An Insight to the Degradation Behaviour of the Parallel Connected Lithium-Ion Battery Cells
Abstract
:1. Introduction
2. Experimental Details
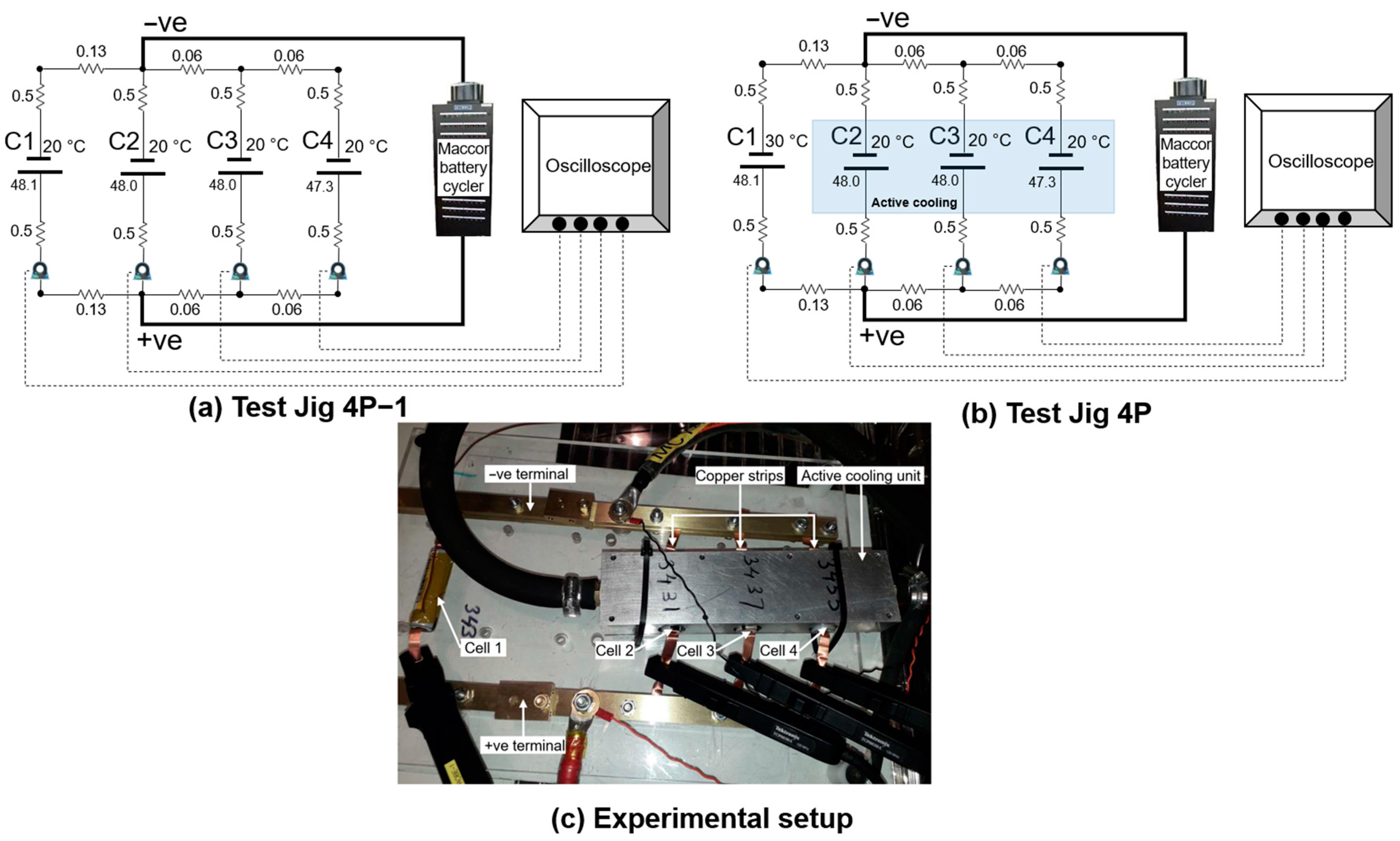
2.1. Battery Cells and Experimental Setup
2.2. Validation of Experimental Jigs
2.3. Ageing Test
