Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR)
Abstract
:1. Introduction
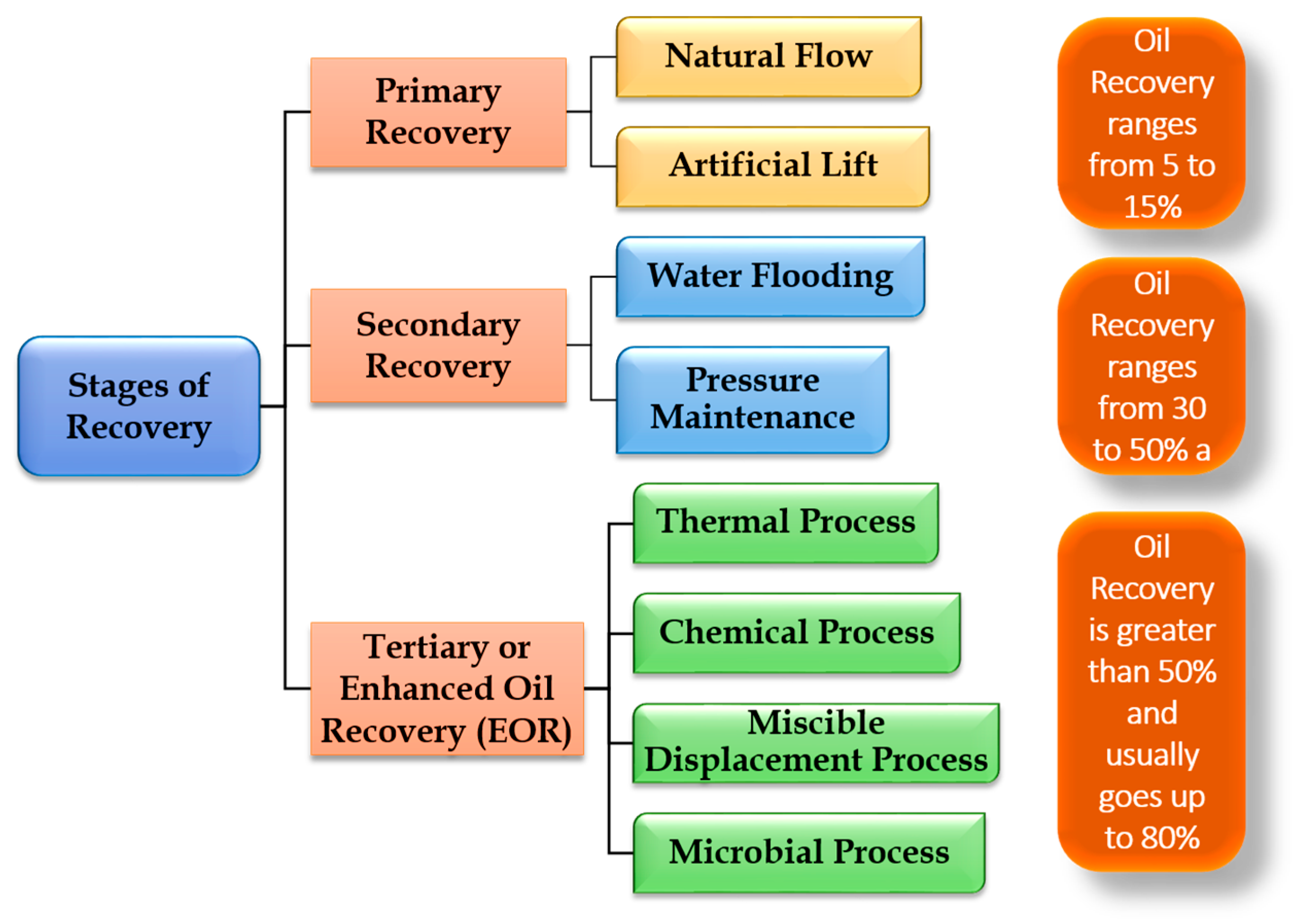
2. Phases of Oil Recovery
2.1. Primary Recovery Stage
2.2. Secondary Recovery Stage
2.3. Tertiary Recovery Stage (Enhanced Oil Recovery)
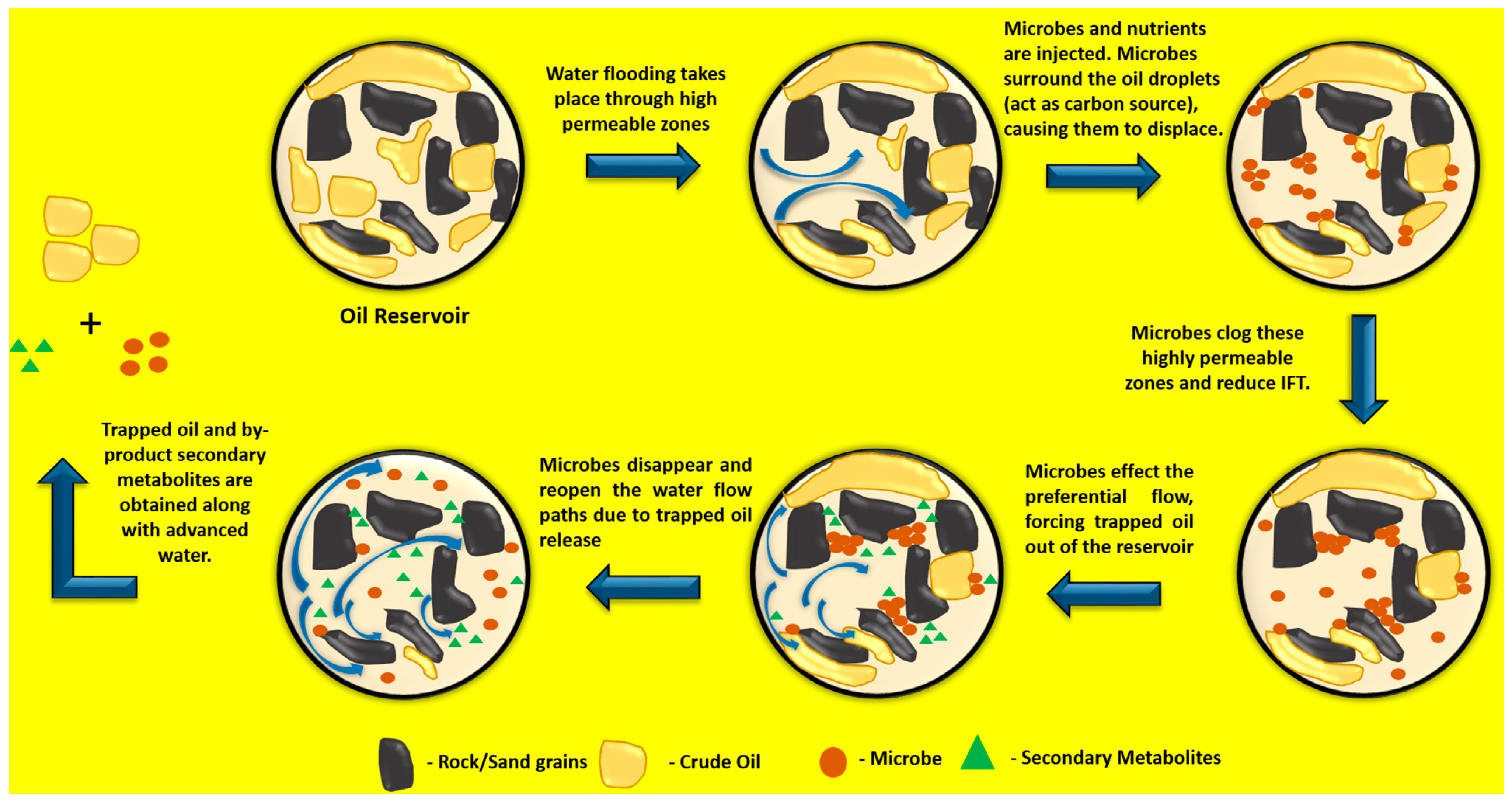
2.3.1. Microbial Method (MEOR)
2.3.2. Microbial Metabolic Products Involved
Biomass
Biosurfactants
Biopolymers
Biogases
Bioacids and Biosolvents
3. MEOR Dynamics
- Chemical factors such as electrolyte composition, redox potential, and nutrient composition;
- Physical factors such as pore geometry, salinity, temperature, porosity, pore size, pressure, dissolved solids, lithology, and permeability;
- Biological factors such as the type of microbe and extracellular product cytotoxicity.
3.1. Selecting the Reservoir
- StructuralAnalysis: The regions of permeability and the area penetrated by oil can only be determined by this analysis for the plugging of specific pore throats to enhance the efficiency of sweeping. The depth of the oil well, the spatial distribution of oil films, and water saturation of water are included. This also comes with a high risk of drilling uncertainties. If the oil reservoir is not properly analyzed, the microbes will begin to destroy the method.
