | 627 | 19.59 |
| 9 | Cl− | mg/L | 7100 | 154,544 | 21.77 |
| 10 | SO42− | mg/L | 11,210 | 22,798 | 2.03 |
| 11 | F− | mg/L | 1010 | 2700 | 2.67 |
| Item | pH Value | Sludge Moisture Content | Mg(OH)2 | CaSO4 | Ca(OH)2 | SiO2 | Other |
|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | ||
| 1# Sludge | 8.26 | 37% | 3.41% | 86.7% | 1.84% | 1.11% | 6.94% |
| 2# Sludge | 8.7 | 50% | 84.51% | 3.8% | 9.44% | 0.28% | 1.97% |
| 3# Sludge | 10.5 | 42% | 44.52% | 1.7% | 51.52% | 0.17% | 2.09% |
| Item | pH | Ca2+ mg/L | Mg2+ mg/L | Na+ mg/L | K+ mg/L | Cl− mg/L | SO42− mg/L |
|---|---|---|---|---|---|---|---|
| 3#Liquid | 10.5 | 80,089 | 671 | 3368 | 726 | 156,827 | 472 |
| Item | CaCl2 | Ca(OH)2 | CaSO4 | Na2O | K2O | MgO |
|---|---|---|---|---|---|---|
| Dried solid | 73.6% | 10.02% | 4.24% | 2.51% | 0.38% | 0.32% |
| Item | Unit | Values | Remark | |
|---|---|---|---|---|
| 1 | Origin wastewater flow from WFGD | |||
| Flow rate | m3/h | 5 | ||
| Chloride irons | mg/L | 20,000 | ||
| PH value | -- | 5.6 | ||
| Density | kg/m3 | 1020 | ||
| 2 | Flue gas for concentrating process scrubber | |||
| Flow rate | Nm3/h | 125,000 | ||
| Scrubber inlet temperature | °C | 120 | ||
| Scrubber outlet temperature | °C | 58 | ||
| Scrubber diameter | m | 4.5 | ||
| 3 | Slurry after concentrating process | |||
| Flow rate | m3/h | 0.5 | ||
| Chloride irons | mg/L | 205,000 | ||
| PH value | -- | 1.24 | ||
| Density | kg/m3 | 1290 | ||
| 4 | Lime consumption for chemical dosing process | |||
| Mass flow rate | kg/h | 15 | ||
| Ca(OH)2 Purity | % | 85% | ||
| 5 | Sludge after chemical dosing and filter press | |||
| Sludge moisture content | % | 40% | ||
| Mass flow rate | kg/h | 55 | wet basis | |
| PH value | -- | 7~9 | ||
| 6 | Final liquid for ash-mixing | |||
| Flow rate | m3/h | 0.51 | ||
| Chloride irons | mg/L | 187,250 | ||
| PH value | -- | 7~9 | ||
| Density | kg/m3 | 1240 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Zhang, M.; Deng, B.; Yang, X.; Yang, H. Primary Research of a New Zero-Liquid-Discharge Technology of Wet Flue Gas Desulfurization Wastewater by Low-Rank Heat from Flue Gas. Energies 2021, 14, 4259. https://doi.org/10.3390/en14144259
Yao X, Zhang M, Deng B, Yang X, Yang H. Primary Research of a New Zero-Liquid-Discharge Technology of Wet Flue Gas Desulfurization Wastewater by Low-Rank Heat from Flue Gas. Energies. 2021; 14(14):4259. https://doi.org/10.3390/en14144259
Chicago/Turabian StyleYao, Xuan, Man Zhang, Boyu Deng, Xinhua Yang, and Hairui Yang. 2021. "Primary Research of a New Zero-Liquid-Discharge Technology of Wet Flue Gas Desulfurization Wastewater by Low-Rank Heat from Flue Gas" Energies 14, no. 14: 4259. https://doi.org/10.3390/en14144259
APA StyleYao, X., Zhang, M., Deng, B., Yang, X., & Yang, H. (2021). Primary Research of a New Zero-Liquid-Discharge Technology of Wet Flue Gas Desulfurization Wastewater by Low-Rank Heat from Flue Gas. Energies, 14(14), 4259. https://doi.org/10.3390/en14144259