2.3.1. Individual Cell Snapshot Testing
2.3.2. Module Level Snapshot Testing
2.3.3. Cycling of 4P Module
3. Results and Discussion
3.1. Validation of Experimental Jig
3.1.1. Testing of Individual Cells
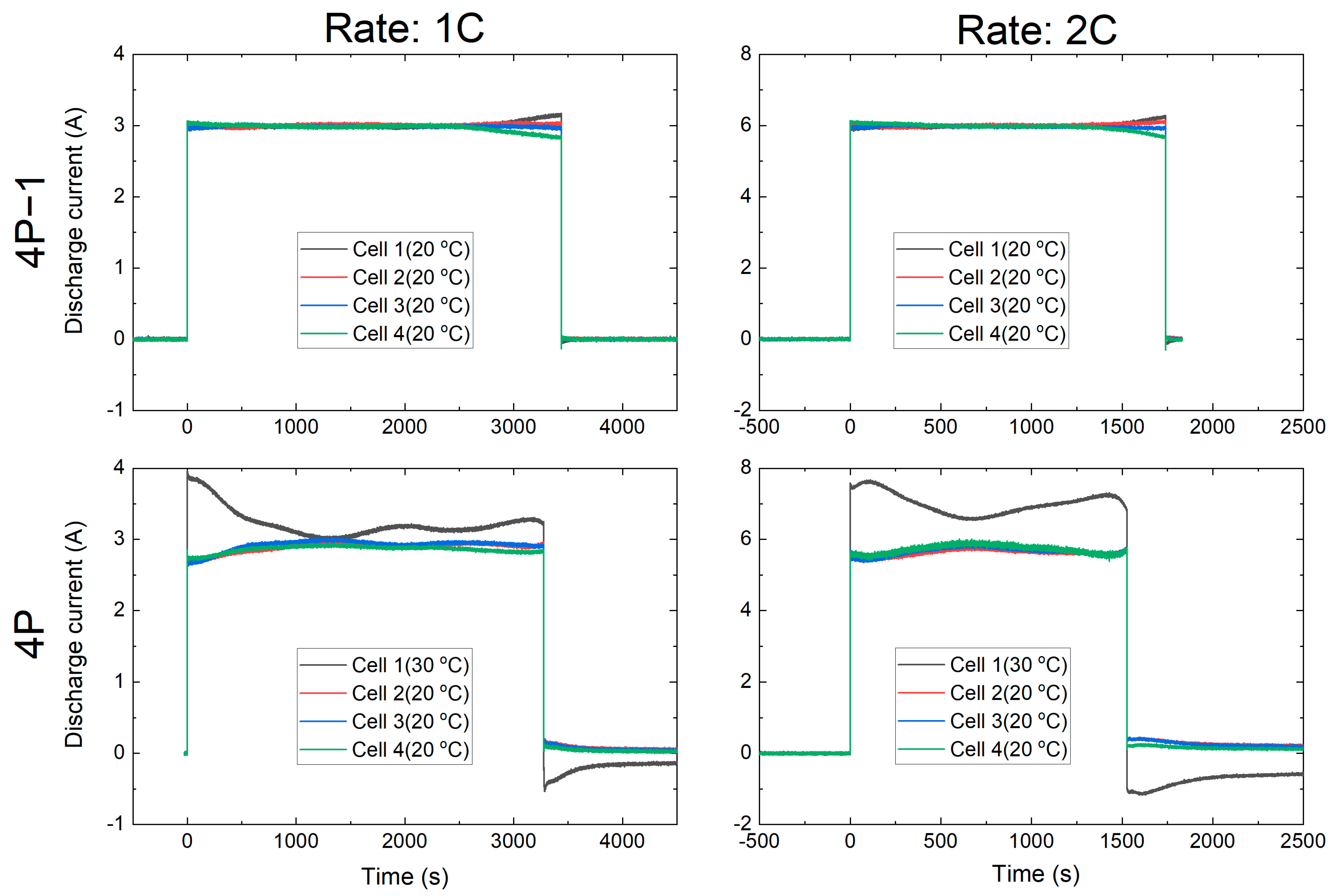
3.1.2. Current Distribution Behaviour with and without Temperature Gradient