- Geological Complexity: This plays a crucial role in the injections of microbes due to the function they perform, and therefore needs to be carefully studied. The alteration in any of the different geological elements of salinity, porosity, wettability, or permeability leads to dysfunctional microbes or their absence.
- Well Pattern to be Drilled: This parameter is used when economic factors are to be considered. The pattern, i.e., a horizontal, directional, or extended range drill, determines the injectors and producers to be used.
- Permeability Analysis: This is critical for choosing the appropriate bacterial strain and the reservoir composition that are suited to the microbe’s survival and feeding pattern. This factor should be reduced to be beneficial, as microbial consortium clogging will occur if the MEOR is not designed or implemented properly. In this case, the microbial metabolites themselves reduce the permeability by favouring plugging of thief zones as observed in Figure 3.
- Temperature: This is one of the key drivers for both the bacterial species variation and the oil reservoir’s hydrocarbon development. Only extremophiles can survive extreme conditions, making them critical for the reservoir. Among these, Pseudomonas (predominantly), Arcobacter, Marinobacter, Halomonas, and Caldicoprobacter are found in the oil pits at high temperatures.
3.2. Selecting the Potential Microbes
3.3. Selecting the Right Nutrients
3.4. Pilot Testing
4. Microbial Metabolic Pathways Involved
5. Concept of Mathematical Modelling in MEOR
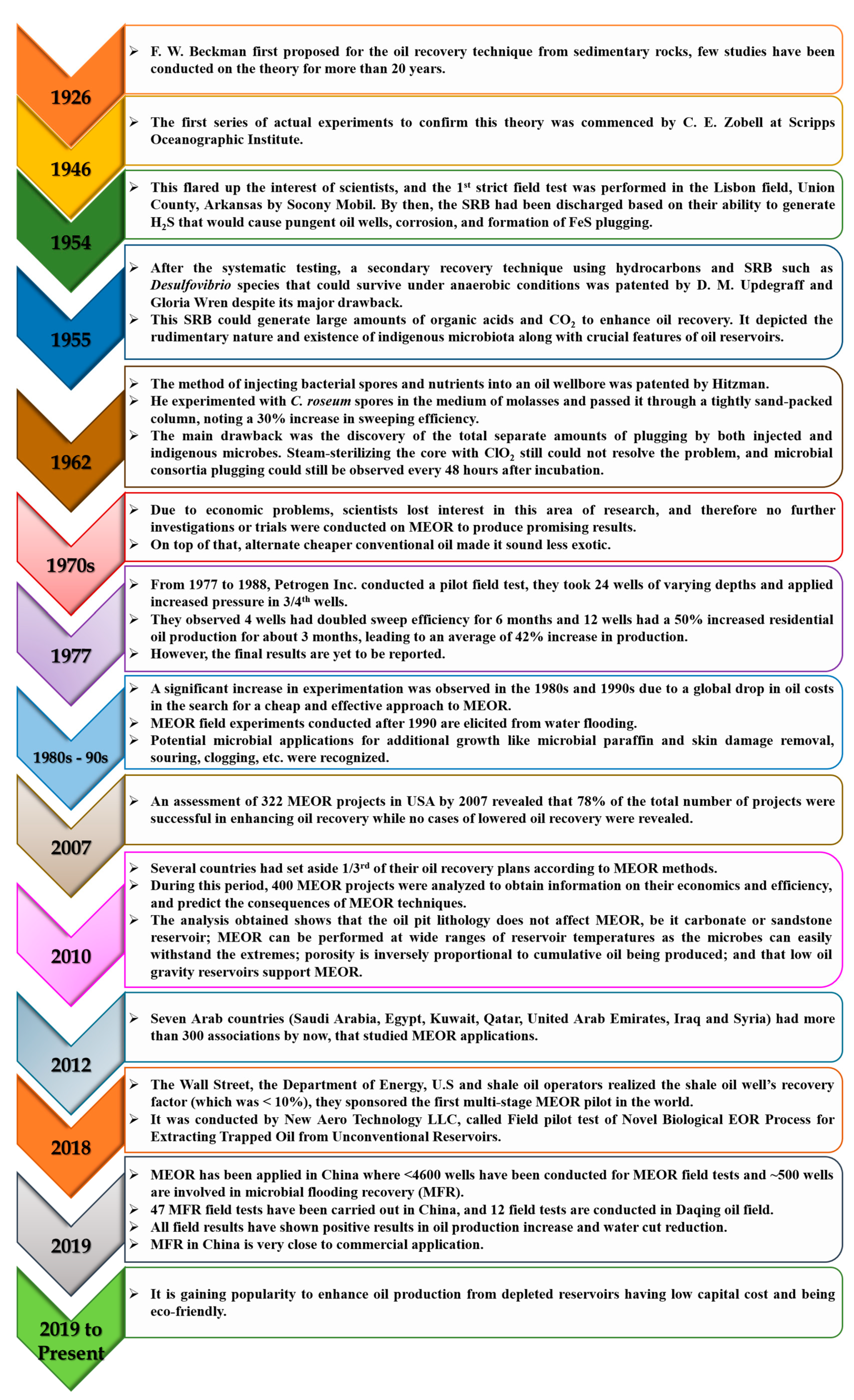
6. A Vast Chronicle of MEOR History and Case Studies
7. Trends and Ventures in MEOR
8. Conclusions
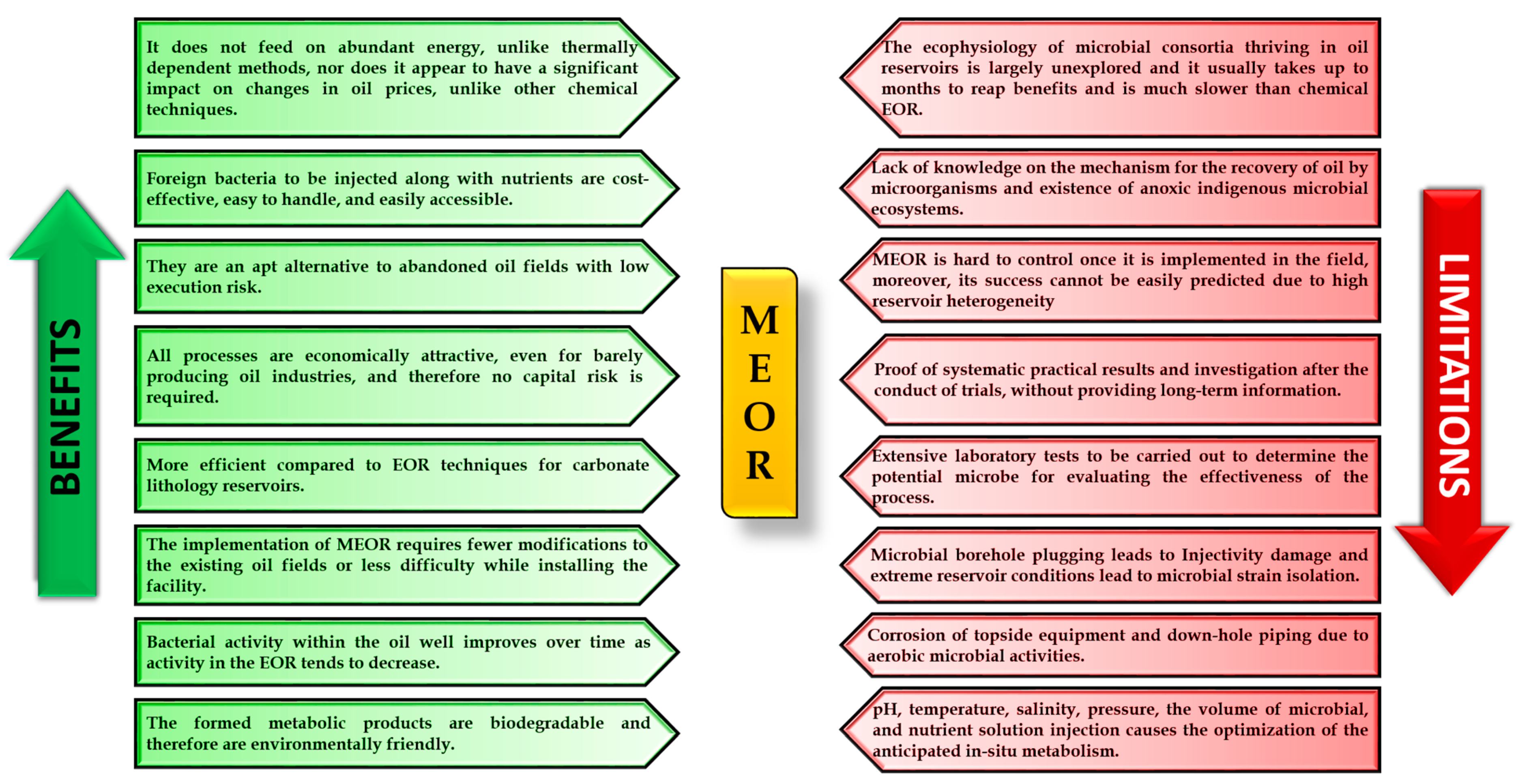
- MEOR is undoubtedly a visionary approach to the field of oil recovery, although concerns have previously been raised about the Industrial Proposal Standards required to implement field microbial processes.