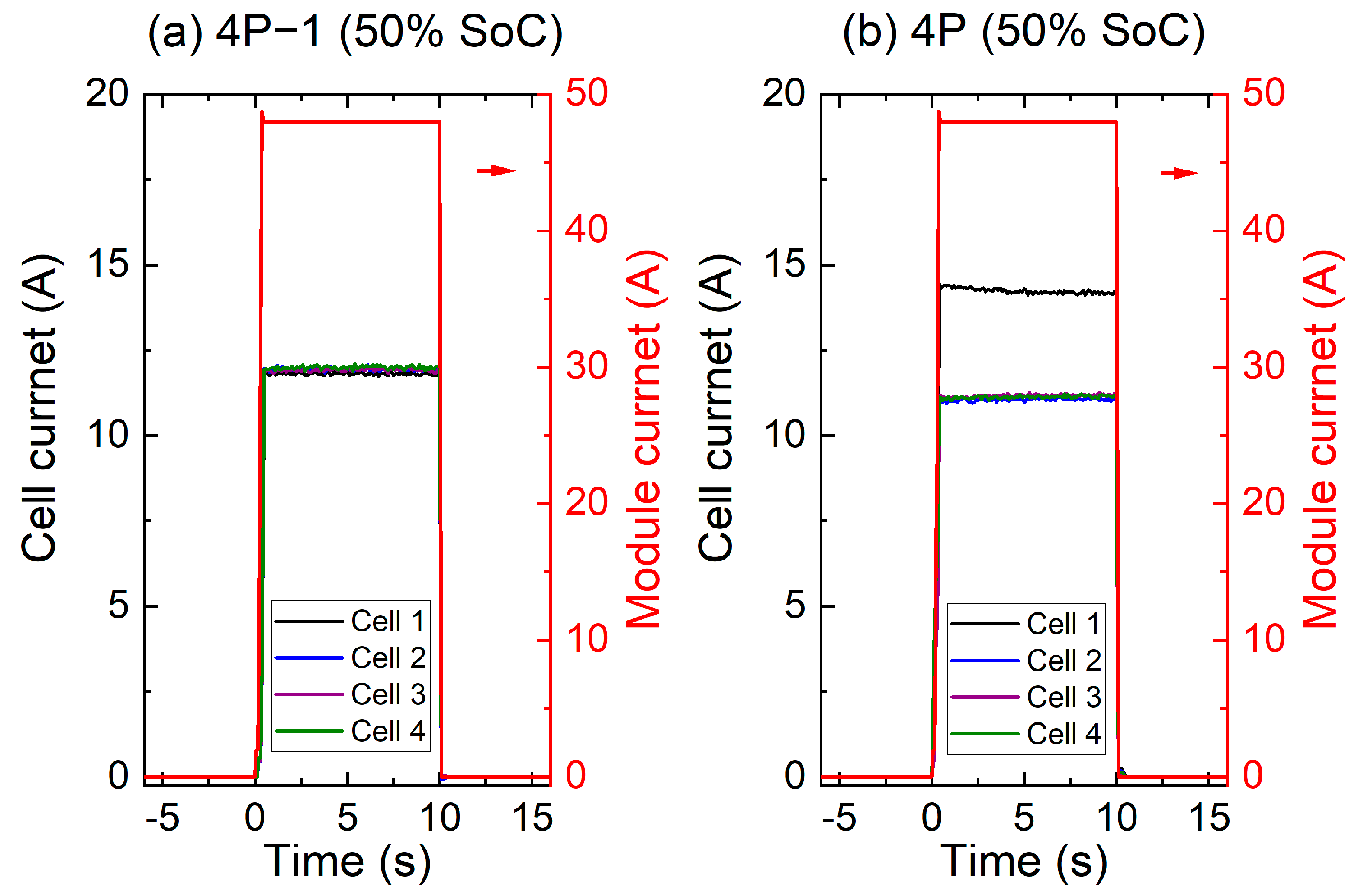
3.1.3. Current Distribution Behaviour during Pulse Power Test
3.2. Ageing of Cells and Module
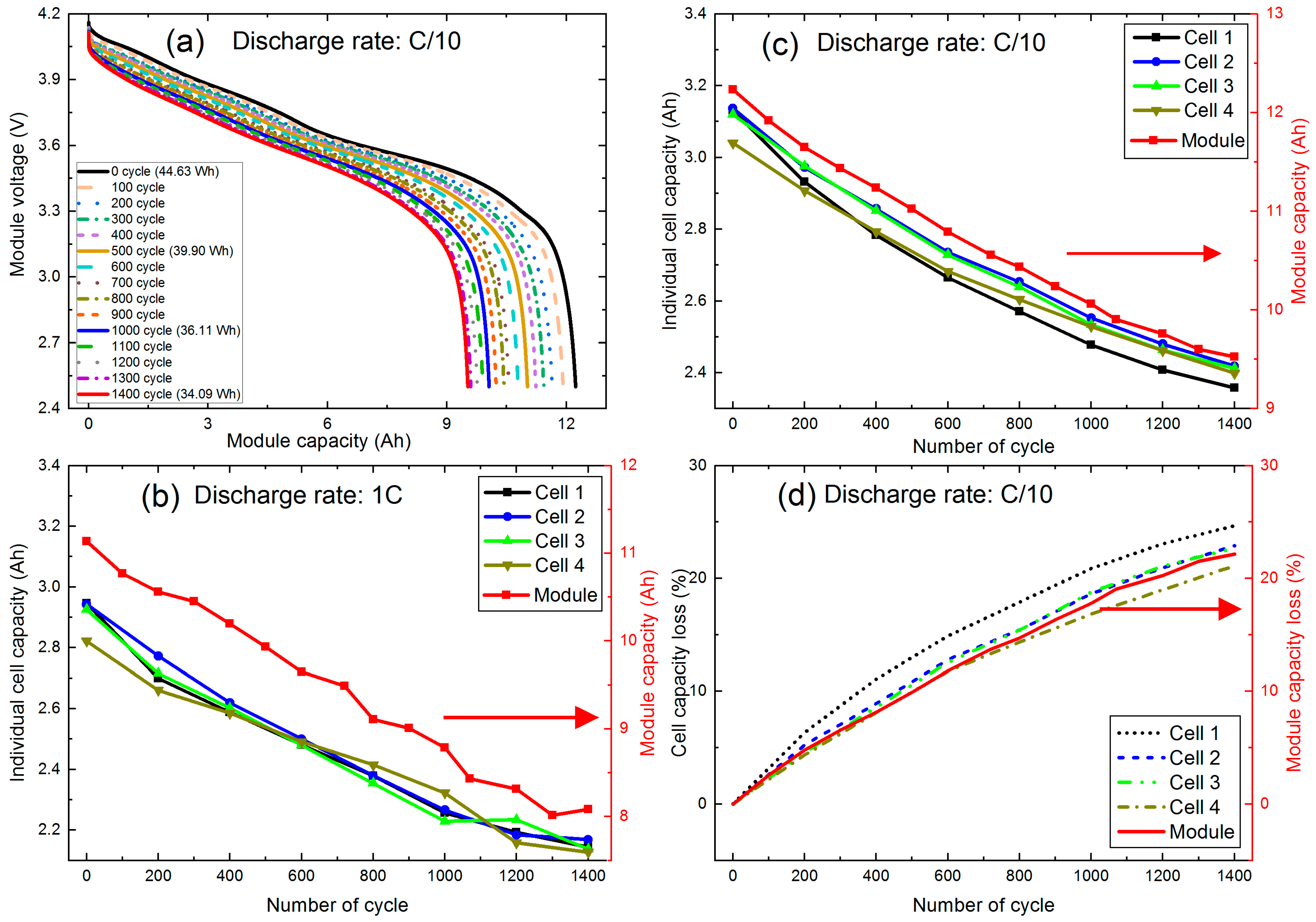
3.2.1. Capacity Degradation of Individual Cells and Module
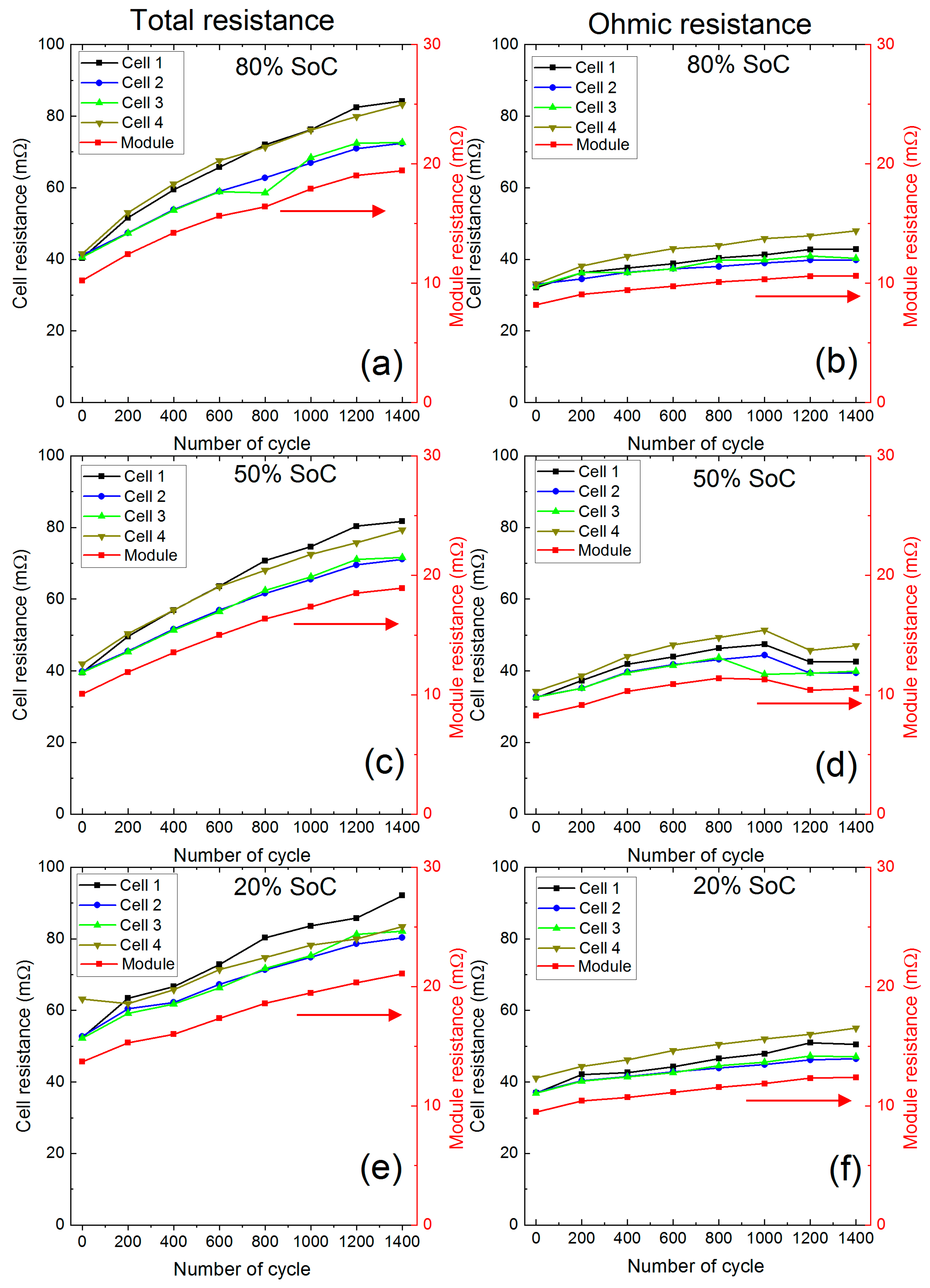
3.2.2. Cell and Module Resistance
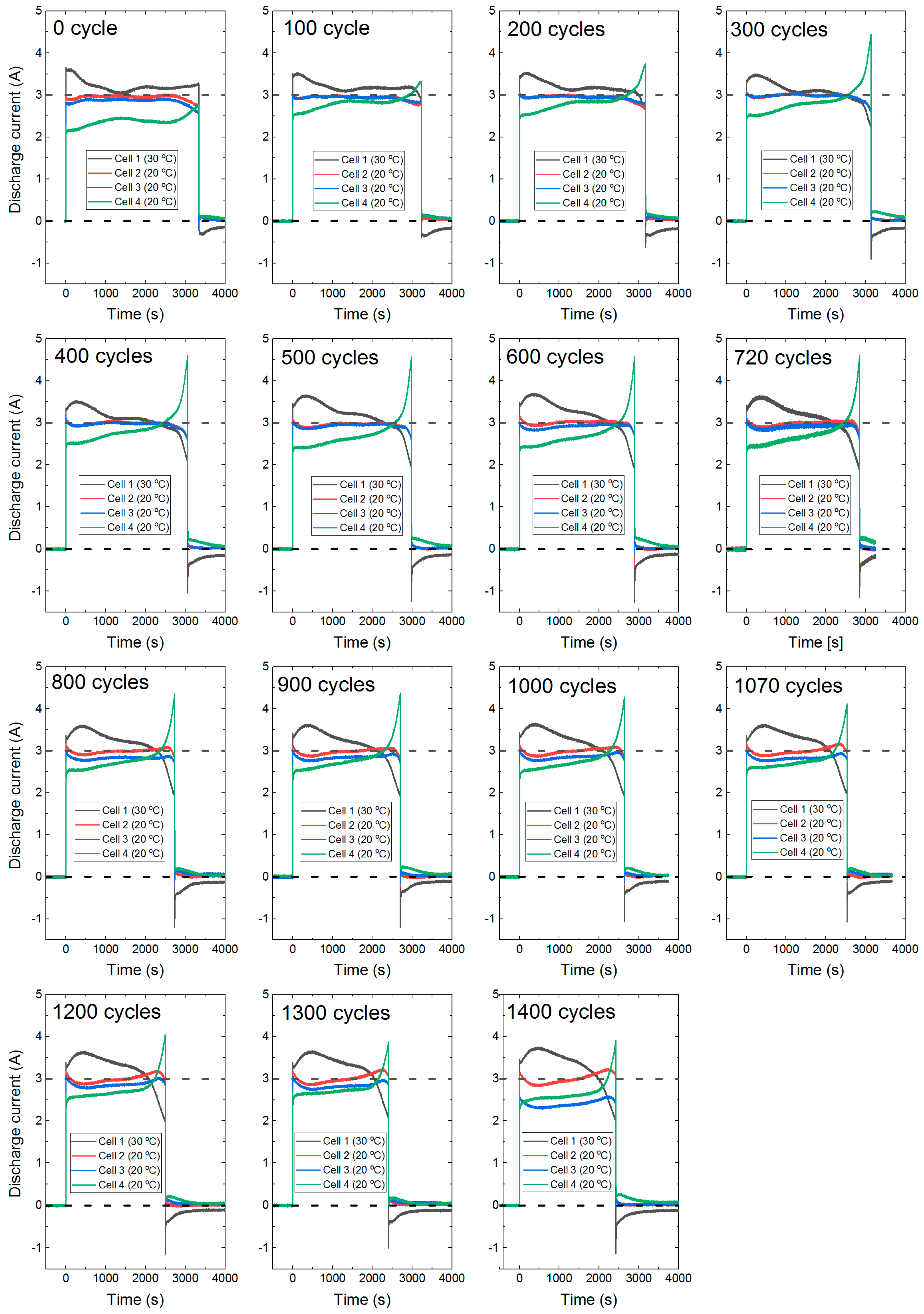
3.3. Current and Energy Distribution in Cycling
- (1)
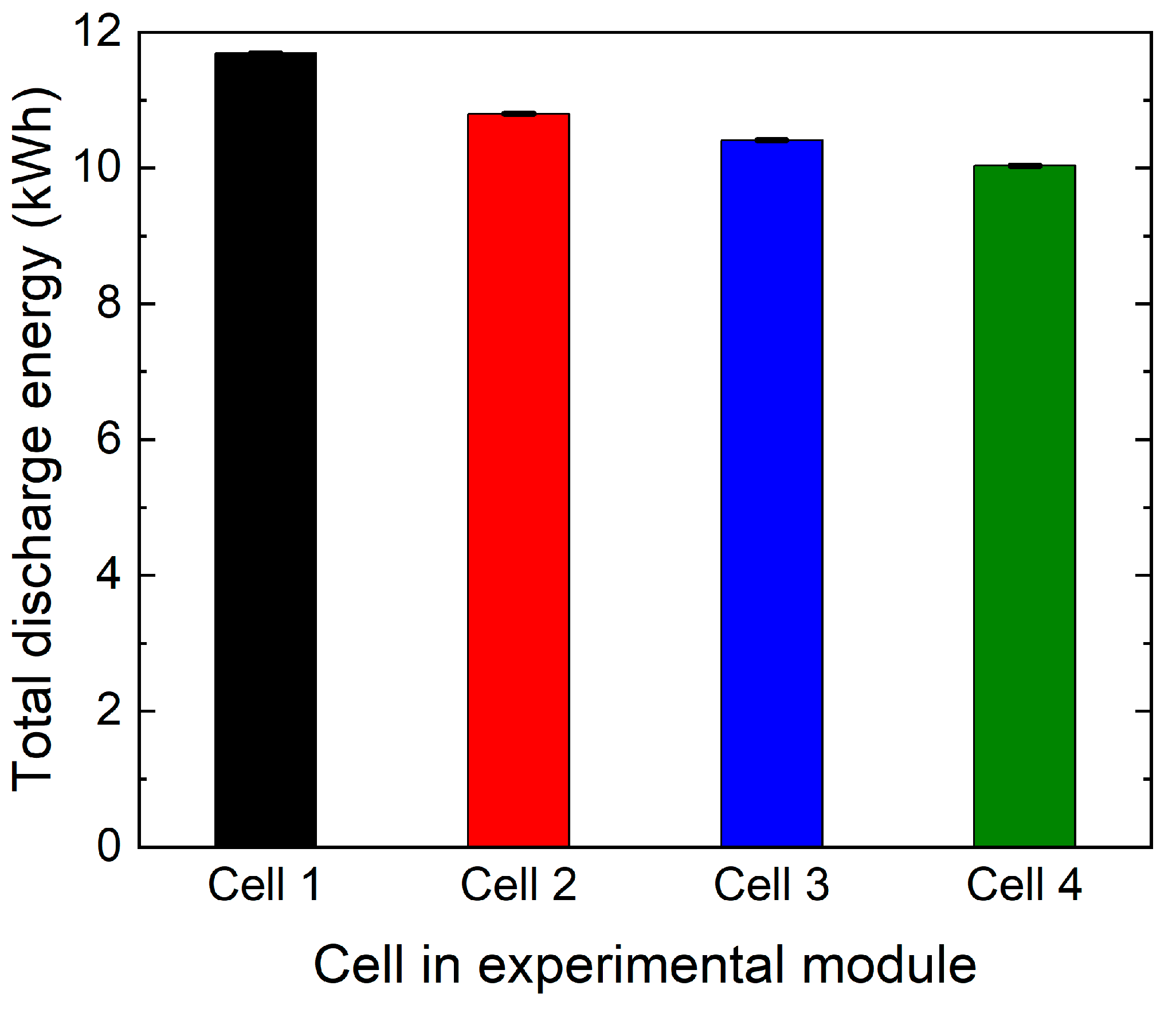
- As C1 discharged at a higher average current, it is likely to have gone through higher energy throughput. A comparison in total discharge energy in each cell over the 1400 cycles is shown in Figure 10. Cell C1 had 8.9% higher than average energy throughput. Wang et al. reported a power-law relationship between energy throughput and degradation between 15 °C to 60 °C [26]. With every watt-hour of energy throughput, active lithium will be lost in SEI layer growth and other side reactions often referred to as loss of active lithium (LLI) [1]. This is likely the key source of higher capacity degradation in C1. For the same reason, C4 experiences the lowest capacity degradation, which has 6.5% lower than the average energy throughput.