- A number of challenges and complications must be addressed before MEOR can be used. Although progress has been made, past approaches should also be scrutinized.
- It is argued that MEOR is cost-effective, eco-friendly, and most suitable for mature oil wells with a high water cut and that it is a feasible alternative to conventional methods.
- New biological trends such as GEMEOR and EEOR may be the innovative strategies required to produce the desired breakthroughs. These advances may tilt the global energy balance towards cheaper prices and encourage domestic production.
- An aspiring advancement program of studies would thus be required to verify the feasibility of MEOR. Integrated research and cross-disciplinary collaboration between the fields of petroleum engineering, economics, geology, bioengineering, and microbiology are highly recommended for better results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, J.; Kazy, S.K.; Gupta, A.; Dutta, A.; Mohapatra, B.; Roy, A.; Bera, P.; Mitra, A.; Sar, P. Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front. Microbiol. 2016, 7, 1407. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Petroleum & Natural Gas. Indian PNG Statistics 2019–2020; Government of India: New Delhi, India, 2020.
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Nnaemeka, O.; Franklin, N.; Stanley, O. A Review of Microbial Enhanced Oil Recovery Applications Projects. Oil Gas Res. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Cheng, M.; Yu, L.; Gao, J.; Lei, G.; Zhang, Z. Isolating, identifying and evaluating of oil degradation strains for the air-assisted microbial enhanced oil recovery process. PLoS ONE 2021, 16, e0243976. [Google Scholar] [CrossRef]
- Yuan, B.; Wood, D.A. Overview of Formation Damage During Improved and Enhanced Oil Recovery. In Formation Damage during Improved Oil Recovery: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–20. [Google Scholar]
- Câmara, J.M.D.A.; Sousa, M.A.S.B.; Barros Neto, E.L.; Oliveira, M.C.A. Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbial-enhanced oil recovery (MEOR). J. Pet. Explor. Prod. Technol. 2019, 9, 2333–2341. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.K.; Choudhary, S. Bacterial and archaeal diversity in oil fields and reservoirs and their potential role in hydrocarbon recovery and bioprospecting. Environ. Sci. Pollut. Res. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, H.; Xue, Q. Potential applications of microbial enhanced oil recovery to heavy oil. Crit. Rev. Biotechnol. 2020, 40, 459–474. [Google Scholar] [CrossRef]
- Shibulal, B.; Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J. Microbial enhanced heavy oil recovery by the aid of inhabitant spore-forming bacteria: An insight review. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew. Sustain. Energy Rev. 2017, 74, 159–172. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Use of Microorganisms in the Recovery of Oil From Recalcitrant Oil Reservoirs: Current State of Knowledge, Technological Advances and Future Perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef] [Green Version]
- She, H.; Kong, D.; Li, Y.; Hu, Z.; Guo, H. Recent Advance of Microbial Enhanced Oil Recovery (MEOR) in China. Geofluids 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.A.H.; Vasconcellos, J.M.; Morales, M.E.O. Factors Affecting Microbial Enhanced Oil Recovery (MEOR). In Proceedings of the 25th Pan-American Conference of Naval Engineering—COPINAVAL; Springer: Berlin/Heidelberg, Germany, 2019; pp. 375–384. [Google Scholar]
- Romero-Zeron, L. Chemical Enhanced Oil Recovery (cEOR)—A Practical Overview; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Bi, Y.; Xiu, J.; Ma, T. Application potential analysis of enhanced oil recovery by biopolymer-producing bacteria and biosurfactant-producing bacteria compound flooding. Appl. Sci. 2019, 9, 5119. [Google Scholar] [CrossRef] [Green Version]
- Haq, B. The Role of Microbial Products in Green Enhanced Oil Recovery: Acetone and Butanone. Polymer 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Alagorni, A.H.; Yaacob, Z.B.; Nour, A.H. An Overview of Oil Production Stages: Enhanced Oil Recovery Techniques and Nitrogen Injection. Int. J. Environ. Sci. Dev. 2015, 6, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Ganat, T. Pumping System of Heavy Oil Production. Process. Heavy Crude Oils Chall. Oppor. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Anuka, A.; Falode, O.; Ogunshe, A.; Udie, C. An Overview of Microbial Enhanced oil Recovery in Nigeria. In Proceedings of the Society of Petroleum Engineers—SPE Nigeria Annual International Conference and Exhibition 2020, Online, 11–13 August 2020. [Google Scholar] [CrossRef]
- Romero-Zerón, L. Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-Contaminated Sites; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Oliveira, X.; Sn, S. The Challenge of the Use the Microorganisms in Enhance Oil Recover. Recent Adv. Petrochem. Sci. 2017, 1, 0034–0036. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Z.; Zhang, Z.; Sun, S.; Li, H.; Fu, P. Stimulation of indigenous microbes by optimizing the water cut in low permeability reservoirs for green and enhanced oil recovery. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ali, N.; Wang, F.; Xu, B.; Safdar, B.; Ullah, A.; Naveed, M.; Wang, C.; Rashid, M.T. Production and application of biosurfactant produced by bacillus licheniformis Ali5 in enhanced oil recovery and motor oil removal from contaminated sand. Molecules 2019, 24, 4448. [Google Scholar] [CrossRef] [Green Version]
- Muggeridge, A.; Cockin, A.; Webb, K.; Frampton, H.; Collins, I.; Moulds, T.; Salino, P. Recovery rates, enhanced oil recovery and technological limits. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372. [Google Scholar] [CrossRef] [Green Version]
- Mokheimer, E.M.A.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. Technol. Trans. ASME 2019, 141. [Google Scholar] [CrossRef]
- Huang, S.; Cao, M.; Cheng, L. Experimental study on the mechanism of enhanced oil recovery by multi-thermal fluid in offshore heavy oil. Int. J. Heat Mass Transf. 2018, 122, 1074–1084. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vardhan, K.H.; Jeevanantham, S.; Karishma, S.B.; Yaashikaa, P.R.; Vellaichamy, P. A review on systematic approach for microbial enhanced oil recovery technologies: Opportunities and challenges. J. Clean. Prod. 2020, 258, 120777. [Google Scholar] [CrossRef]
- Pang, Z.; Liu, H.; Zhu, L. A laboratory study of enhancing heavy oil recovery with steam flooding by adding nitrogen foams. J. Pet. Sci. Eng. 2015, 128, 184–193. [Google Scholar] [CrossRef]
- Kirmani, F.U.D.; Raza, A.; Gholami, R.; Haidar, M.Z.; Fareed, C.S. Analyzing the effect of steam quality and injection temperature on the performance of steam flooding. Energy Geosci. 2021, 2, 83–86. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. J. Pet. Explor. Prod. Technol. 2018, 8, 1373–1387. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.J. A comprehensive review of alkaline-surfactant-polymer (ASP) flooding. Asia Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Mandal, A. Chemical flood enhanced oil recovery: A review. Int. J. Oil Gas Coal Technol. 2015, 9, 241–264. [Google Scholar] [CrossRef]
- Kokal, S.; Al-Kaabi, A. Enhanced oil recovery: Challenges & opportunities. World Pet. Counc. Off. Publ. 2010, 64, 64–69. [Google Scholar]
- Sun, C.; Guo, H.; Guo, H.; Guo, H.; Li, Y.; Jiang, G.; Ma, R. Alkali Effect on Alkali-Surfactant-Polymer (ASP) Flooding Enhanced Oil Recovery Performance: Two Large-Scale Field Tests’ Evidence. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Alamooti, A.M.; Malekabadi, F.K. An Introduction to Enhanced Oil Recovery. In Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Nazina, T.; Sokolova, D.; Grouzdev, D.; Semenova, E.; Babich, T.; Bidzhieva, S.; Serdukov, D.; Volkov, D.; Bugaev, K.; Ershov, A.; et al. The potential application of microorganisms for sustainable petroleum recovery from heavy oil reservoirs. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Wooden, B. Seismic Stimulation: An Eco-Friendly, Effective EOR Alternative. Available online: https://jpt.spe.org/seismic-stimulation-eco-friendly-effective-eor-alternative (accessed on 19 June 2021).