- (2)
- Higher charge-discharge current within a lithium-ion battery cell activates different ageing mechanisms such as particle cracking, loss of electrical contact, structural disordering, graphite exfoliation [1]. All these mechanisms will lead to loss of active materials (LAM) and thus internal resistance growth. Cell C1 experiences around 3.5 A discharge current during cycling, which was 0.5 A higher than the average. During charge, C1 also experiences 8.2% higher than the average current over the entire charge duration. While at the beginning of charging, the C1 current is even higher than 70% of the average current, which reduces as the charge progress. The charging protocol of this experiment used the highest charge rate of C/3 for cycling. This led to charging the C1 at a much higher than rated current continuously and for a short period in every charge step. Use of high current, during both charge and discharge, will activate most of the degradation mechanisms related to LAM [1]. This explains higher resistance growth in tandem with higher capacity degradation in cell C1. During charging, 70% higher current than the average value will accelerate lithium plating [27], which accelerates degradation and impose safety risk.
- (3)
- For cell C4, although the average discharge current was lower, the current increased dramatically towards the last part of the discharge. From 200 cycles onwards, at the end of discharge, the current in C4 was much higher than the cell C1. The maximum end of discharge current in C4 was 4.2 A at the 660th cycle, which decreased to 3.7 A at 1300th cycle. This most likely will activate degradation mechanisms related to LAM [1]. Therefore, C4 experienced higher than average resistance growth, but a lower capacity degradation.
- (4)
- The change of current amplitude in cells C1 and C4 can provide an interesting insight into the degradation. C1 experienced the highest current amplitude at the first part of the discharge throughout the experiment. From the 100 cycle onwards, the C1 current value initially increased (visible hump in Figure 9) then gradually decreased till the last part of the discharge. The hump increased and become more pronounced as the experiment progressed to 1400 cycles. This is likely to be because of the thermal management of cell C1. During cycling, C1 was at 30 °C within the thermal chamber, whereas C2, C3 and C4 were at 20 °C actively cooled using a liquid cooling system. When discharge started C1 heated up at a higher rate and poorly cooled compared to C2, C3 and C4. This led to further reduction of C1 internal resistance and higher current. However, with reduced resistance the heat generation rate is also reduced, and at a point, this mechanism stabilises, when the peak in C1 current can be observed.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Somerville, L. Post-Mortem Analysis of Lithium-Ion Cells after Accelerated Lifetime Testing. Ph.D. Thesis, University of Warwick, Coventry, UK, 2017. [Google Scholar]
- Rothgang, S.; Baumhöfer, T.; van Hoek, H.; Lange, T.; De Doncker, R.W.; Sauer, D.U. Modular battery design for reliable, flexible and multi-technology energy storage systems. Appl. Energy 2015, 137, 931–937. [Google Scholar] [CrossRef]
- Bruen, T.; Marco, J. Modelling and experimental evaluation of parallel connected lithium ion cells for an electric vehicle battery system. J. Power Sources 2016, 310, 91–101. [Google Scholar] [CrossRef]
- Brand, M.J.; Hofmann, M.H.; Steinhardt, M.; Schuster, S.F.; Jossen, A. Current distribution within parallel-connected battery cells. J. Power Sources 2016, 334, 202–212. [Google Scholar] [CrossRef]
- Gogoana, R.; Pinson, M.B.; Bazant, M.Z.; Sarma, S.E. Internal resistance matching for parallel-connected lithium-ion cells and impacts on battery pack cycle life. J. Power Sources 2014, 252, 8–13. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Marco, J.; Jennings, P. Combined electrical and electrochemical-thermal model of parallel connected large format pouch cells. J. Energy Storage 2019, 22, 194–207. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Arias, S.; Krishna, M.; Worwood, D.; Barai, A.; Widanalage, D.; Marco, J. Quantifying cell-to-cell variations of a parallel battery module for different pack configurations. Appl. Energy 2021, 282, 115859. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Czyrka, K.; Brand, M.J.; Steinhardt, M.; Noel, A.; Spingler, F.B.; Jossen, A. Dynamics of current distribution within battery cells connected in parallel. J. Energy Storage 2018, 20, 120–133. [Google Scholar] [CrossRef]
- Fill, A.; Koch, S.; Pott, A.; Birke, K.-P. Current distribution of parallel-connected cells in dependence of cell resistance, capacity and number of parallel cells. J. Power Sources 2018, 407, 147–152. [Google Scholar] [CrossRef]