- Rezaei Dehshibi, R.; Mohebbi, A.; Riazi, M.; Niakousari, M. Experimental investigation on the effect of ultrasonic waves on reducing asphaltene deposition and improving oil recovery under temperature control. Ultrason. Sonochem. 2018, 45, 204–212. [Google Scholar] [CrossRef]
- Shafiai, S.H.; Gohari, A. Conventional and electrical EOR review: The development trend of ultrasonic application in EOR. J. Pet. Explor. Prod. Technol. 2020, 10, 2923–2945. [Google Scholar] [CrossRef]
- Saikia, U.; Bharanidharan, R.; Vendhan, E.; Kumar Yadav, S.; Siva Shankar, S. A brief review on the science, mechanism and environmental constraints of microbial enhanced oil recovery (MEOR). Int. J. Chem. Techol. Res. 2013, 5, 1205–1212. [Google Scholar]
- Parihar, J.; Bagaria, A. The Extremes of Life and Extremozymes. Divers Perspect. 2020, 3, 2581–3226. [Google Scholar] [CrossRef]
- Moreno, M.D.L.; Pérez, D.; García, M.T.; Mellado, E. Halophilic Bacteria as a Source of Novel Hydrolytic Enzymes. Life 2013, 3, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Irwin, J.A. Overview of extremophiles and their food and medical applications. Physiol. Biotechnol. Asp. Extrem. 2020, 65–87. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Evaluation of rhamnolipid production by a halotolerant novel strain of Pseudomonas aeruginosa. Bioresour. Technol. 2019, 288, 121577. [Google Scholar] [CrossRef]
- Volk, H.; Hendry, P. 3° Oil Recovery: Fundamental Approaches and Principles of Microbially Enhanced Oil Recovery. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2727–2738. [Google Scholar]
- Yi, L.N.; Li, Z.P.; Liu, L.; Bi, Y.Q.; Wang, X.T.; Yi, J.P. Functional microbial stimulation for oil recovery enhancement based on microbial community analysis. Biotechnol. Biotechnol. Equip. 2018, 32, 1468–1476. [Google Scholar] [CrossRef]
- Shibulal, B.; Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J. Microbial-Enhanced Heavy Oil Recovery under Laboratory Conditions by Bacillus firmus BG4 and Bacillus halodurans BG5 Isolated from Heavy Oil Fields. Colloids Interfaces 2018, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, A.; Almohsin, A.; Varavei, A.; Delshad, M.; Bai, B.; Sepehrnoori, K. New Experiments and Models for Conformance Control Microgels. In Proceedings of the Conference Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; pp. 1–17. [Google Scholar]
- Wood, D.A. Microbial improved and enhanced oil recovery (Mieor): Review of a set of technologies diversifying their applications. Adv. Geo Energy Res. 2019, 3, 122–140. [Google Scholar] [CrossRef]
- Kögler, F.; Dopffel, N.; Mahler, E.; Hartmann, F.S.F.; Schulze-Makuch, D.; Visser, F.; Frommherz, B.; Herold, A.; Alkan, H. Influence of surface mineralogy on the activity of Halanaerobium sp. during microbial enhanced oil recovery (MEOR). Fuel 2021, 290, 119973. [Google Scholar] [CrossRef]
- Gaol, C.L.; Ganzer, L.; Mukherjee, S.; Alkan, H. Parameters govern microbial enhanced oil recovery (MEOR) performance in real-structure micromodels. J. Pet. Sci. Eng. 2021, 205, 108814. [Google Scholar] [CrossRef]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2008, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Glagoleva, O.F.; Kapustin, V.M. Physicochemical aspects of primary oil processing technology (Review). Pet. Chem. 2018, 58, 1–7. [Google Scholar] [CrossRef]
- Prabhakar, S.; Melnik, R. Wettability alteration of calcite oil wells: Influence of smart water ions. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Kwon, T.-H.; Park, T.; Jeong, M.S. Introduction. In Theory and Practice in Microbial Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–25. [Google Scholar]
- Miller, R.G.; Sorrell, S.R. The future of oil supply. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Ma, D.; Wu, K.; Wei, X.; Xu, Y. A review Study: Development of Microbial Enhancing oil Recovery Technology. In Proceedings of the 2016 5th International Conference on Environment, Materials, Chemistry and Power Electronics, Zhengzhou, China, 11–12 April 2016; pp. 619–625. [Google Scholar] [CrossRef] [Green Version]
- Azarhava, H.; Bajestani, M.I.; Jafari, A.; Vakilchap, F.; Mousavi, S.M. Production and physicochemical characterization of bacterial poly gamma- (glutamic acid) to investigate its performance on enhanced oil recovery. Int. J. Biol. Macromol. 2020, 147, 1204–1212. [Google Scholar] [CrossRef]
- Ashish Debnath, M. Application of biosurfactant produced by an adaptive strain of C.tropicalis MTCC230 in microbial enhanced oil recovery (MEOR) and removal of motor oil from contaminated sand and water. J. Pet. Sci. Eng. 2018, 170, 40–48. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 2019, 7, 581. [Google Scholar] [CrossRef] [Green Version]
- Tatar, A. Microbial Enhanced Oil Recovery: Microbiology and Fundamentals. In Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 291–508. [Google Scholar]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine biosurfactants: Biosynthesis, structural diversity and biotechnological applications. Mar. Drugs 2019, 17, 408. [Google Scholar] [CrossRef] [Green Version]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int. J. Mol. Sci. 2020, 21, 2152. [Google Scholar] [CrossRef] [Green Version]
- Putra, W.; Hakiki, F. Microbial enhanced oil recovery: Interfacial tension and biosurfactant-bacteria growth. J. Pet. Explor. Prod. Technol. 2019, 9, 2353–2374. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Kryachko, Y. Novel approaches to microbial enhancement of oil recovery. J. Biotechnol. 2018, 266, 118–123. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [Green Version]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; RAMU Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Randhawa, K.K.S.; Rahman, P.K.S.M. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef] [Green Version]
- Albokari, M.; Mashhour, I.; Alshehri, M.; Boothman, C.; Al-Enezi, M. Characterization of microbial communities in heavy crude oil from Saudi Arabia. Ann. Microbiol. 2015, 65, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Yong Ng, H.; Taherzadeh, M.J.; Hao Ngo, H.; Chang, J.S.; Wong, J.W.C.; You, S.; Teixeira, J.A.; Bui, X.T. Bio-based rhamnolipids production and recovery from waste streams: Status and perspectives. Bioresour. Technol. 2021, 319, 124213. [Google Scholar] [CrossRef]
- Renard, P.; Canet, I.; Sancelme, M.; Matulova, M.; Uhliarikova, I.; Eyheraguibel, B.; Nauton, L.; Devemy, J.; Traïkia, M.; Malfreyt, P.; et al. Cloud Microorganisms, an Interesting Source of Biosurfactants. In Surfactants and Detergents; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Lin, J.; Wang, W.; Li, S. High-Yield Di-Rhamnolipid Production by Pseudomonas aeruginosa YM4 and its Potential Application in MEOR. Molecules 2019, 24, 1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eruke, O.; Udoh, A. Potentials for Biosurfactant Enhanced Bioremediation of Hydrocarbon Contaminated Soil and Water–A Review. Adv. Res. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Martínez-Toledo, A.; Rodríguez-Vázquez, R. Response surface methodology (Box-Behnken) to improve a liquid media formulation to produce biosurfactant and phenanthrene removal by Pseudomonas putida. Ann. Microbiol. 2011, 61, 605–613. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Odibo, F.J.C. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J. Microbiol. Biotechnol. 2012, 28, 937–942. [Google Scholar] [CrossRef]
- Singh, P.; Tiwary, B.N. Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines, India. Bioresour. Bioprocess. 2016, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Shreve, G.S.; Makula, R. Characterization of a new rhamnolipid biosurfactant complex from pseudomonas isolate DYNA270. Biomolecules 2019, 9, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haloi, S.; Sarmah, S.; Gogoi, S.B.; Medhi, T. Characterization of Pseudomonas sp. TMB2 produced rhamnolipids for ex-situ microbial enhanced oil recovery. 3 Biotech 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Nejad, Y.S.; Jaafarzadeh, N.; Ahmadi, M.; Abtahi, M.; Ghafari, S.; Jorfi, S. Remediation of oily sludge wastes using biosurfactant produced by bacterial isolate Pseudomonas balearica strain Z8. J. Environ. Health Sci. Eng. 2020, 18, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Bharal, P.; Konwar, B.K. Production and physico-chemical characterization of a biosurfactant produced by Pseudomonas aeruginosa OBP1 isolated from petroleum sludge. Appl. Biochem. Biotechnol. 2011, 164, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.J.; Luo, Z.B.; Dong, H.P.; Yu, L.; Cui, Q.F.; Bi, Y.Q. Synthesis, characterization, and oil recovery application of biosurfactant produced by indigenous Pseudomonas aeruginosa WJ-1 using waste vegetable oils. Appl. Biochem. Biotechnol. 2012, 166, 1148–1166. [Google Scholar] [CrossRef]
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of microbial rhamnolipid by Pseudomonas aeruginosa MM1011 for Ex situ enhanced oil recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Bharali, P.; Singh, S.P.; Dutta, N.; Gogoi, S.; Bora, L.C.; Debnath, P.; Konwar, B.K. Biodiesel derived waste glycerol as an economic substrate for biosurfactant production using indigenous Pseudomonas aeruginosa. RSC Adv. 2014, 4, 38698–38706. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Mohamed, M.S.; Samak, N. Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz. J. Chem. Eng. 2014, 31, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Deepika, K.V.; Kalam, S.; Ramu Sridhar, P.; Podile, A.R.; Bramhachari, P.V. Optimization of rhamnolipid biosurfactant production by mangrove sediment bacterium Pseudomonas aeruginosa KVD-HR42 using response surface methodology. Biocatal. Agric. Biotechnol. 2016, 5, 38–47. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Moreira, F.S.; Cardoso, V.L.; de Resende, M.M. Soy molasses as a fermentation substrate for the production of biosurfactant using Pseudomonas aeruginosa ATCC 10145. Environ. Sci. Pollut. Res. 2017, 24, 18699–18709. [Google Scholar] [CrossRef]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef]
- Liu, W.J.; Duan, X.D.; Wu, L.P.; Masakorala, K. Biosurfactant Production by Pseudomonas aeruginosa SNP0614 and its Effect on Biodegradation of Petroleum. Appl. Biochem. Microbiol. 2018, 54, 155–162. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Verma, N. Optimization of rhamnolipid production from Pseudomonas aeruginosa PBS towards application for microbial enhanced oil recovery. 3 Biotech 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, P.; Guo, C.; Shi, R.J.; Zhang, Y. Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresour. Technol. 2018, 251, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Hajfarajollah, H.; Noghabi, K.A.; Manteghian, M. Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process. RSC Adv. 2019, 9, 7932–7947. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xue, R.; Liu, S.; Xu, N.; Xin, F.; Zhang, W.; Jiang, M.; Dong, W. High Di-rhamnolipid Production Using Pseudomonas aeruginosa KT1115, Separation of Mono/Di-rhamnolipids, and Evaluation of Their Properties. Front. Bioeng. Biotechnol. 2019, 7, 245. [Google Scholar] [CrossRef]
- Zhao, F.; Han, S.; Zhang, Y. Comparative studies on the structural composition, surface/interface activity and application potential of rhamnolipids produced by Pseudomonas aeruginosa using hydrophobic or hydrophilic substrates. Bioresour. Technol. 2020, 295, 122269. [Google Scholar] [CrossRef]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. Adv. Exp. Med. Biol. 2010, 672, 135–145. [Google Scholar] [CrossRef]
- Qazi, M.A.; Subhan, M.; Fatima, N.; Ali, M.I.; Ahmed, S. Role of Biosurfactant Produced By Fusarium sp. BS-8 in Enhanced Oil Recovery (EOR) Through Sand Pack Column. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 598–604. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Ismail, S. Biosurfactant production by Bacillus salmalaya for lubricating oil solubilization and biodegradation. Int. J. Environ. Res. Public Health 2015, 12, 9848–9863. [Google Scholar] [CrossRef] [Green Version]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.P.; Silva, M.D.S.; Costa, E.V.L.; Rufino, R.D.; Santos, V.A.; Ramos, C.S.; Sarubbo, L.A.; Porto, A.L.F. Production and characterization of a biosurfactant produced by Streptomyces sp. DPUA 1559 isolated from lichens of the Amazon region. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Araújo, H.W.C.; Andrade, R.F.S.; Montero-Rodríguez, D.; Rubio-Ribeaux, D.; Alves Da Silva, C.A.; Campos-Takaki, G.M. Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb. Cell Fact. 2019, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.A.O.S.; Santos, B.L.P.; Vieira, I.M.M.; Ramos, L.C.; de Souza, R.R.; Silva, D.P.; Ruzene, D.S. Evaluation of a new strategy in the elaboration of culture media to produce surfactin from hemicellulosic corncob liquor. Biotechnol. Rep. 2019, 24, e00364. [Google Scholar] [CrossRef] [PubMed]
- Purwasena, I.A.; Astuti, D.I.; Syukron, M.; Amaniyah, M.; Sugai, Y. Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J. Pet. Sci. Eng. 2019, 183, 106383. [Google Scholar] [CrossRef]
- Hu, X.; Qiao, Y.; Chen, L.Q.; Du, J.F.; Fu, Y.Y.; Wu, S.; Huang, L. Enhancement of solubilization and biodegradation of petroleum by biosurfactant from Rhodococcus erythropolis HX-2. Geomicrobiol. J. 2020, 37, 159–169. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential Food Application of a Biosurfactant Produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Campos Guerra, J.M.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ 2020, 2020, e9064. [Google Scholar] [CrossRef] [Green Version]
- Purwasena, I.A.; Sugai, Y.; Sasaki, K. Estimation of the potential of an anaerobic thermophilic oil-degrading bacterium as a candidate for MEOR. J. Pet. Explor. Prod. Technol. 2014, 4, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Godwin Jacob, C. The Effect of Salt Concentration on Microbes during Microbial Enhanced Oil Recovery. J. Eng. Res. Appl. 2014, 4, 244–247. [Google Scholar]
- Bachmann, R.T.; Johnson, A.C.; Edyvean, R.G.J. Biotechnology in the petroleum industry: An overview. Int. Biodeterior. Biodegrad. 2014, 86, 225–237. [Google Scholar] [CrossRef]
- Cui, Q.F.; Sun, S.S.; Luo, Y.J.; Yu, L.; Zhang, Z.Z. Comparison of in-situ and ex-situ microbial enhanced oil recovery by strain Pseudomonas aeruginosa WJ-1 in laboratory sand-pack columns. Pet. Sci. Technol. 2017, 35, 2044–2050. [Google Scholar] [CrossRef]
- Gao, G.; Ji, K.; Zhang, Y.; Liu, X.; Dai, X.; Zhi, B.; Cao, Y.; Liu, D.; Wu, M.; Li, G.; et al. Microbial enhanced oil recovery through deep profile control using a conditional bacterial cellulose-producing strain derived from Enterobacter sp. FY-07. Microb. Cell Fact. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of polysaccharide biopolymer in petroleum recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayung, M.; Aung, M.M.; Azhar, S.C.; Abdullah, L.C.; Su’ait, M.S.; Ahmad, A.; Jamil, S.N.A.M. Bio-based polymer electrolytes for electrochemical devices: Insight into the ionic conductivity performance. Materials 2020, 13, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, N.A.; Valdez, A.L.; Fariña, J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Wang, T.; Li, Y.; Gao, N.L.; Zhang, L.; Chen, W.H.; Yin, X. Optimization of scleroglucan production by Sclerotium rolfsii by lowering pH during fermentation via oxalate metabolic pathway manipulation using CRISPR/Cas9. Fungal Biol. Biotechnol. 2021, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tomulescu, C.; Stoica, R.; Sevcenco, C.; Căşărică, A.; Moscovici, M.; Vamanu, A. Levan-A Mini Review. Sci. Bull. Ser. F. Biotechnol. 2016, 20, 309–317. [Google Scholar]