- Luca, R.; Whiteley, M.; Neville, T.; Tranter, T.; Weaving, J.; Marco, J.; Shearing, P.R.; Brett, D.J.L. Current Imbalance in Parallel Battery Strings Measured Using a Hall-Effect Sensor Array. Energy Technol. 2021, 9, 2001014. [Google Scholar] [CrossRef]
- Liu, X.; Ai, W.; Naylor Marlow, M.; Patel, Y.; Wu, B. The effect of cell-to-cell variations and thermal gradients on the performance and degradation of lithium-ion battery packs. Appl. Energy 2019, 248, 489–499. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Lin, S.; Bai, F.; Tanveer, W.H.; Cha, S.; Wu, X.; Feng, W. Nonuniform current distribution within parallel-connected batteries. Int. J. Energy Res. 2018, 42, 2835–2844. [Google Scholar] [CrossRef]
- Grün, T.; Stella, K.; Wollersheim, O. Influence of circuit design on load distribution and performance of parallel-connected Lithium ion cells for photovoltaic home storage systems. J. Energy Storage 2018, 17, 367–382. [Google Scholar] [CrossRef]
- Hunt, I.; Zhang, T.; Patel, Y.; Marinescu, M.; Purkayastha, R.; Kovacik, P.; Walus, S.; Swiatek, A.; Offer, G.J. The Effect of Current Inhomogeneity on the Performance and Degradation of Li-S Batteries. J. Electrochem. Soc. 2017, 165, A6073–A6080. [Google Scholar] [CrossRef] [Green Version]
- Dubarry, M.; Devie, A.; Liaw, B.Y. Cell-balancing currents in parallel strings of a battery system. J. Power Sources 2016, 321, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Xiong, R.; Mi, C.C. Study of the characteristics of battery packs in electric vehicles with parallel-connected lithium-ion battery cells. IEEE Trans. Ind. Appl. 2015, 51, 1872–1879. [Google Scholar] [CrossRef]
- Jocher, P.; Steinhardt, M.; Ludwig, S.; Schindler, M.; Martin, J.; Jossen, A. A novel measurement technique for parallel-connected lithium-ion cells with controllable interconnection resistance. J. Power Sources 2021, 503, 230030. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. A Mathematical Model of Stress Generation and Fracture in Lithium Manganese Oxide. J. Electrochem. Soc. 2006, 153, A1019–A1030. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The effects of elastic stiffening on the evolution of the stress field within a spherical electrode particle of lithium-ion batteries. Int. J. Appl. Mech. 2013, 5, 1350040. [Google Scholar] [CrossRef]
- Sieg, J.; Bandlow, J.; Mitsch, T.; Dragicevic, D.; Materna, T.; Spier, B.; Witzenhausen, H.; Ecker, M.; Sauer, D.U. Fast charging of an electric vehicle lithium-ion battery at the limit of the lithium deposition process. J. Power Sources 2019, 427, 260–270. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Barai, A.; Uddin, K.; Widanalage, W.D.; McGordon, A.; Jennings, P. The effect of average cycling current on total energy of lithium-ion batteries for electric vehicles. J. Power Sources 2016, 303, 81–85. [Google Scholar] [CrossRef]
- Uddin, K.; Moore, A.D.; Barai, A.; Marco, J. The effects of high frequency current ripple on electric vehicle battery performance. Appl. Energy 2016, 178, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model for graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.Y.; García, V.M.; Viera, J.C.; González, M. Operando lithium plating quantification and early detection of a commercial LiFePO 4 cell cycled under dynamic driving schedule. J. Power Sources 2017, 356, 36–46. [Google Scholar] [CrossRef] [Green Version]



| Jig | SoC % | Module Resistance (mΩ) | Calculated Module Resistance (mΩ) | Cell 1 (mΩ) | Cell 2 (mΩ) | Cell 3 (mΩ) | Cell 4 (mΩ) |
|---|---|---|---|---|---|---|---|
| 4P−1 | 80 | 11.5 | 11.6 | 46.5 | 46.2 | 46.2 | 46.4 |
| 4P | 12.0 | 11.9 | 39.6 | 51.4 | 50.8 | 51.3 | |
| 4P−1 | 50 | 10.8 | 11.0 | 45.6 | 43.4 | 43.4 | 43.3 |
| 4P | 11.1 | 11.2 | 37.5 | 48.3 | 47.8 | 48.0 | |
| 4P−1 | 20 | 14.7 | 14.8 | 59.0 | 58.8 | 59.4 | 60.2 |
| 4P | 15.0 | 15.0 | 47.9 | 64.4 | 64.4 | 67.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Amin, M.; Barai, A.; Ashwin, T.R.; Marco, J. An Insight to the Degradation Behaviour of the Parallel Connected Lithium-Ion Battery Cells. Energies 2021, 14, 4716. https://doi.org/10.3390/en14164716
Al-Amin M, Barai A, Ashwin TR, Marco J. An Insight to the Degradation Behaviour of the Parallel Connected Lithium-Ion Battery Cells. Energies. 2021; 14(16):4716. https://doi.org/10.3390/en14164716
Chicago/Turabian StyleAl-Amin, Mohammad, Anup Barai, T.R. Ashwin, and James Marco. 2021. "An Insight to the Degradation Behaviour of the Parallel Connected Lithium-Ion Battery Cells" Energies 14, no. 16: 4716. https://doi.org/10.3390/en14164716
APA StyleAl-Amin, M., Barai, A., Ashwin, T. R., & Marco, J. (2021). An Insight to the Degradation Behaviour of the Parallel Connected Lithium-Ion Battery Cells. Energies, 14(16), 4716. https://doi.org/10.3390/en14164716