- Ağçeli, G.K.; Cihangir, N. Synthesis, characterization and antimicrobial performance of novel nanostructured biopolymer film based on levan/clay/LL-37 antimicrobial peptide. Biocatal. Agric. Biotechnol. 2020, 23, 101421. [Google Scholar] [CrossRef]
- Jeong, M.S.; Lee, Y.W.; Lee, H.S.; Lee, K.S. Simulation-Based Optimization of Microbial Enhanced Oil Recovery with a Model Integrating Temperature, Pressure, and Salinity Effects. Energies 2021, 14, 1131. [Google Scholar] [CrossRef]
- Jinfang, W.; Zhengmao, W.; Jun, T.; Chengfang, S.; Jian, G. An Evaluation of Microbial Flooding for Enhanced Oil Recovery, Fuyu Oilfield, China. In Proceedings of the Society of Petroleum Engineers—SPE Annual Caspian Technical Conference and Exhibition, Baku, Azerbaijan, 1–3 November 2017. [Google Scholar]
- Phetcharat, T.; Dawkrajai, P.; Chitov, T.; Wongpornchai, P.; Saenton, S.; Mhuantong, W.; Kanokratana, P.; Champreda, V.; Bovonsombut, S. Effect of inorganic nutrients on bacterial community composition in oil-bearing sandstones from the subsurface strata of an onshore oil reservoir and its potential use in Microbial Enhanced Oil Recovery. PLoS ONE 2018, 13, e0198050. [Google Scholar] [CrossRef]
- Ma, T.T.; Liu, L.Y.; Rui, J.P.; Yuan, Q.; Feng, D.S.; Zhou, Z.; Dai, L.R.; Zeng, W.Q.; Zhang, H.; Cheng, L. Coexistence and competition of sulfate-reducing and methanogenic populations in an anaerobic hexadecane-degrading culture. Biotechnol. Biofuels 2017, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Omoniyi, O. A review of microbial enhanced oil recovery: Current development and future prospects. Int. J. Sci. Eng. Res. 2015, 6, 1378–1389. [Google Scholar]
- Jahanbani Veshareh, M.; Ayatollahi, S. Microorganisms’ effect on the wettability of carbonate oil-wet surfaces: Implications for MEOR, smart water injection and reservoir souring mitigation strategies. J. Pet. Explor. Prod. Technol. 2020, 10, 1539–1550. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhang, C.; Yang, K.L.; He, J. Unique genetic cassettes in a thermoanaerobacterium contribute to simultaneous conversion of cellulose and monosugars into butanol. Sci. Adv. 2018, 4, e1701475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N.; Ferreira, T. Clostridium sp. as Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 1–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazina, T.N.; Sokolova, D.S.; Babich, T.L.; Semenova, E.M.; Ershov, A.P.; Bidzhieva, S.K.; Borzenkov, I.A.; Poltaraus, A.B.; Khisametdinov, M.R.; Tourova, T.P. Microorganisms of low-temperature heavy oil reservoirs (Russia) and their possible application for enhanced oil recovery. Microbiology 2017, 86, 773–785. [Google Scholar] [CrossRef]
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial Processes in Oil Fields. Culprits, Problems, and Opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [CrossRef]
- Siegert, M.; Sitte, J.; Galushko, A.; Krüger, M. Starting up microbial enhanced oil recovery. Adv. Biochem. Eng. Biotechnol. 2014, 142, 1–94. [Google Scholar] [CrossRef]
- Olajire, A.A.; Essien, J.P. Aerobic Degradation of Petroleum Components by Microbial Consortia. J Pet Env. Biotechnol 2014, 5, 1. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, J. New insights into bioremediation strategies for oil-contaminated soil in cold environments. Int. Biodeterior. Biodegrad. 2019, 142, 58–72. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root Exudation: The Ecological Driver of Hydrocarbon Rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Erdner, D.L.; Rosenheim, B.E.; Shetty, P.; Seitz, K.W.; Baker, B.J.; Liu, Z. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J. 2018, 12, 2532–2543. [Google Scholar] [CrossRef]
- Sierra-García, I.N.; Alvarez, J.C.; De Vasconcellos, S.P.; De Souza, A.P.; Dos Santos Neto, E.V.; De Oliveira, V.M. New hydrocarbon degradation pathways in the microbial metagenome from brazilian petroleum reservoirs. PLoS ONE 2014, 9, 90087. [Google Scholar] [CrossRef] [Green Version]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Varjani, S.J.; Gnansounou, E. Microbial dynamics in petroleum oilfields and their relationship with physiological properties of petroleum oil reservoirs. Bioresour. Technol. 2017, 245, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Natalia, I.; de Oliveir, V.M. Microbial Hydrocarbon Degradation: Efforts to Understand Biodegradation in Petroleum Reservoirs. In Biodegradation—Engineering and Technology; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Mbadinga, S.M.; Wang, L.Y.; Zhou, L.; Liu, J.F.; Gu, J.D.; Mu, B.Z. Microbial communities involved in anaerobic degradation of alkanes. Int. Biodeterior. Biodegrad. 2011, 65, 1–13. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum hydrocarbon contamination in terrestrial ecosystems—fate and microbial responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [Green Version]
- Okpala, G.N.; Voordouw, G. Comparison of nitrate and perchlorate in controlling sulfidogenesis in heavy oil-containing bioreactors. Front. Microbiol. 2018, 9, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voordouw, G.; Agrawal, A.; Park, H.S.; Gieg, L.M.; Jack, T.R.; Cavallaro, A.; Granli, T.; Miner, K. Souring Treatment with Nitrate in Fields from Which Oil is Produced by Produced Water Reinjection. In Proceedings of the Proceedings—SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011; Volume 2, pp. 651–653. [Google Scholar]
- Lin, J.; Hao, B.; Cao, G.; Wang, J.; Feng, Y.; Tan, X.; Wang, W. A study on the microbial community structure in oil reservoirs developed by water flooding. J. Pet. Sci. Eng. 2014, 122, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Alkan, H.K.; Gutierrez, I.; Bültemeier, H.; Mahler, E. Design and Performance Prediction of an MEOR Field Pilot by Numerical Methods. In Proceedings of the IOR 2015—18th European Symposium on Improved Oil Recovery, Dresden, Germany, 14–16 April 2015; p. cp-445. [Google Scholar]
- Jeong, M.S.; Lee, J.H.; Lee, K.S. Critical review on the numerical modeling of in-situ microbial enhanced oil recovery processes. Biochem. Eng. J. 2019, 150, 107294. [Google Scholar] [CrossRef]
- Hong, E.; Jeong, M.S.; Kim, T.H.; Lee, J.H.; Cho, J.H.; Lee, K.S. Development of coupled biokinetic and thermal model to optimize cold-water microbial enhanced oil recovery (MEOR) in homogenous reservoir. Sustainability 2019, 11, 1652. [Google Scholar] [CrossRef] [Green Version]
- Gang, H.Z.; Liu, M.T.; Mu, B.Z. Modeling of microorganisms transport in a cylindrical pore. J. Ind. Microbiol. Biotechnol. 2008, 35, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Trefry, M.G.; Park, J.; Liu, K.; Haq, B.; Johnston, C.D.; Volk, H. Interactions of Microbial-Enhanced Oil Recovery Processes. Transp. Porous Media 2011, 87, 77–104. [Google Scholar] [CrossRef]
- Sivasankar, P.; Kumar, G.S. Numerical modelling of enhanced oil recovery by microbial flooding under non-isothermal conditions. J. Pet. Sci. Eng. 2014, 124, 161–172. [Google Scholar] [CrossRef]
- Al-Sulaimani, H.; Joshi, S.; Al-Wahaibi, Y.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A. Microbial biotechnology for enhancing oil recovery: Current developments and future prospects. Invit. Rev. Biotechnol. Bioinf. Bioeng. 2011, 1, 147–158. [Google Scholar]
- Ansah, E.O.; Sugai, Y.; Nguele, R.; Sasaki, K. Integrated microbial enhanced oil recovery (MEOR) simulation: Main influencing parameters and uncertainty assessment. J. Pet. Sci. Eng. 2018, 171, 784–793. [Google Scholar] [CrossRef]
- Wang, T.; Yu, L.; Xiu, J.; Ma, Y.; Lin, W.; Ma, T.; Wang, X.; Wang, L. A mathematical model for microbial enhanced oil recovery using biopolymer-producing microorganism. Fuel 2018, 216, 589–595. [Google Scholar] [CrossRef]
- Sugai, Y.; Owaki, Y.; Sasaki, K. Simulation study on reservoir souring induced by injection of reservoir brine containing sulfate-reducing bacteria. Sustainability 2020, 12, 4603. [Google Scholar] [CrossRef]
- Sivasankar, P.; Govindarajan, S.K. Modelling the Coupled Effects of Temperature, Injection Rate and Microbial Kinetic Parameters on Oil Recovery by Microbial Flooding. In Proceedings of the Society of Petroleum Engineers—SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 25–28 April 2016. [Google Scholar]
- De Souza, P.M.; Goulart, F.R.D.V.; Marques, J.M.; Bizzo, H.R.; Blank, A.F.; Groposo, C.; De Sousa, M.P.; Vólaro, V.; Alviano, C.S.; Moreno, D.S.A.; et al. Growth inhibition of sulfate-reducing bacteria in produced water from the petroleum industry using essential oils. Molecules 2017, 22, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, H.; Jia, W.; Ren, S.; Wang, J. Application of Bacillus subtilis strain for microbial-enhanced oil recovery. Int. J. Green Energy 2019, 16, 530–539. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Nesterov, I.; Shapiro, A.A. Microbial enhanced oil recovery—A modeling study of the potential of spore-forming bacteria. Comput. Geosci. 2016, 20, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Henni, A. Oman Champions EOR in the Middle East. J. Pet. Technol. 2014, 66, 86–89. [Google Scholar] [CrossRef]
- Volk, H.; Liu, K. 3° Oil Recovery: Experiences and Economics of Microbially Enhanced Oil Recovery (MEOR). In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2739–2751. [Google Scholar]
- Guo, H.; Li, Y.; Yiran, Z.; Wang, F.; Wang, Y.; Yu, Z.; Haicheng, S.; Yuanyuan, G.; Chuyi, J.; Xian, G. Progress of Microbial Enhanced Oil Recovery in China. In Proceedings of the Society of Petroleum Engineers—SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015; pp. 1422–1437. [Google Scholar]
- Speight, J.; El-Gendy, N.S. Introduction to Petroleum Biotechnology. In Introduction to Petroleum Biotechnology, 1st ed.; Speight, J.G., El-Gendy, N.S., Eds.; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- Liu, Q.; Niu, J.; Yu, Y.; Wang, C.; Lu, S.; Zhang, S.; Lv, J.; Peng, B. Production, characterization and application of biosurfactant produced by Bacillus licheniformis L20 for microbial enhanced oil recovery. J. Clean. Prod. 2021, 307, 127193. [Google Scholar] [CrossRef]
- Ansah, E.O.; Vo Thanh, H.; Sugai, Y.; Nguele, R.; Sasaki, K. Microbe-induced fluid viscosity variation: Field-scale simulation, sensitivity and geological uncertainty. J. Pet. Explor. Prod. Technol. 2020, 10, 1983–2003. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Wei, X. Oil Recovery: Experiences and Economics of Microbially Enhanced Oil Recovery (MEOR). In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Lee, S.Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–18. [Google Scholar]
- Astuti, D.I.; Purwasena, I.A.; Putri, R.E.; Amaniyah, M.; Sugai, Y. Screening and characterization of biosurfactant produced by Pseudoxanthomonas sp. G3 and its applicability for enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2019, 9, 2279–2289. [Google Scholar] [CrossRef] [Green Version]


| Metabolic Bioproducts | Preferred Reservoir Type | Microbes | Role in Oil Recovery |
|---|---|---|---|
| Cell biomass | Stratified reservoir swith different permeable zones | Bacillus licheniformis, Leuconostoc mesenteroides, Xanthomonas campestris | Selective and as non-selective plugging, viscosity relaxation, wettability alteration, oil degradation, and emulsification. |
| Biosurfactants | Mature water flooded reservoirs, sandstone or carbonate reservoirs with less than 50 °C (moderate temperatures) | Acinetobacter calcoacetiens, Arthrobacter paraffeninues, Bacillus sp., Clostridium sp., Pseudomonas sp. | Emulsification, IFT reduction, viscosity relaxation, and wettability alteration. |
| Biopolymers | Stratified reservoirs with different permeable zones | Bacillus polymyxa, Brevibacterium viscogenes, Leuconostoc mesenteroides Xanthomonas campestris | Injectivity profile and viscosity modification, mobility checking, and decrease in permeability in water-swept regions. |
| Biosolvents | Highly oil-wet, water flooded reservoirs | Clostridium sp., Enterobacter aerogens, Klebsiella sp., Zymomonas mobilis | Increase in permeability and oil viscosity relaxation with long-chain hydrocarbon removal from pore throats. |
| Biogases | Heavy oil reservoirs | Clostridium sp., Enterobacter aerogens, Methanobacterium sp. | Oil swelling, IFT, and viscosity relaxation increase pressure and permeability. |
| Bioacids | Carbonate or Carbonaceous reservoirs | Clostridium sp., Enterobacter sp., Mixed acidogens | Permeability increases, emulsification, CO2 production, and dissolves minerals present in the reservoirs. |
| Microbe | BS Type | ST (mN/m) | CMC (mg/L) | AOR (%) | E24 (%) | IFT (mN/m) | Temp (°C) | P (atm) | API G (°) | OV (cP) | Por | PV | Sub | FR (ml/min) | BS Yield | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Various Pseudomonas species | ||||||||||||||||
| Pseudomonas putida CB-100 | Rhamnolipid | 47.5 ± 1.32 | 430 | - | 20 | - | 37 ± 2.0 | - | - | - | - | - | Phenanthrene | 0.8 | 27 | [82] |
| Pseudomonas nitroreducens | Rhamnolipid | 37 | 28 | - | - | - | 30 | - | - | - | - | - | Glucose | - | 5.46 | [83] |
| Pseudomonas otitidis P4 | Glycolipid | 33.4 | 40 | - | 68.7 | - | 40 | - | - | - | - | - | 2% Sodium acetate | 100 | 2.75 ± 0.07 | [84] |
| Pseudomonas DYNA270 | Rhamnolipid | 22 | 20 | - | - | 0.005 | 120 | 1.22 | - | - | - | - | 4% Mannitol | - | 5.32 | [85] |
| Pseudomonas sp. TMB2 | Rhamnolipid | 33.4 | 120 | 16.7 | 78.6 | 0.8 | 30 | 40.7 | 34.2 | 12.43 | 22, 21.3 19.7 | 21.5, 20.81, 19.25 | 2% Glucose | 0.8 | 2.8 | [86] |
| Pseudomonas balearica Z8 | Rhamnolipid | 41 | 90 | - | 44 | - | 40 | - | - | - | - | - | Oily sludge waste | 1 | - | [87] |
| (b) Various Pseudomonas aeruginosa strains | ||||||||||||||||
| Pseudomonas aeruginosa OBP1 | Rhamnolipid | 31.1 | 45 | - | 82 | 1.5 | 25 ± 1 | - | - | - | - | - | 2% n-hexadecane | 0.4 | 4.57 | [88] |
| Pseudomonas aeruginosa WJ-1 | Rhamnolipid | 24.5 | 14 | 23.02 | 95 | - | 37 | - | - | - | 36.77 | - | 5% Waste Sun flower oil | - | 50.2 | [89] |
| Pseudomonas aeruginosa MM1011 | Rhamnolipid | 26 | 120 | - | 80 | 2 | 25 | 0.05 | 19 | - | 23 | 12.6 | Sun flower oil | 0.14 | 0.7 | [90] |
| Pseudomonas aeruginosa JBK1 | Rhamnolipid | 33.7 | 540 | 10.8 | 62 | 4.7 (d) 3.4 (k) | 90 | - | - | - | - | - | 3% Raw Bio-glycerol | 0.5 | 3.9 | [91] |
| Pseudomonas aeruginosa TMN | Rhamnolipid | 34 | 18.75 | - | 46 | - | 25 to 37 | - | - | - | - | - | 40 g/L Glucose or Glycerol | - | 0.30.25 | [92] |
| Pseudomonas aeruginosa KVD-HR42 | Rhamnolipid | 30.14 | 83 | - | - | 100 | 37 | - | - | - | - | - | 23.85 g/L Karanja oil | - | 5.90 ± 2.1 | [93] |
| Pseudomonas aeruginosa ATCC-10145 | Rhamnolipid | 31.9 | 80 | - | 97.4 | - | 25 to 30 | - | - | - | - | - | Soy molasses | 0.5 | 11.70 | [94] |
| Pseudomonas aeruginosa | Rhamnolipid | - | 55.87 | - | 58.43 ± 0.3 | 1.17 ± 0.01 | 55 | - | - | - | - | - | Kitchen waste oil | 0.5 | 2.47 ± 0.03 | [95] |
| Pseudomonas aeruginosa SNP0614 | Lipopeptide | 25.4 | 45 | - | 90 | - | 37 | - | - | - | - | - | Crude oil | - | - | [96] |
| Pseudomonas aeruginosa PBS | Rhamnolipid | 23.76 | - | 56.18 ± 1.59 | 50 to 60 | - | 100 | - | - | - | - | 29.83 ±0.3 | 2.17% Sodium citrate | 0.4 | 2.65 | [97] |
| Pseudomonas aeruginosa DQ3 | Rhamnolipid | 33.8 | - | 5.22 | 58 | - | 42 | - | - | 10 | 15.26 | 93 | - | 0.2 | - | [98] |
| Pseudomonas aeruginosa | Rhamnolipid | - | 9.25 ± 0.27 | 35.26 | 69 | 127 | 30 | - | 21.9 | - | 20.49 ± 0.69 | - | 125 g/L Glycerol | 1 | 0.877 | [7] |
| Pseudomonas aeruginosa HAK01 | Rhamnolipid | 28.1 | 120 | 43 | 60 | 2.52 | 40 to 121 | 1 | 19.5 | 1.8 | - | 0.4 | 20 g/L Sun flower oil | - | 2.07 | [99] |
| Pseudomonas aeruginosa YM4 | Di-Rhamnolipid | 28 | 50, 60 | - | 61, 57 | <1 | 25 | - | - | - | - | - | Glycerol Soybean Oil | - | 79.7 ± 4.083.5 ± 4.6 | [80] |
| Pseudomonas aeruginosa KT1115 | Rhamnolipid (Mono as well as di) | 28 | 167, 8 | - | 41.4, 52.1 | <1 | 20 to 80 | - | - | - | - | - | Rapeseed oil | - | 44.39 | [100] |
| Pseudomonas aeruginosa SG | Rhamnolipid | 28.1 | 60 | - | 76.1 | 2.09 | 4 to 100 | - | - | 118.9 | - | - | Soybean oil | 0.6 | 10.32 | [101] |
| (c) Other bacterial strains | ||||||||||||||||
| Bacillus mojavensis JF-2 | Lipopeptide | - | 10 to 40 | 14 | - | 1.0 | 37 | - | 32 | 6.0 | 16.7 | 29 | DNA with medium E | 0.515 | - | [102] |
| Fusarium sp. BS-8 | Glycolipid | 32 | - | 46 | 71 | - | 30 | - | - | - | - | 400 | 9% Sucrose | 36 | 5.25 | [103] |
| Bacillus salmalaya 139SI | Cyclic lipopeptide | 27 | 5% | - | 65 ± 1.1 | - | 36 | - | - | - | - | 10 to 11 | 1% Sun flower oil | - | 1.9 | [104] |
| Candida tropicalis MTCC230 | Lipopeptide, Surfactin | 32 | 32.5 | 39.80 | 62 | - | 30 to 90 | - | - | - | - | 31 | 0.5% Glucose + 1.5% Petrol | 1 | - | [62] |
| Bacillus subtilis ANSKLAB03 | Surfactin | 38 | 0.01 | - | 82 | - | 40 | - | - | - | - | - | 2% Sucrose | - | 0.324 | [105] |
| Bacillus atrophaeus L193 | Fengycin, Surfactin, Bacillomycin Iturin | 33 | 9.38 | - | 51.53 ± 1.39 | - | 28 | - | - | - | - | - | 1% Colloidal chitin | - | 2.04 | [94] |
| Streptomyces sp. DPUA1559 | Glycoproteic Surfactant | 25.34 | 10,000 | - | 41, 95 | - | 4 to 80 | - | - | - | - | - | 1% Residual frying soybean oil, Residual motor oil | - | 1.74 | [106] |
| Serratia marcescens UCP 1549 | Polymeric compounds | 25.92 | 1.5% | - | - | - | 28 | - | - | - | - | - | 0.2% Lactose 5% Corn oil | - | - | [107] |
| Bacillus subtilis ICF-PC | Surfactin | 27 | 100 | - | 65.74 | - | 30 to 45 | - | - | - | - | - | HCCL + Glucose | - | 3.95 | [108] |
| Bacillus licheniformis DS1 | Lipopeptide | - | 157.5 | 5.4 | 65.19 | 12.0 | 40 | 1 | - | - | 12.84 | 14.84 | 2% Crude oil | 0.2 | - | [109] |
| Rhodococcus Erythropolis HX-2 | NK | 28.89 | 100 | - | 90 | - | 20 to 100 | - | - | - | - | - | 2% Petroleum | - | 5.0 | [110] |
| Saccharomyces cerevisiae URM 6670 | Glycolipid | 26.64 ± 0.06 | - | - | - | 9.12 ± 0.04 | 40 to 400 | - | - | - | - | - | 1% Waste Soybean oil | 50 | 5.84 ± 0.17 | [111] |
| Candida bombicola URM 3718 | Sophorolipid | 30.79 ± 0.04 | 0.5 | - | 66.77 ± 0.15 | 0.73 ± 0.05 | 28 to 50 | 1 | - | - | - | - | - | - | 2 ± 1.02 | [112] |
| Parameters | San Andres | Queen Sand | Tupungato-Refugio | Huabei | Xinjiang |
|---|---|---|---|---|---|
| Location and discovery | Hockley County, Texas, USA (in 1945) | Ector County, Texas, USA (in 1984) | Tupungato County, Medoza, Argentina (in 1930) | No. 3 plant, Renqiu, China (in 1987) | No. 1 plant, Xinjiang Uygur Autonomous Region, China (in 1991) |
| Comprises | 30 producer and 15 injection wells | 18 producer and 18 injector wells | Three producer wells (Victor Oscuro formation) | Seven producer wells | 10 producer wells |
| Lithology | Fractured dolomite | Porous grey sandstone and siltstone interbedded with anhydrite and salt | Fractured sandstone | Sandstone | Sandstone |
| Depth (ft.) | 4745 | 4450 | 5700 | 6900 | 4900 |
| Porosity (fraction) | 0.079 | 0.182 | 0.18 | 0.232 | 0.30 |
| Permeability [Range] (mD) | 1.7 [0.10–10.0] | 13.0 [0.6–300] | 300 [150–1500] | 240 [20–640] | 70 [0.2–440] |
| Temperature (°F) | 115 | 110 | 160 | 180 | 110 |
| Oil Density (API) | 29 | 30 | 28 | 28 | 29.6 |
| Viscosity (cp) | 4.5 | 11 | 9 | 14 | 50 |
| Pressure (psi) | 1000 | - | 50 | 732 | - |
| Water cut (%) | 91 | 74 | 63.5 to 62 | 60 | 64 to 54 |
| Drive Mechanism | Solution Gas (After 1967 water flooding was initiated) | Solution Gas replaced by water flooding in 1984 | Gas drive, water flooding and water drive combination | Scattered water flooding | Dispersed water flooding |
| Treatment | 19 months of microbe treating | 24 months of Microbe treating | 14 months of microbe treating | 12 months of microbe treating (each well thrice) | 6 months of microbe treating (each well thrice) |
| Shut-in period | 3 days then overnight shut-in for 3 months | 3 days; later, batch treating took 6 to 12 h | 48 h on two wells and 24 h on the next | Not shut | Not shut |
| Spacing | 25 acres | 30 acres | 42 acres | - | - |
| Avg. OP per well | 14 bpd | 42 bpd | 90 bpd | - | - |
| % Decline (%/year) | Initially 6.5, after MEOR, it flattened to 0.6 | Initially 39, after MEOR, it flattened for a few months then resumed at 31 | Initially 7.1, after MEOR, it inclined to 7.3 | Inclined and flattened at 150 bpd | Sustained a rate of about 300 bpd |
| OP Rate | Increased by 10% (40 bpd) | Increased by 47% | Increased by 29% (60 bpd) | Increased by 552% | Increased by 36% (80 bpd) |
| BL Production | OP of 440 bpd is 10% over BL | OP of 1000 bpd is 43% over BL | OP of 270 bpd is 29% over BL | OP of 150 bpd is 552% above BL | OP of 300 bpd is 36% over BL |
| IP | 17,000 bbl, i.e., 7% over the BL | 240,000 bbl, i.e., 34% above BL | 19,000 bbl, i.e., 19% above BL | 41,000 bbl, i.e., 216% over BL | 14,000 bbl, i.e., 43% over BL |
| OIP after WF and MEOR (bbl/ac-ft.) | 205 and 199 | 691 and 660 | 509 and 442 | Due to short duration of the microbe treatment, no samples were available for the field treated oil. | Due to the short duration of the microbe treatment, no samples were available for the field treated oil. |
| ROS under WF | Drops from 35% to 34.1% | Falls from 51.4% to 49.1% | Drops from 38.3% to 33.3% | ||
| Improvement with MEOR (%) | 2.5 | 4.5 | 13 |
| Parameters | Institute of Reservoir Studies | U.S. Department of Energy | China National Petroleum Company |
|---|---|---|---|
| Location | Ahmedabad, India | Washington, DC, U.S. | Beijing, China |
| Lithology | Sandstone | Sandstone | Sandstone |
| Depth (ft.) | 8000 | 10,000 | - |
| Porosity (fraction) | - | - | 0.17 to 0.25 |
| Permeability (mD) | Less than 50 | Less than 100 | More than 150 |
| Temperature (°F) | 194 | 160 | 86 to 140 |
| Oil Density (API) | 20 | 18 to 40 | - |
| Brine Salinity (g/L) | More than 10 | More than 10 | More than 100 |
| pH | 6 to 9 | - | - |
| Viscosity (cp) | 20 | - | 30 to 150 |
| Residual Oil Saturation (%) | Less than 25 | Less than 25 | - |
| Pressure (psi) | Less than 4267 | - | - |
| Water cut (%) | 30 to 90 | - | 60 to 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quraishi, M.; Bhatia, S.K.; Pandit, S.; Gupta, P.K.; Rangarajan, V.; Lahiri, D.; Varjani, S.; Mehariya, S.; Yang, Y.-H. Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR). Energies 2021, 14, 4684. https://doi.org/10.3390/en14154684
Quraishi M, Bhatia SK, Pandit S, Gupta PK, Rangarajan V, Lahiri D, Varjani S, Mehariya S, Yang Y-H. Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR). Energies. 2021; 14(15):4684. https://doi.org/10.3390/en14154684
Chicago/Turabian StyleQuraishi, Marzuqa, Shashi Kant Bhatia, Soumya Pandit, Piyush Kumar Gupta, Vivek Rangarajan, Dibyajit Lahiri, Sunita Varjani, Sanjeet Mehariya, and Yung-Hun Yang. 2021. "Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR)" Energies 14, no. 15: 4684. https://doi.org/10.3390/en14154684
APA StyleQuraishi, M., Bhatia, S. K., Pandit, S., Gupta, P. K., Rangarajan, V., Lahiri, D., Varjani, S., Mehariya, S., & Yang, Y.-H. (2021). Exploiting Microbes in the Petroleum Field: Analyzing the Credibility of Microbial Enhanced Oil Recovery (MEOR). Energies, 14(15), 4684. https://doi.org/10.3390/en14154684












